Abstract
We performed a systematic review of publications describing a correlation between oral anticoagulant medications and intravesical BCG outcome. We collected information on the impact of such medications on tumour recurrence and progression and we excluded papers not reporting outcome correlations. Patients were divided into group 1 and 2 based on whether they were taking or not taking any anticoagulant medications. A total of 7 manuscripts published between 1990 and 2009 were included in this study. Data heterogeneity precluded meta-analysis. In studies combining all anticoagulant medications, 3 out of 5 (60%) publications did not identify any difference in outcome, while 2 (40%) documented significantly more recurrences in group 1 patients. In studies performing multivariate analysis and only examining the intake of 1 medication, warfarin alone seemed to be associated with increased risk of bladder tumour recurrences and progression following intravesical BCG treatment, while ASA alone seemed to be associated with more protective effects. There is no strong evidence to support the allegations of a protective role of ASA and a deleterious role for warfarin. Further, well-designed experimental and clinical studies are needed to clarify the mechanism of action of intravesical BCG along with possible drug interactions.
Introduction
Bladder cancer is the sixth most common non-cutaneous cancer and the ninth most common cause of overall cancer death in Canada.1 Eighty percent of urothelial cancers present as non-invasive disease. Transurethral resection of bladder tumours (TURBT), along with intravesical Bacillus Calmette-Guérin (BCG) management, is presently considered the most effective management for intermediate and high-risk non-invasive tumours. Despite a significant reduction in recurrence and progression rates following BCG, still up to 40% of patients will not respond and up to 50% may even progress.2,3 Consequently, studies have attempted to define factors that may influence BCG success.
The precise mechanism of BCG antitumour response remains undefined. BCG binding to the extracellular matrix was documented to be a key step in the initiation of its antitumour response.4,5 In experimental studies, medications that inhibit fibrin clot formation have significantly decreased its antitumour activity,4 while antifibrinolytic medications improved its outcome.6,7
A concern regarding the efficacy of intravesical BCG administration thus emerges when taking into account that urothelial cancers are more common in older patients who often have a significant history of vascular diseases and are frequently taking systemic anticoagulants or antiplatelet agents. Multiple publications have tested the outcome of BCG in patients receiving anticoagulant medications with variable results.
The goal of the current study was to perform a systematic review of the literature to determine if administering intravesical BCG in patients on oral anticoagulant or antiplatelet medications affects the prognosis.
Methods
A systematic review was performed in conformity to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement.8
Finding relevant studies
We systematically searched MEDLINE (1946 to January 2013) using both Ovid SP and PubMed search interfaces, EMBASE (1974 to Week 1, 2013), BIOSIS Previews (January 2013), Web of Science with Conference Proceedings (January 2013), and the Cochrane Central Register of Controlled Trials (January 2013) databases; we looked for studies assessing the effect of anticoagulants and antiplatelet agents on the efficacy of BCG intravesical therapy in patients with bladder cancer. We used various combinations of subject headings and text words, such as BCG vaccine, Bacillus Calmette-Guérin, anticoagulants, antiplatelet, antiaggregant, platelet aggregation inhibitors, blood platelets, platelet aggregation, warfarin, acetylsalicylic acid, clopidogrel, fibrin clot inhibitor, urinary bladder neoplasms, transitional cell carcinoma (TCC), urothelial cell carcinoma (UCC), bladder cancer, bladder neoplasm, and bladder tumour.
The search strategies were modified for each electronic database using database-specific search terms, field names and syntax. In addition, the bibliographies of relevant publications, internet-based registry of clinical trials (clinicaltrials.gov), Health Technology Assessment (HTA) agencies, Google and Google Scholar were also searched to identify additional citations and ongoing trials. We excluded papers in languages other than English.
Criteria for considering articles for review
Studies were included if they met the following criteria: (1) the article involves patients with bladder tumours managed with an intravesical BCG instillation; (2) the study entails a cohort of patients who are on at least one oral anticoagulant or antiplatelet medication; and (3) the manuscript included information on prognosis.
For publications studying the outcome of different management modalities, if shown, only data concerning outcome of intravesical BCG instillation was included. Since the goal of this study was to assess association of oral anticoagulant or antiplatelet medication in BCG treated patients with prognosis, all papers not reporting any outcome correlations were excluded. Manuscripts involving anticoagulant medications administered by routes other than orally (e.g., intravesically) were also excluded.
Relevant data were extracted into predesigned custom-made spreadsheets. If available, the outcomes related to a single distinct anticoagulant or antiplatelet medications were extracted separately. Studies were assessed for inclusion by 1 reviewer and corroborated by 2 other independent reviewers. Differences were resolved by consensus.
For descriptive purposes, the cohort of patients taking an anticoagulant or antiplatelet medication was labelled group 1 and those not on an anticoagulant or antiplatelet medication were called group 2.
The primary endpoint was tumour recurrence. The secondary endpoints were (1) recurrence stage and/or grade; (2) tumour progression; and (3) disease-free interval (time to recurrence or progression).
Statistical analysis
A formal meta-analysis was not conducted due to the heterogeneity amongst the publications regarding the reported study populations, oral anticoagulants or antiplatelet drugs used, tumour pathologies, BCG regimens and outcomes. Data were extracted and summarized using central tendency measures and ranges as provided by the authors.
Results
Search results and characteristics of included studies
We identified 375 citations; after removing duplicate citations, 311 records were assessed for relevance. In total, 303 records were excluded. One pertinent record that was only published as a conference abstract was also excluded.9 In total, 7 studies met the inclusion criteria (Fig. 1) and were included in this analysis. Four (57%) of these studies were North American,10–13 2 (29%) Australian14,15 and one (14%) European.16 These papers described investigations conducted between 1981 and 2006 and were published between1990 and 2009. For all manuscripts, the outcome analysis in relation to fibrin clot inhibitors was computed retrospectively. The included studies featured a total of 1423 patients with primary or recurrent bladder urothelial carcinoma treated with intravesical BCG. None of the publications included patients with upper tract urothelial carcinoma. They involved 341 patients on at least 1 anticoagulant or antiplatelet medication (Group 1).
Fig. 1.
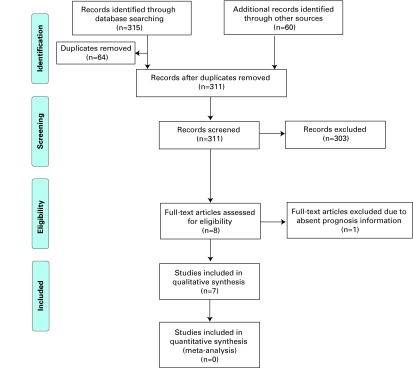
PRISMA Study Selection Flow Diagram. Adapted from Moher et al.8
Baseline patient characteristics and study endpoints for all studies are shown in Table 1; medication history, tumour characteristics and BCG information are in Table 2.
Table 1.
Baseline patient characteristics, study design and study endpoints
| Hudson et al.13 1990 | P’ng et al.15 1993 | Witjes et al.16 1993 | Rogerson14 1994 | Heiner et al.12 2008 | Gee et al.11 2008 | Boorjian et al.10 2009 | |
|---|---|---|---|---|---|---|---|
| Study years included | Dec 1981–June 1989 | 1985–1990 | Apr 1987–Dec 1991 | Dec 1987–Oct 1991 | Dec 1999–July 2004 | June 1991–Sep 2003 | July 1978–Nov 2006 |
| Study type Institution | Retrospective
|
Retrospective
|
Retrospective
|
Retrospective Heidelberg Repatriation Hospital |
Retrospective
|
Retrospective
|
Retrospective
|
| Province/country | Missouri, USA | Queensland, Australia | Netherlands | Victoria, Australia | Georgia, USA | Wisconsin, USA | New York, USA |
| Total patients | 162 | 45 | 313 | 56 | 58 | 154 | 1218 |
| Included patients | 149 | 45 | 183 | 38 | 58 | 43 | 907 |
| Inclusion selection criteria | Available medication record | All patients included | N/A | Available medication record | N/A | Available records |
|
| Anticoagulant G1 | 29 | 9 | 42 | 13 | 7 | 20 | 221 |
| Control G2 | 120 | 36 | 141 | 25 | 51 | 23 | 686 |
| Male: Female ratio Statistical significance | N/A | 33:12 | N/A | N/A | N/A | 36:7 More males in G1 (NS) |
721:186 More males in G1 (p < 0.01) |
| Age, years (range) Statistical significance | N/A | Mean both groups: 69 (36–92) | N/A | N/A | N/A | Mean
p < 0.01 |
Median
p < 0.01 |
| Follow-up (range) | Mean
|
Mean both groups 20.3 months Median both groups 16 months (3–56) |
Mean
|
Mean varied among stages ranging from 6 to 28 months (2-36) | N/A | N/A | Median 4.2 years in patients without recurrence and 3.7 years without progression |
| Primary endpoint studied | Tumour recurrence | Persistent or recurrence tumour at first follow-up in 3 months | Disease (recurrence) free interval | Persistent or recurrence disease at 6 weeks | Response to therapy categorized in relation to age
|
Recurrence-free and progression-free intervals | Tumour recurrence and progression to open surgery |
G: group; N/A: not available
Table 2.
Medication history, tumour characteristics and BCG information
| Hudson et al.13 1990 | P’ng et al.15 1993 | Witjes et al.16 1993 | Rogerson14 1994 | Heiner et al.12 2008 | Gee et al.11 2008 | Boorjian et al.10 2009 | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Gp1/Gp2 | 29/120 | 9/36 | 42/141 | 13/25 | 7/51 | 20/23 | 221/686 | |
| Medication history | Anticoagulant medication used in G1 |
|
|
|
|
|
ASA (n=20) |
|
| Other concomitant medications | Antibiotics, CVS, respiratory, DM, CNS, GIT, steroids, thyroid, vitamins, antihistaminics | Unknown, No Antibiotics | Antibiotics (more significant in G1), CVS, CNS, respiratory, paracetamol and others | N/A | N/A | N/A | N/A | |
|
| ||||||||
| Tumour characteristics | Primary/recurrent urothelial carcinoma pT stage | Primary or recurrent | N/A | Primary or recurrent | Primary or recurrent | N/A | N/A | N/A |
| CIS, pTa, pT1, G1: 24%, 38%, 38% G2: 22%, 42%, 36%
|
CIS, pTa, (not stratified by anticoag. gps) | CIS, pTa, pT1 G1: 9%, 60%, 31% G2: 11%, 64%, 25%
|
CIS, pTa, pT1,p T4 (not stratified by anticoag. group) [1 pt had history of pT2, treated with ERT and rec as T1) |
CIS, pTa, pT1 (not stratified by anticoag group) | CIS, pure papillary bladder cancer G1: 75%, 40% G2: 91%, 13%
|
Stages imputed as:
G1: 69%, 2%, 29% G2: 70%, 5%, 26%
|
||
| Grade | Grade I, II, III G1: 17%, 69%, 14% G2: 21%, 63%, 16% |
Grade I, II, III (not stratified by anticoagulant groups) | N/A | N/A | N/A | N/A | Grades imputed as: Low, high and unknown G1: 9%, 50%, 42% G2: 13%, 48%, 39% |
|
|
| ||||||||
| BCG information | BCG strain | Pasteur | Pasteur strain | TICE* (n=25) and RIVM±(n=17) | Pasteur strain | TICE* | N/A | N/A |
| Dose | 120 mg | 120 mg | 5 × 108 | 60 mg or 120 mg | 50 mg | N/A | N/A | |
| Dilution | in 50 cc | in N/A | in 50 cc | in 50 cc | N/A | N/A | ||
| Time in Bladder | normal saline 2 hours | N/A | normal saline N/A |
in 60 cc normal saline 1.5 hours | normal saline N/A |
N/A | N/A | |
| Interval given post-TURBT | within 6 months | N/A | 7 to 15 d | N/A | N/A | N/A | N/A | |
| Course induction cycle |
|
1/week × 6 weeks |
|
|
1/week × 6 weeks | N/A | 1/week × 6 weeks | |
| Maintenance BCG | N/A | N/A | N/A | N/A |
|
Not all patients G1: 3 (15%) G2: 6 (26%) |
N/A | |
| BCG complications | N/A | N/A | Bacterial cystitis G1>G2 | Not stratified by anticoagulant group |
|
N/A | N/A | |
G: group; TICE: Merck & Co. Inc., Whitehouse Station, NJ; ±RIVM: A Dutch BCG preparation. BCG: Bacille Calmette-Guérin; ASA: acetylsalicylic acid; NSAID: nonsteroidal antiinflammatory drugs; CVS: cardiovascular; DM: diabetes mellitus; CNS: central nervous system; GIT: gastrointestinal tract; N/A: not available; CIS: carcinoma in situ.
While 5 studies10,13–16 combined the outcome of different medications taken either separately or together (Table 3), only 3 articles10–12 tested the outcome of only 1 type of anticoagulant or antiplatelet medication (Table 3, Table 4). All manuscripts reported tumour recurrence following intravesical BCG. Tumour progression was evaluated in 3 studies.10,11,13 Multivariate analyses was only performed in 3 studies10–12 (Table 3, Table 4).
Table 3.
Studies combining the outcome of different anticoagulant or antiplatelet medications
| Hudson et al.13 1990 | P’ng et al.15 1993 | Witjes et al.16 1993 | Rogerson14 1994 | Heiner et al.12 2008 | Gee et al.11 2008 | Boorjian et al.10 2009 | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| G1/G2 Medication | 29/120 Combined | 9/36 Combined | 42/141 Combined | 13/25 Combined | 7/51 Warfarin | 20/23 ASA | 221/686 Combined |
| - Recurrence in G1 | -15/29 (52%)* | - 8/9 (88.9%) | - 13/42 (31%)* | - 11/13 (84.6%)* | - N/A | - 8/20 (40%)* | - 189/221 (85.5%)* |
| - Time to recurrence (range) | - N/A | - NA | - Mean: 9.7 months** (2.9-20.4) | - N/A | - N/A | - 5-year risk: 36%* | - Median: 7 months* |
| - Progression or recurrence stage/grade |
|
- N/A | - N/A | - N/A | - N/A | - 4/20 (20%)** (stages not shown) | - 80/221 to open surgery* (stages not shown) |
| - Time to progression | - N/A | - N/A | - N/A | - N/A | - N/A | - N/A | - 5-year risk: 40%* |
| - Recurrence in G2 | - 40/120 (33%)* | - 11/36 (30.6%)* | - 56/141 (40%)* | -12/25 (48%)* | - N/A | - 17/23 (74%)* | - 566/686 (82.5%)* |
| - Time to recurrence (range) | - N/A | - NA | - Mean: 9.4 months** (2.4–43.4) | - N/A | - N/A | - 5-year risk: 73%* | - Median: 6 months* |
| -10/120 | - N/A | - N/A | - N/A | - N/A | - 7/23 (30%)** (stages not shown) | - 270/686 to open surgery* (stages not shown) | |
| - Progression or recurrence stage/grade | (8%)** recurrence as superficial/CIS
|
||||||
| - N/A | - N/A | - N/A | - N/A | - N/A | - N/A | - 5-year risk: 43%* | |
| -Time to progression | |||||||
| Statistical Tests: | Chi square test | Fisher’s exact test | Kaplan-Meier/log-rank test | Chi-square test | - N/A | Kaplan-Meier/log-rank test | Kaplan-Meier/log-rank test |
| p value |
*p = 0.065 **p < 0.01 |
*x2 = 7.79, *p < 0.01 |
p = 0.28 (NS) |
*p < 0.05 |
*p = 0.03 **p = 0.4 (NS) |
*p = 0.6 (NS) | |
| Univariate results summary |
|
|
|
|
- N/A |
|
|
| Multivariate analyses results | |||||||
| - Recurrences | N/A | N/A | N/A | N/A |
|
|
N/A |
| - Progression | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| - Statistical tests | N/A | N/A | N/A | N/A | - ANOVA | - Multivariate analysis | N/A |
| - Controlled covariates |
|
|
|||||
Asterisks indicate data used for p value calculations.
G: group; BCG: Bacille Calmette-Guérin; ASA: acetylsalicylic acid; CIS: carcinoma in situ; N/A: not available; NS: non-significant; HR: hazard ratio; ANOVA: analysis of variance.
Table 4.
Study reporting the outcome of ASA or warfarin intake alone in a subgroup cohort
| Boorjian et. al.10 2009 | ||||
|---|---|---|---|---|
|
| ||||
| G1/G2 | 170/737 | 52/855 | ||
| Anticoagulant | ASA | Warfarin | ||
| Univariate analyses using Kaplan-Meier /log-rank test | - Recurrence in G1 | - N/A | - N/A | |
| - Time to recurrence (range) | - Median: 7 months* | - Median: 7 months* | ||
| - 5-year risk of recurrence | - N/A | - N/A | ||
| - Progression | - N/A | - N/A | ||
| - Time to progression | - N/A | - Median: 2.1 years | ||
| - 5-year risk of progression | - 34%** | - 65%** | ||
| - Recurrence in G2 | - N/A | - N/A | ||
| - Time to recurrence (range) | - Median: 6 months* | - Median: 6 months* | ||
| - 5-year risk of recurrence | - N/A | - N/A | ||
| - Progression | - N/A | - N/A | ||
| - Time to progression | - N/A | - Median: 9 years | ||
| - 5-year risk of progression | - 44%** | - 41%** | ||
| p value | *p = 0.6 (NS) | *p = 0.14 (NS) | ||
| **p = 0.029 | **p = <0.01 | |||
| Results summary: | - Similar recurrence in both groups | - Similar recurrence in both groups | ||
| - Sig. more prog. in Gp2. | - Significantly more progression in G1 | |||
|
| ||||
| Multivariate analyses | Recurrence | Impact of 1 anticoagulant medication in a cohort of patients taking any 1 or more anticoagulant | - NS difference among both groups | - NS difference among both groups |
| - HR: 0.91 (0.75- 1.10) | - HR: 1.19 (0.89-1.59) | |||
| - p = 0.3 | - p = 0.2 | |||
| Impact of 1 anticoagulant medication in a cohort of patients only taking 1 anticoagulant | - NS difference among both groups (data not shown) | - Significantly increased risks in G1 | ||
| - HR: 1.39, 95% CI (1-1.94) | ||||
| - p = 0.047 | ||||
| - Progression | - Significantly decreased risk of progression to open surgery | - Significantly increased risks in G1 | ||
| - HR: 1.89, 95% CI (1.31-2.74) | ||||
| - HR: 0.71, 95%CI (0.52-0.96) | - p = 0.0007 | |||
| - p = 0.024 | ||||
| - Statistical tests | - Cox proportional hazards regression model | |||
| - Controlled covariates | - Age, initial tumour stage (superficial vs. invasive) and anticoagulation medication used | |||
Asterisks indicate data used for p value calculations. N/A: not available; NS: non-significant; G: group; HR: hazard ratio; CI: confidence interval.
Effects of anticoagulant or antiplatelet medications on tumor recurrence
Studies combining patients on multiple anticoagulant or antiplatelet medications taken either separately or together
Two studies14,15 documented significantly higher overall tumour recurrence rates in Group 1. One study16 showed a non-significant tendency towards more recurrences in Group 2 patients, while 2 other manuscripts did not show differences among both groups10,13 even after controlling for the type of anticoagulant or antiplatelet medication used.10 When excluding tumour progression from overall recurrences, 1 of the 2 latter studies13 revealed a significantly increased rate of superficial tumour recurrences in Group 1 patients. Time to recurrence was assessed in 2 studies10,16 and was not significantly different between both groups (Table 3).
Studies assessing patients taking only one type of anticoagulant or anti-platelet medication
For patients taking oral warfarin alone, 1 study did not document any difference in recurrence and in time to recurrence among both groups by univariate analysis.10 In 2 studies using multivariate analyses, warfarin intake alone was associated with significantly increased risks of recurrences in Group 1 patients following intravesical BCG treatment.10,12 In patients taking only systemic acetylsalicylic acid (ASA), by means of univariate analysis, 1 study reported significantly higher risk of tumour recurrence in Group 2 patients,11 whereas another study did not.8 When using multivariate analyses, 1 study documented evidence of significantly decreased risk of recurrence within Group 1 patients after controlling for age, sex, smoking status, maintenance BCG, and the presence of carcinoma in situ (CIS) or papillary tumour.11 Another study however failed to show any significant difference in recurrence rates among both groups after controlling for age, initial tumour stage (superficial vs. invasive), and anticoagulation medication used.10
Effects of anticoagulant or antiplatelet medications on tumour progression
Studies combining patients on multiple anticoagulant or antiplatelet medications taken either separately or together
There was neither a significant difference in tumour progression nor in time to progression in both groups when tested by univariate analyses.10,13
Studies assessing patients taking only one type of anticoagulant or anti-platelet medication
Among patients taking oral warfarin alone, using both univariate and multivariate analyses, 1 study indicated significantly higher progression risk in Group 1 patients with a shorter median time to progression.10 In patients taking only systemic ASA, using univariate analyses, 1 study indicated no difference in tumour progression among both groups,11 while another study documented significantly decreased risk of progression among Group 1 patients.10 On multivariate analysis, only 1 study reported a significantly decreased risk of progression to open surgery following intravesical BCG instillation in patients taking ASA.10
Discussion
Along with TURBT, intravesical BCG has become a common treatment to manage intermediate- and high-risk non-invasive bladder cancer. Intravesical BCG was shown to significantly decrease tumour recurrence and progression when compared to TURBT alone. Still, up to 40% of patients receiving intravesical BCG failed to respond and as high as 50% of patients even progressed.2,3 Thus, it becomes intuitively evident to try and examine potential risks influencing BCG treatment success.
The exact mechanism by which BCG creates its response remains unclear. It may be achieved through several mechanisms.17 TURBT initially exposes the lamina propria and basement membrane of the urothelium leading to fibrin clot formation, altogether rich in fibronectin. The live attenuated strain of Mycobacterium bovis can thus adhere to the newly exposed fibronectin and undergo endocytosis resulting into a massive release of cytokines, chemokines and leads to activation of natural killer cells and cytotoxic T lymphocytes.18 This BCG-fibronectin binding capacity directly correlates with BCG anti-tumor activity.19 This key role of fibronectin in initiating an antitumour response was documented in a murine model, when administering anti-fibronectin antibodies lead to a total loss of BCG anti-tumour activity.4 Likewise, intravesical heparin administration inhibited BCG-fibronectin binding in a rabbit model.7 Conversely, antifibrinolytic agents given intravenously6 or intravesically7 significantly improved BCG antitumour responses in experimental models. It was thus postulated that medications inhibiting fibrin clot formation would adversely influence BCG efficacy. Based on these findings, antifibrinolytic agents were administered intravesically (to prevent their systemic adverse effects) in a randomized prospective double blinded controlled study including 257 non-invasive low- to intermediate-risk bladder cancer patients. This study reported significantly favourable outcomes on a short median follow-up of 2 years.20
Since bladder tumours are more common in older patients who frequently suffer from cardiac and/or cerebrovascular diseases and are often dependent on anticoagulant or antiplatelet medications, the efficacy of intravesical BCG in these patients has been questioned. Therefore, we systematically reviewed the literature to try and identify if concomitant intake of anticoagulant/antiplatelet medications affects the prognosis of intravesical BCG. For descriptive analyses, patients were divided into Groups 1 and 2 based on whether they were taking anticoagulant or antiplatelet medications or not, respectively.
The ideal method to test our outcomes is to design randomized, controlled prospective studies. However, if no randomized studies exist, observational studies showing consistent results can still be used to reasonably draw valid conclusion; there is a caveat: if such studies are retrospective and inadequately conducted, they are prone to inherent selection biases.
Given the heterogeneity of these studies we could not perform a meta-analysis. Reviewing the literature has revealed significant differences:
Failure to reveal critical baseline patient characteristics;
Limited number of study patients;
Exclusions of up to more than half of patients due to unavailability of medication records;
Different mean age among tested groups;
Different duration and follow-up schedules;
Different use of anticoagulant or antiplatelet medication type, dosage and duration;
Combining the outcome of different anticoagulant or antiplatelet medications groups whether taken separately or together;
Failure to take into account the concomitant intake of other medications;
Diverse risk factors for bladder cancer prognosis;
Different pre-treatment tumour stages and grades;
Variable BCG strains used along with multiple BCG regimens;
Unavailability of BCG complications;
Unknown recurrence stage and grade;
Different tested endpoints using variable statistical analyses; and
An ill-defined time to recurrence with possible introduction of lead time biases.
Despite all these differences, most investigators did not control for the vast variation in their examined variables. Among those studies that performed univariate analysis, 5 combined the different groups of anticoagulant or antiplatelet medications.10,13–16 Only 1 manuscript tested individual recurrences based on invasion stage and reported more superficial recurrences in group 1 patients.13 However, most publications only compared overall recurrences and 60% of the studies failed to show any statistical difference in disease recurrence among both groups.10,13,16 The conference abstract that was excluded from this study also failed to show any significant difference in recurrence-free survival rates following antiplatelet administration.9 Two studies examined tumour progression following BCG and both found no differences among both groups.10,13
Only 2 published manuscripts reported the outcome of patients taking oral warfarin alone. One of the studies used multivariate analyses in a cohort of patients taking 1 or more anticoagulant medications and did not find any significant difference in recurrence rates among both groups after controlling for the type of anticoagulant medication used. It was only when the authors sub-selected a cohort (using only 1 anticoagulant medication) and compared those to a group of controls that a significantly increased risk of recurrence following BCG was found among patients on warfarin (Table 4).10 Such analytical strategy raises serious concerns regarding the possibility of selection bias.
This manuscript had further limitations some of which were acknowledged by the authors, given the difficult nature of conducting and planning such studies. These limitations involved combining pTa, pT1 and CIS tumour stages that are known to have dissimilar risks and prognosis under one category. It also included the inclusion of a significant proportion of patients with unknown stage and grade. Furthermore authors did not control for other important risk factors, like smoking history and duration of warfarin medication use. Moreover these patients never received a maintenance intravesical BCG.
Similarly, another study compared the outcome of warfarin intake alone by ranking the response to BCG therapy into an ordinal scale of 1 to 4 and averaging those responses.12 The authors reported a poorer response rank of 2.6 following BCG in patients on warfarin independent of age. This study also had many limitations, including drawing conclusions from only 7 patients in the warfarin group and not controlling for all of the other risk factors. Furthermore, these scale numbers represent a rank with no numerical measurable differences between groups, thus computing means becomes very confusing and caution should be taken prior to drawing conclusion from such statistical analysis. Additionally, analysis of variance (ANOVA) for such categorical values may lead to misleading results that go beyond the normal chance of Type I and Type II errors.21
By means of multivariate analyses, 2 publications tested intravesical BCG outcome in patients only taking ASA and both described a seemingly protective effect.10,11 While 1 study reported significantly fewer recurrences with no difference in progression in patients taking ASA,11 the other manuscript failed to find any significant difference in recurrence and found a significantly decreased risk of progression to open surgery in the ASA group.10 Several mechanisms have been suggested to elucidate the possible ASA beneficiary effects.10 It may be in part explained by the fact that local or systemic anticoagulants may prevent tumour cell adhesion and implantation to injured urothelium.22,23 Furthermore, ASA may exert its protective role through its non-selective cyclooxygenase (COX)-2 inhibition. COX-inhibiting drugs have antitumour activity in both canine and rodent models of urinary bladder cancer.24 Additionally COX-2 was found to be expressed in human CIS and invasive urothelial carcinoma, but not in the normal bladder.24 Moreover, COX inhibition in a murine model enhanced the production of interleukin-12 by BCG activated dendritic cells and generated type 1 T-helper response that may partially justify the improved BCG efficacy.25
The limitations of this study are similar to those in previously published studies. This study highlights the fact that our cumulative understanding in this area is very limited and insufficient to justify the practice of discontinuing warfarin prior to intravesical BCG therapy. We also suggest that patients continue their ASA medications for their cardiovascular benefits rather than for any other potential benefits in changing the prognosis of their bladder cancer.
Conclusions
A total of 7 published manuscripts examined the outcome of intravesical BCG in patients receiving anticoagulant or antiplatelet medications with contradictory results. Based on published data, there is no strong evidence to either encourage or discourage anticoagulant or antiplatelet medications while on intravesical BCG therapy yet. Further experimental and properly conducted clinical studies are still needed to further explore drug interactions and elucidate the mechanism of action of BCG.
Footnotes
Competing interests: Dr. Lazo-Langner has received honoraria for Advisory Board membership from Pfizer and LeoPharma. He has also received an unrestricted educational grant and honoraria from Alexion and Pfizer. He has participated in clinical trials for Pfizer, LeoPharma, Bayer and Daiichi-Sankyo. Dr. Fahmy, Ms. Iansavichene and Dr. Pautler all declare no competing financial or personal interests.
This paper has been peer-reviewed.
References
- 1. Canadian Cancer Society’s Steering Committee on Cancer Statistics: Canadian Cancer Statistics (2012). http://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/Canadian-Cancer-Statistics-2012---English.pdf. Accessed October 29, 2013.
- 2.Witjes JA. Management of BCG failures in superficial bladder cancer: a review. Eur Urol. 2006;49:790–7. doi: 10.1016/j.eururo.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 3.Zlotta AR, Fleshner NE, Jewett MA. The management of BCG failure in non-muscle-invasive bladder cancer: an update. Can Urol Assoc J. 2009;3:S199–205. doi: 10.5489/cuaj.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kavoussi LR, Brown EJ, Ritchey JK, et al. Fibronectin-mediated Calmette-Guerin bacillus attachment to murine bladder mucosa. Requirement for the expression of an antitumor response. J Clin Invest. 1990;85:62–7. doi: 10.1172/JCI114434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ratliff TL, Palmer JO, McGarr JA, et al. Intravesical Bacillus Calmette-Guerin therapy for murine bladder tumors: initiation of the response by fibronectin-mediated attachment of Bacillus Calmette-Guerin. Cancer Res. 1987;47:1762–6. [PubMed] [Google Scholar]
- 6.Hudson MA, Brown EJ, Ritchey JK, et al. Modulation of fibronectin-mediated Bacillus Calmette-Guerin attachment to murine bladder mucosa by drugs influencing the coagulation pathways. Cancer Res. 1991;51:3726–32. [PubMed] [Google Scholar]
- 7.Shen ZJ, Wang Y, Ding GQ, et al. Study on enhancement of fibronectin-mediated bacillus Calmette-Guerin attachment to urinary bladder wall in rabbits. World J Urol. 2007;25:525–9. doi: 10.1007/s00345-007-0198-z. [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Yuge K, Kikuchi E, Tanaka N, et al. Clinical impact of anti-platelet medication in patients with non-muscle invasive bladder cancer treated with BCG therapy. Urology. 2012;80:S218. [Google Scholar]
- 10.Boorjian SA, Berglund RK, Maschino AC, et al. Fibrin clot inhibitor medication and efficacy of bacillus Calmette-Guerin for bladder urothelial cancer. J Urol. 2009;182:1306–12. doi: 10.1016/j.juro.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 11.Gee JR, Jarrard DF, Bruskewitz RC, et al. Reduced bladder cancer recurrence rate with cardioprotective aspirin after intravesical bacille Calmette-Guerin. BJU Int. 2009;103:736–9. doi: 10.1111/j.1464-410X.2008.08123.x. [DOI] [PubMed] [Google Scholar]
- 12.Heiner JG, Terris MK. Effect of advanced age on the development of complications from intravesical bacillus Calmette-Guerin therapy. Urol Oncol. 2008;26:137–40. doi: 10.1016/j.urolonc.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Hudson MA, Yuan JJ, Catalona WJ, et al. Adverse impact of fibrin clot inhibitors on intravesical bacillus Calmette-Guerin therapy for superficial bladder tumors. J Urol. 1990;144:1362–4. doi: 10.1016/s0022-5347(17)39741-0. [DOI] [PubMed] [Google Scholar]
- 14.Rogerson JW. Intravesical bacille Calmette-Guerin in the treatment of superficial transitional cell carcinoma of the bladder. Br J Urol. 1994;73:655–8. doi: 10.1111/j.1464-410x.1994.tb07551.x. [DOI] [PubMed] [Google Scholar]
- 15.P’Ng K B, Walsh MD, Seymour GJ, et al. The adverse effect of fibrin-clot inhibiting drugs on intravesical bacillus Calmette-Guerin efficacy for superficial bladder cancer. Aust N Z J Surg. 1993;63:127–30. doi: 10.1111/j.1445-2197.1993.tb00060.x. [DOI] [PubMed] [Google Scholar]
- 16.Witjes JA, vd Meijden AP, Doesburg W, et al. Influence of fibrin clot inhibitors on the efficacy of intravesical Bacillus Calmette-Guerin in the treatment of superficial bladder cancer. The Dutch Southeast Cooperative Urological Group. Eur Urol. 1993;23:366–70. doi: 10.1159/000474631. [DOI] [PubMed] [Google Scholar]
- 17.Zuiverloon TC, Nieuweboer AJ, Vekony H, et al. Markers predicting response to bacillus Calmette-Guerin immunotherapy in high-risk bladder cancer patients: a systematic review. Eur Urol. 2012;61:128–45. doi: 10.1016/j.eururo.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 18.Alexandroff AB, Jackson AM, O’Donnell MA, et al. BCG immunotherapy of bladder cancer: 20 years on. Lancet. 1999;353:1689–94. doi: 10.1016/S0140-6736(98)07422-4. [DOI] [PubMed] [Google Scholar]
- 19.Hudson MA, Ritchey JK, Catalona WJ, et al. Comparison of the fibronectin-binding ability and antitumor efficacy of various mycobacteria. Cancer Res. 1990;50:3843–7. [PubMed] [Google Scholar]
- 20.Pan CW, Shen ZJ, Ding GQ. The effect of intravesical instillation of antifibrinolytic agents on bacillus Calmette-Guerin treatment of superficial bladder cancer: a pilot study. J Urol. 2008;179:1307–11. doi: 10.1016/j.juro.2007.11.045. [DOI] [PubMed] [Google Scholar]
- 21.Jaeger TF. Categorical Data Analysis: Away from ANOVAs (transformation or not) and towards Logit Mixed Models. J Mem Lang. 2008;59:434–46. doi: 10.1016/j.jml.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.See WA, Chapman PH. Heparin prevention of tumor cell adherence and implantation on injured urothelial surfaces. J Urol. 1987;138:182–6. doi: 10.1016/s0022-5347(17)43040-0. [DOI] [PubMed] [Google Scholar]
- 23.See WA, Miller JS, Williams RD. Pathophysiology of transitional tumor cell adherence to sites of urothelial injury in rats: mechanisms mediating intravesical recurrence due to implantation. Cancer Res. 1989;49:5414–8. [PubMed] [Google Scholar]
- 24.Mohammed SI, Knapp DW, Bostwick DG, et al. Expression of cyclooxygenase-2 (COX-2) in human invasive transitional cell carcinoma (TCC) of the urinary bladder. Cancer Res. 1999;59:5647–50. [PubMed] [Google Scholar]
- 25.Dovedi SJ, Kirby JA, Atkins H, et al. Cyclooxygenase-2 inhibition: a potential mechanism for increasing the efficacy of bacillus calmette-guerin immunotherapy for bladder cancer. J Urol. 2005;174:332–7. doi: 10.1097/01.ju.0000161589.85869.ae. [DOI] [PubMed] [Google Scholar]


