Abstract
Both serine/threonine and tyrosine phosphorylation of receptor proteins have been implicated in the process of long-term potentiation (LTP), but there has been no direct demonstration of a change in receptor phosphorylation after LTP induction. We show that, after induction of LTP in the dentate gyrus of anesthetized adult rats, there is an increase in the tyrosine phosphorylation of the 2B subunit of the N-methyl-D-aspartate (NMDA) receptor (NR2B), as well as several other unidentified proteins. Tyrosine phosphorylation of NR2B was measured in two ways: binding of antiphosphotyrosine antibodies (PY20) to glycoprotein(s) of 180 kDa (GP180) purified on Con A-Sepharose and binding of anti-NR2B antibodies to tyrosine-phosphorylated proteins purified on PY20-agarose. Three hours after LTP induction, anti-NR2B binding to tyrosine phosphorylated proteins, expressed as a ratio of tetanized to control dentate (Tet/Con), was 2.21 +/- 0.50 and PY20 binding to GP180 was 1.68 +/- 0.16. This increase in the number of tyrosine phosphorylated NR2B subunits occurred without a change in the total number of NR2B subunits. When the induction of LTP was blocked by pretreatment of the animal with the NMDA receptor antagonist MK801, the increase in PY20 binding to GP180 was also blocked (Tet/Con = 1.09 +/- 0.26). The increased PY20 binding to GP180 was also apparent 15 min after LTP induction (Tet/Con = 1.41 +/- 0.16) but not detectable 5 min after LTP induction (Tet/Con = 1.01 +/- 0.19). These results suggest that tyrosine phosphorylation of the NMDA receptor contributes to the maintenance of LTP.
Full text
PDF

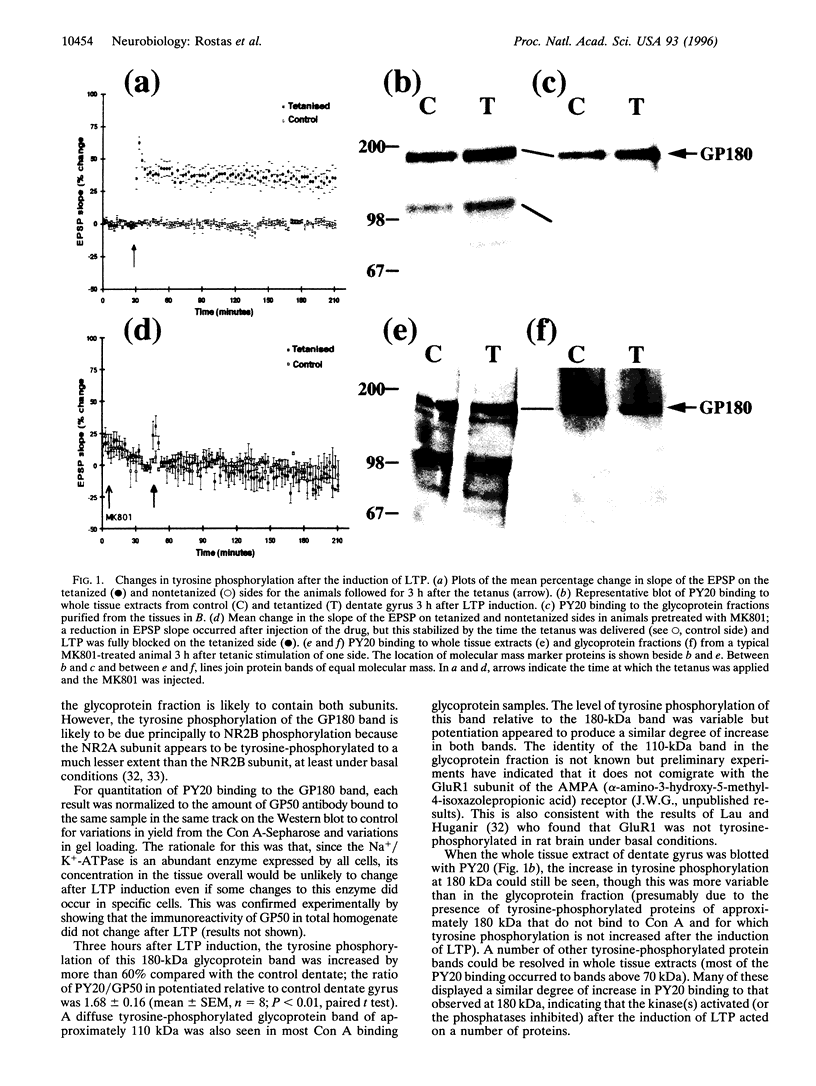

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Saito H. Tyrosine kinase inhibitors, herbimycin A and lavendustin A, block formation of long-term potentiation in the dentate gyrus in vivo. Brain Res. 1993 Sep 3;621(1):167–170. doi: 10.1016/0006-8993(93)90315-e. [DOI] [PubMed] [Google Scholar]
- Abeliovich A., Chen C., Goda Y., Silva A. J., Stevens C. F., Tonegawa S. Modified hippocampal long-term potentiation in PKC gamma-mutant mice. Cell. 1993 Dec 31;75(7):1253–1262. doi: 10.1016/0092-8674(93)90613-u. [DOI] [PubMed] [Google Scholar]
- Abraham W. C., Dragunow M., Tate W. P. The role of immediate early genes in the stabilization of long-term potentiation. Mol Neurobiol. 1991;5(2-4):297–314. doi: 10.1007/BF02935553. [DOI] [PubMed] [Google Scholar]
- Au K. N., Gurd J. W. Tyrosine phosphorylation in a model of ischemia using the rat hippocampal slice: specific, long-term decrease in the tyrosine phosphorylation of the postsynaptic glycoprotein PSD-GP180. J Neurochem. 1995 Oct;65(4):1834–1841. doi: 10.1046/j.1471-4159.1995.65041834.x. [DOI] [PubMed] [Google Scholar]
- Beesley P. W., Paladino T., Gravel C., Hawkes R. A., Gurd J. W. Characterization of gp 50, a major glycoprotein present in rat brain synaptic membranes, with a monoclonal antibody. Brain Res. 1987 Apr 7;408(1-2):65–78. doi: 10.1016/0006-8993(87)90359-3. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993 Jan 7;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudmore S. B., Gurd J. W. Postnatal age and protein tyrosine phosphorylation at synapses in the developing rat brain. J Neurochem. 1991 Oct;57(4):1240–1248. doi: 10.1111/j.1471-4159.1991.tb08285.x. [DOI] [PubMed] [Google Scholar]
- Grant S. G., O'Dell T. J., Karl K. A., Stein P. L., Soriano P., Kandel E. R. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992 Dec 18;258(5090):1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- Gurd J. W., Bissoon N. In vivo phosphorylation of the postsynaptic density glycoprotein gp180. J Neurochem. 1985 Oct;45(4):1136–1140. doi: 10.1111/j.1471-4159.1985.tb05533.x. [DOI] [PubMed] [Google Scholar]
- Gurd J. W., Bissoon N., Kelly P. T. Synaptic junctional glycoproteins are phosphorylated by cyclic-AMP-dependent protein kinase. Brain Res. 1983 Jun 20;269(2):287–296. doi: 10.1016/0006-8993(83)90138-5. [DOI] [PubMed] [Google Scholar]
- Gurd J. W., Bissoon N. Phosphorylation of proteins of the postsynaptic density: effect of development on protein tyrosine kinase and phosphorylation of the postsynaptic density glycoprotein, PSD-GP180. J Neurosci Res. 1990 Mar;25(3):336–344. doi: 10.1002/jnr.490250310. [DOI] [PubMed] [Google Scholar]
- Gurd J. W. Phosphorylation of the postsynaptic density glycoprotein gp180 by Ca2+/calmodulin-dependent protein kinase. J Neurochem. 1985 Oct;45(4):1128–1135. doi: 10.1111/j.1471-4159.1985.tb05532.x. [DOI] [PubMed] [Google Scholar]
- Gurd J. W. Phosphorylation of the postsynaptic density glycoprotein gp180 by endogenous tyrosine kinase. Brain Res. 1985 May 6;333(2):385–388. doi: 10.1016/0006-8993(85)91599-9. [DOI] [PubMed] [Google Scholar]
- Hvalby O., Hemmings H. C., Jr, Paulsen O., Czernik A. J., Nairn A. C., Godfraind J. M., Jensen V., Raastad M., Storm J. F., Andersen P. Specificity of protein kinase inhibitor peptides and induction of long-term potentiation. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4761–4765. doi: 10.1073/pnas.91.11.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T., Moriyoshi K., Sugihara H., Sakurada K., Kadotani H., Yokoi M., Akazawa C., Shigemoto R., Mizuno N., Masu M. Molecular characterization of the family of the N-methyl-D-aspartate receptor subunits. J Biol Chem. 1993 Feb 5;268(4):2836–2843. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lau L. F., Huganir R. L. Differential tyrosine phosphorylation of N-methyl-D-aspartate receptor subunits. J Biol Chem. 1995 Aug 25;270(34):20036–20041. doi: 10.1074/jbc.270.34.20036. [DOI] [PubMed] [Google Scholar]
- Lledo P. M., Hjelmstad G. O., Mukherji S., Soderling T. R., Malenka R. C., Nicoll R. A. Calcium/calmodulin-dependent kinase II and long-term potentiation enhance synaptic transmission by the same mechanism. Proc Natl Acad Sci U S A. 1995 Nov 21;92(24):11175–11179. doi: 10.1073/pnas.92.24.11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G., Larson J., Kelso S., Barrionuevo G., Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 1983 Oct 20;305(5936):719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Kauer J. A., Perkel D. J., Mauk M. D., Kelly P. T., Nicoll R. A., Waxham M. N. An essential role for postsynaptic calmodulin and protein kinase activity in long-term potentiation. Nature. 1989 Aug 17;340(6234):554–557. doi: 10.1038/340554a0. [DOI] [PubMed] [Google Scholar]
- Malinow R., Schulman H., Tsien R. W. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science. 1989 Aug 25;245(4920):862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- McGlade-McCulloh E., Yamamoto H., Tan S. E., Brickey D. A., Soderling T. R. Phosphorylation and regulation of glutamate receptors by calcium/calmodulin-dependent protein kinase II. Nature. 1993 Apr 15;362(6421):640–642. doi: 10.1038/362640a0. [DOI] [PubMed] [Google Scholar]
- Menegoz M., Lau L. F., Hervé D., Huganir R. L., Girault J. A. Tyrosine phosphorylation of NMDA receptor in rat striatum: effects of 6-OH-dopamine lesions. Neuroreport. 1995 Dec 29;7(1):125–128. [PubMed] [Google Scholar]
- Monyer H., Burnashev N., Laurie D. J., Sakmann B., Seeburg P. H. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994 Mar;12(3):529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Monyer H., Sprengel R., Schoepfer R., Herb A., Higuchi M., Lomeli H., Burnashev N., Sakmann B., Seeburg P. H. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992 May 22;256(5060):1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Moon I. S., Apperson M. L., Kennedy M. B. The major tyrosine-phosphorylated protein in the postsynaptic density fraction is N-methyl-D-aspartate receptor subunit 2B. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3954–3958. doi: 10.1073/pnas.91.9.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyoshi K., Masu M., Ishii T., Shigemoto R., Mizuno N., Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991 Nov 7;354(6348):31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992 Oct 23;258(5082):597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- O'Connor J. J., Rowan M. J., Anwyl R. Tetanically induced LTP involves a similar increase in the AMPA and NMDA receptor components of the excitatory postsynaptic current: investigations of the involvement of mGlu receptors. J Neurosci. 1995 Mar;15(3 Pt 1):2013–2020. doi: 10.1523/JNEUROSCI.15-03-02013.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell T. J., Kandel E. R., Grant S. G. Long-term potentiation in the hippocampus is blocked by tyrosine kinase inhibitors. Nature. 1991 Oct 10;353(6344):558–560. doi: 10.1038/353558a0. [DOI] [PubMed] [Google Scholar]
- Pawson T. Protein modules and signalling networks. Nature. 1995 Feb 16;373(6515):573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- Qian Z., Gilbert M. E., Colicos M. A., Kandel E. R., Kuhl D. Tissue-plasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature. 1993 Feb 4;361(6411):453–457. doi: 10.1038/361453a0. [DOI] [PubMed] [Google Scholar]
- Rosenblum K., Dudai Y., Richter-Levin G. Long-term potentiation increases tyrosine phosphorylation of the N-methyl-D-aspartate receptor subunit 2B in rat dentate gyrus in vivo. Proc Natl Acad Sci U S A. 1996 Sep 17;93(19):10457–10460. doi: 10.1073/pnas.93.19.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A. J., Stevens C. F., Tonegawa S., Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992 Jul 10;257(5067):201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Okumura-Noji K. NMDA receptor subunits epsilon 1 (NR2A) and epsilon 2 (NR2B) are substrates for Fyn in the postsynaptic density fraction isolated from the rat brain. Biochem Biophys Res Commun. 1995 Nov 13;216(2):582–588. doi: 10.1006/bbrc.1995.2662. [DOI] [PubMed] [Google Scholar]
- Tan S. E., Wenthold R. J., Soderling T. R. Phosphorylation of AMPA-type glutamate receptors by calcium/calmodulin-dependent protein kinase II and protein kinase C in cultured hippocampal neurons. J Neurosci. 1994 Mar;14(3 Pt 1):1123–1129. doi: 10.1523/JNEUROSCI.14-03-01123.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. A., Leonard J. P. Effect of protein kinase-C activation on the Mg(2+)-sensitivity of cloned NMDA receptors. Neuropharmacology. 1996 Jan;35(1):29–36. doi: 10.1016/0028-3908(95)00177-8. [DOI] [PubMed] [Google Scholar]
- Wang J. H., Feng D. P. Postsynaptic protein kinase C essential to induction and maintenance of long-term potentiation in the hippocampal CA1 region. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2576–2580. doi: 10.1073/pnas.89.7.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. H., Kelly P. T. Postsynaptic injection of CA2+/CaM induces synaptic potentiation requiring CaMKII and PKC activity. Neuron. 1995 Aug;15(2):443–452. doi: 10.1016/0896-6273(95)90048-9. [DOI] [PubMed] [Google Scholar]
- Wang Y. T., Salter M. W. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature. 1994 May 19;369(6477):233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]