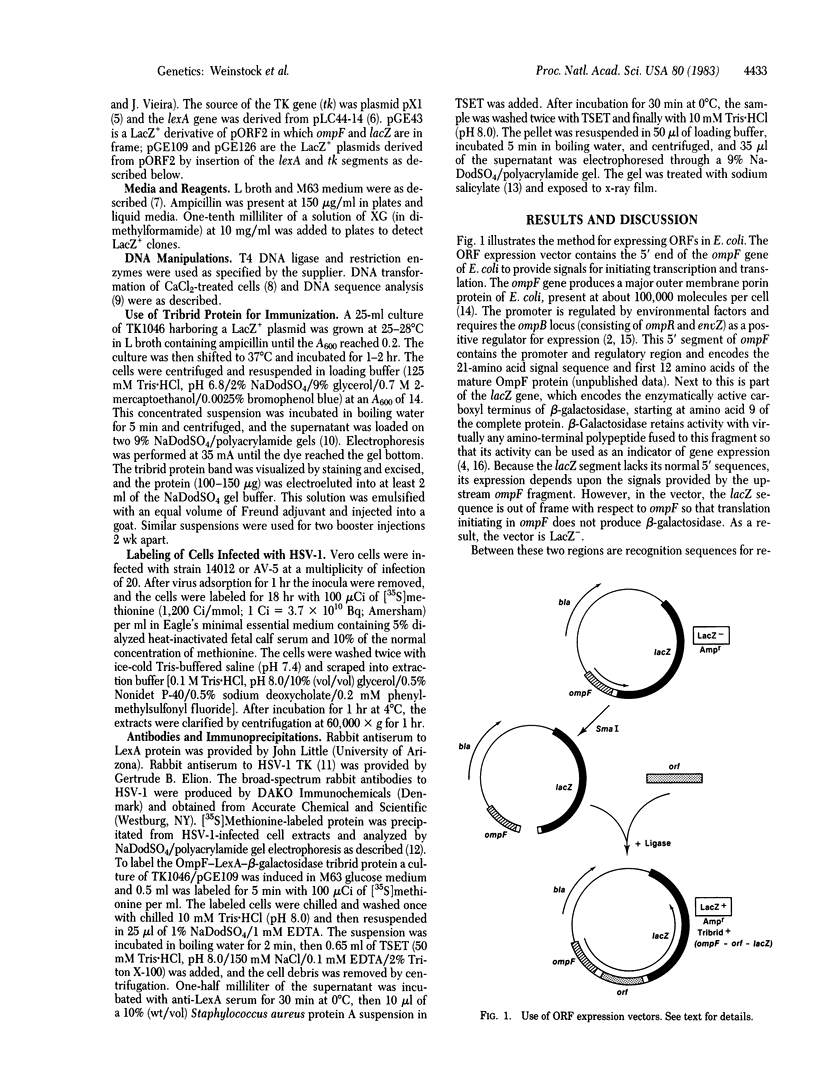
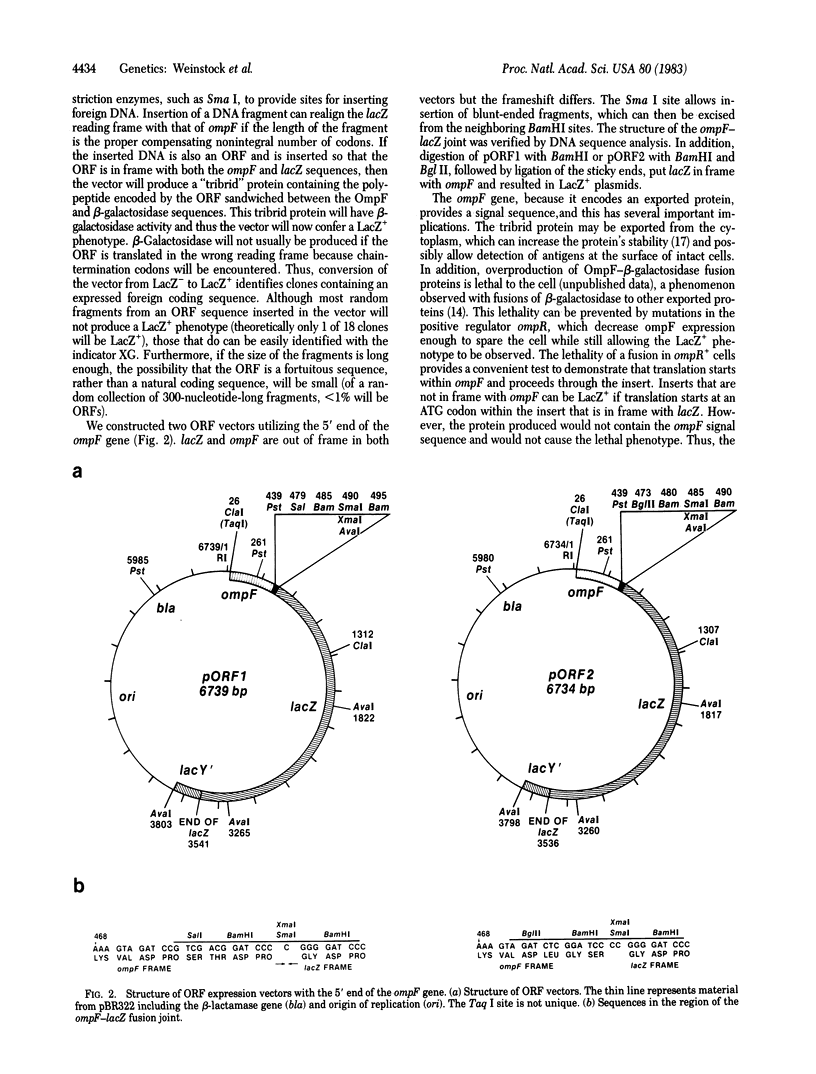
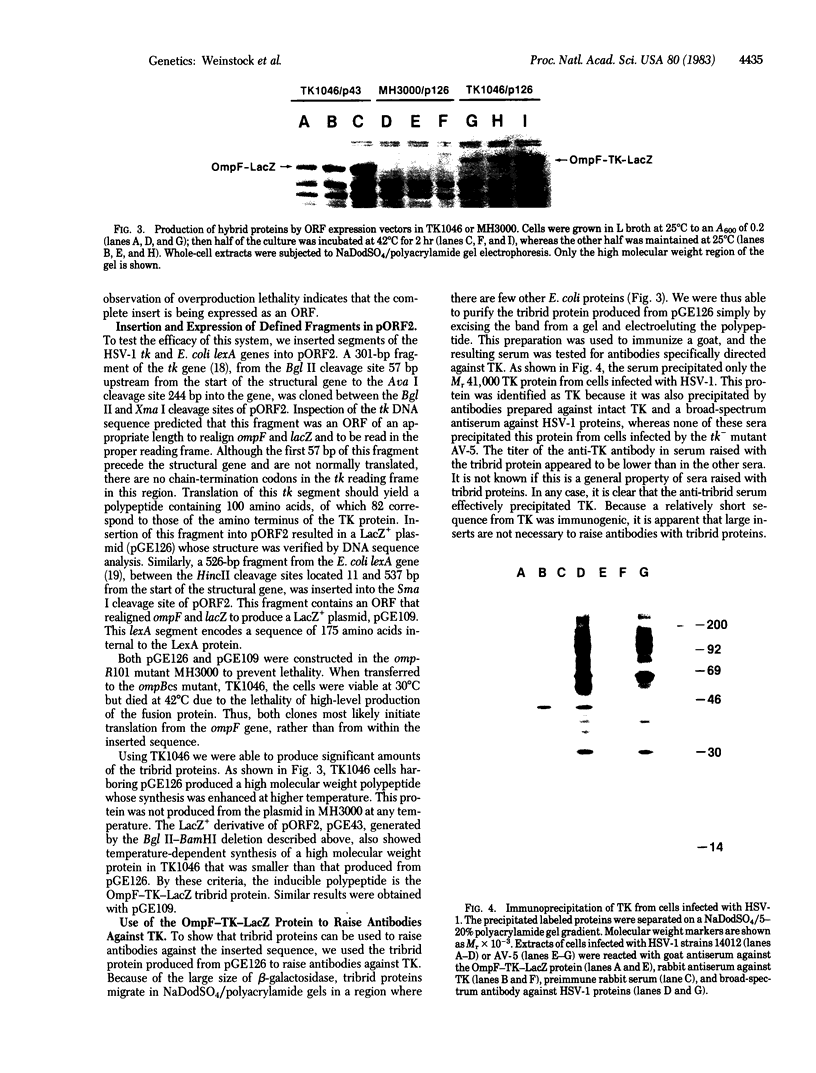
Abstract
We have developed an Escherichia coli plasmid vector for the identification and expression of foreign DNA segments that are open reading frames (ORFs). The 5' end of ompF, an E. coli gene encoding an abundant outer membrane protein, is used to provide a strong, regulated promoter, translation initiation site, and signal sequence for export from the cytoplasm. This sequence is coupled to the lacZ gene of E. coli so that expression of beta-galactosidase requires ompF transcription and translation signals. However, this hybrid gene is LacZ- because lacZ is out of frame with respect to ompF. Restriction enzyme recognition sites are located between ompF and lacZ to allow convenient insertion of DNA fragments. If an insert is an ORF of the correct length, ompF and lacZ become realigned in frame, resulting in a LacZ+ gene that produces a tribrid protein with the translation product of the insert sandwiched between OmpF and beta-galactosidase. The LacZ+ phenotype thus identifies clones containing an expressed ORF. To demonstrate the vector's utility we inserted a fragment from the herpes virus thymidine kinase gene and used the resulting tribrid protein to raise antibodies that precipitate thymidine kinase from herpes virus-infected cells. We also inserted a fragment from the E. coli lexA gene to produce a tribrid protein that is precipitated by antiserum raised with LexA protein. Thus, tribrid fusion proteins can be used to produce or detect antibodies and also to identify the product of a cloned gene.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. Shotgun DNA sequencing using cloned DNase I-generated fragments. Nucleic Acids Res. 1981 Jul 10;9(13):3015–3027. doi: 10.1093/nar/9.13.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchel D. E., Gronenborn B., Müller-Hill B. Sequence of the lactose permease gene. Nature. 1980 Feb 7;283(5747):541–545. doi: 10.1038/283541a0. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Enquist L. W., Vande Woude G. F., Wagner M., Smiley J. R., Summers W. C. Construction and characterization of a recombinant plasmid encoding the gene for the thymidine kinase of Herpes simplex type 1 virus. Gene. 1979 Nov;7(3-4):335–342. doi: 10.1016/0378-1119(79)90052-0. [DOI] [PubMed] [Google Scholar]
- Gray M. R., Colot H. V., Guarente L., Rosbash M. Open reading frame cloning: identification, cloning, and expression of open reading frame DNA. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6598–6602. doi: 10.1073/pnas.79.21.6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Genetic analysis of the major outer membrane proteins of Escherichia coli. Annu Rev Genet. 1981;15:91–142. doi: 10.1146/annurev.ge.15.120181.000515. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Genetic analysis of the ompB locus in Escherichia coli K-12. J Mol Biol. 1981 Sep 5;151(1):1–15. doi: 10.1016/0022-2836(81)90218-7. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. The ompB locus and the regulation of the major outer membrane porin proteins of Escherichia coli K12. J Mol Biol. 1981 Feb 15;146(1):23–43. doi: 10.1016/0022-2836(81)90364-8. [DOI] [PubMed] [Google Scholar]
- Horii T., Ogawa T., Ogawa H. Nucleotide sequence of the lexA gene of E. coli. Cell. 1981 Mar;23(3):689–697. doi: 10.1016/0092-8674(81)90432-3. [DOI] [PubMed] [Google Scholar]
- Koenen M., Rüther U., Müller-Hill B. Immunoenzymatic detection of expressed gene fragments cloned in the lac Z gene of E. coli. EMBO J. 1982;1(4):509–512. doi: 10.1002/j.1460-2075.1982.tb01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Little J. W. Isolation of recombinant plasmids and phage carrying the lexA gene of Escherichia coli K-12. Gene. 1980 Aug;10(3):237–247. doi: 10.1016/0378-1119(80)90053-0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuirt P. V., Keller P. M., Elion G. B. A radioimmunoassay for herpes simplex virus (HSV) thymidine kinase. Virology. 1982 Jan 30;116(2):489–498. doi: 10.1016/0042-6822(82)90142-8. [DOI] [PubMed] [Google Scholar]
- Rüther U., Koenen M., Sippel A. E., Müller-Hill B. Exon cloning: immunoenzymatic identification of exons of the chicken lysozyme gene. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6852–6855. doi: 10.1073/pnas.79.22.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman H. A., Silhavy T. J., Beckwith J. R. Labeling of proteins with beta-galactosidase by gene fusion. Identification of a cytoplasmic membrane component of the Escherichia coli maltose transport system. J Biol Chem. 1980 Jan 10;255(1):168–174. [PubMed] [Google Scholar]
- Talmadge K., Gilbert W. Cellular location affects protein stability in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1830–1833. doi: 10.1073/pnas.79.6.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M. J., Sharp J. A., Summers W. C. Nucleotide sequence of the thymidine kinase gene of herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1441–1445. doi: 10.1073/pnas.78.3.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig M., Heilman C. J., Jr, Rabin H., Hopkins R. F., 3rd, Neubauer R. H., Hampar B. Production of monoclonal antibodies against nucleocapsid proteins of herpes simplex virus types 1 and 2. J Virol. 1979 Nov;32(2):676–678. doi: 10.1128/jvi.32.2.676-678.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]