Abstract
Purpose
The aim of the study was to evaluate the prognostic value of prostate specific antigen (PSA) response to neoadjuvant androgen deprivation therapy (ADT) prior to dose-escalated radiation therapy (RT) and long-term ADT in high-risk prostate cancer.
Methods and Materials
We reviewed the charts of all patients diagnosed with high-risk prostate cancer and treated with a combination of long-term ADT (median, 24 months) and dose-escalated (median, 75.6 Gy) RT between 1990 and 2007. The association of patient, tumor and treatment characteristics with biochemical response to neoadjuvant ADT, and their effect on failure-free survival (FFS), time to distant metastasis (TDM), prostate cancer-specific mortality (PCSM) and overall survival (OS) were examined.
Results
A total of 196 patients met the criteria for inclusion. Median follow up time for patients alive at last contact was 7.0 years (range, 0.5–18.1 years). Multivariate analysis identified the pre-radiation therapy PSA level (pre-RT PSA; <0.5 vs. ≥0.5 ng/ml) as a significant independent predictor of FFS (p=0.021), TDM (p=0.009), PCSM (p=0.039) and OS (p=0.037). On multivariate analysis, pre-treatment PSA (iPSA) and African-American race were significantly associated with failure to achieve a pre-RT PSA <0.5 ng/ml.
Conclusions
For high-risk prostate cancer patients treated with long-term ADT and dose-escalated RT, a pre-RT PSA level ≥0.5 ng/ml after neoadjuvant ADT predicts for worse survival measures. Both elevated iPSA and Afrian-American race are associated with increased risk of having a pre-RT PSA level ≥0.5 ng/ml. These patients should be considered for clinical trials testing newer more potent androgen depleting therapies such as abiraterone and MDV3100 in combination with radiation.
Keywords: pre-RT PSA, androgen deprivation therapy, radiation therapy, prostate cancer
INTRODUCTION
Multiple randomized trials have established the benefit of combined modality therapy with androgen deprivation therapy (ADT) and radiation therapy (RT) for men with clinically localized, high-risk prostate cancer (1–5). Randomized data also support the benefit of dose-escalated (≥74 Gy) RT in the treatment of intermediate- and high-risk prostate cancer (6,7), and the current standard of care in the treatment of high-risk prostate cancer is long-term (≥2 years) ADT combined with dose-escalated RT. ADT is commonly initiated in the neoadjuvant setting, administered concurrently with RT and continued in an adjuvant setting after RT completion.
Amidst the growing appreciation of cardiovascular and metabolic risks associated with ADT (8), defining the appropriate length and intensity of ADT to maximize the therapeutic benefit and yet minimize toxicities is of great interest. One approach to individually tailor an optimal ADT regimen is to identify surrogate markers of ADT effectiveness. A recent Canadian randomized phase III trial comparing the effect of 3 vs. 8 months of neoadjuvant ADT preceding conventional-dose (66 Gy) RT in clinically localized prostate cancer patients demonstrated no difference in 5-year failure-free survival (FFS) between the two arms overall, but did show a benefit of 8 months for 5-year disease-free survival in the high-risk sub-group (9). However, a subsequent re-analysis examining biochemical response to neoadjuvant ADT prior to initiating RT found that patients who achieved a pre-RT PSA ≤0.1 ng/ml, had improved biochemical disease-free survival (BDFS) at a median follow up of 6.4 years (10). These findings suggest that biochemical response to neoadjuvant ADT rather than duration may be the critical factor. The importance of pre-RT PSA level after neoadjuvant ADT in patients with clinically localized prostate cancer has been similarly demonstrated in other studies, albeit primarily in the conventional-dose era (≤ 70 Gy; 11–14).
Given the potential prognostic and therapeutic implications of stratifying patients based on serum PSA response after neoadjuvant ADT, we sought to evaluate the role of pre-RT PSA in predicting survival measures in patients with clinically localized high-risk prostate cancer treated in the current era of long-term ADT and dose-escalated RT. We additionally sought to investigate the patient, tumor and treatment characteristics that influence biochemical response to neoadjuvant ADT.
MATERIALS AND METHODS
Study population
We reviewed the charts of all patients diagnosed with clinically localized high-risk prostate cancer treated with a combination of ADT (neoadjuvant, concurrent and adjuvant setting) and RT at XXXX between 1990 and 2007. High-risk disease was defined in accordance with the D’Amico risk group stratification, i.e. having at least one of the following criteria: pre-treatment PSA (iPSA) >20 ng/ml, or Gleason score ≥8, or Clinical T Stage ≥T2c (15). Additional inclusion criteria were (a) no evidence of metastatic disease, (b) 45 to 365 days of neoadjuvant ADT at the treating physician’s discretion, (c) a pre-RT PSA level determined between 45 days and 365 days after start of neoadjuvant ADT. A total of 196 patients met the criteria for inclusion. The study protocol was approved by the Institutional Review Board of the XXXX with a waiver of consent.
Hormone therapy
The hormonal ablative therapies were chosen at the treating physician’s discretion and included luteinizing hormone-releasing hormone (LH-RH) agonist monotherapy, orchiectomy, or total androgen blockade (androgen receptor antagonist combined with LH-RH agonist). Total duration of hormone therapy (median duration, 24 months) included hormone therapy in the neoadjuvant period (median duration, 2.9 months), concurrent with RT, and in the adjuvant period (median duration, 20.7 months).
Radiation therapy
RT techniques used included four-field box, 3-dimensional conformal RT or intensity-modulated RT. The minimal target volume was the prostate and seminal vesicles. Radiation was delivered with 6–18 MV photons in 180–200 cGy per fraction to a median dose of 75.6 Gy (range, 63.0–79.2 Gy).
Statistical Analysis
We applied the methods of Williams and colleagues (16) to identify an optimal cut-point of pre-RT PSA for discriminating FFS in this population. This pre-RT PSA cut-point was also used to subsequently investigate time to distant metastasis (TDM), prostate cancer-specific mortality (PCSM) and overall survival (OS).
Summary statistics were used to describe patient (age, race), tumor (iPSA, clinical T stage, Gleason score) and treatment (duration of neoadjuvant ADT, pre-RT PSA, total duration of ADT) characteristics. We used Fisher’s exact test to test for differences between pre-RT PSA groups for categorical variables and the Wilcoxon rank-sum test to compare medians between pre-RT PSA groups for continuous variables.
Estimates of FFS and OS were calculated using the Kaplan-Meier method. For OS, an event was death from any cause. For FFS, an event was any of the following: biochemical failure (defined as nadir PSA + 2 ng/ml), local relapse, nodal relapse, distant metastasis, salvage therapy or death from any cause. Because death was included as an event in the definition of FFS, Cox proportional hazards regression was performed to model the impact of patient, tumor and treatment characteristics on the FFS and OS outcomes. The cumulative incidence of TDM and PCSM were calculated using the methods of Marubini and Valsecchi (17). Confidence intervals for the cumulative incidence at event times were estimated using the method of Choudhury (18). For TDM, an event was distant metastasis. For PCSM, an event was death from prostate cancer. For TDM and PCSM, death from any cause and death from causes other than prostate cancer, respectively, were considered competing events. A Fine and Gray competing risk multivariable regression model was used to asses the impact of patient, tumor and treatment characteristics on the TDM and PCSM outcomes (19). For each of the outcomes, time was measured from the start of RT until the date of the event or last follow-up. To identify factors associated with pre-RT PSA dichotomized on the cut-point, we used logistic regression methods.
All analyses were performed with S-PLUS 7.0 for Windows (Insightful Corp., Seattle, Washington), SAS 9.1 for Windows (SAS Institute Inc., Cary, North Carolina) and STATA™ 11.0 for Windows (StataCorp LP, College Station, Texas).
RESULTS
Using the methods of Williams and colleagues (15) we identified a pre-RT PSA cut-point of 0.5 ng/ml as optimally discriminating FFS, TDM, PCSM, and OS in this population. Patient, tumor, and treatment characteristics for the entire cohort and according to pre-RT PSA level (<0.5 vs. ≥0.5 ng/ml) are summarized in Table 1. Median age at diagnosis was 67 years (range, 49–86 years). Median follow-up for patients alive at last contact was 7.0 years (range, 0.5–18.1 years). The median duration of neoadjuvant ADT was 2.9 months in both groups. The median dose of radiation was 75.6 Gy for both groups. The median iPSA was significantly higher in the pre-RT PSA ≥0.5 ng/ml compared with pre-RT PSA <0.5 ng/ml group (25.7 ng/ml vs. 9.8 ng/ml, p<0.001). The proportion of men with a pre-RT PSA ≥0.5 ng/ml compared with pre-RT PSA <0.5 ng/ml was significantly higher for African-American men (85.2% vs. 14.8%) than other men (54.4% vs. 45.6%, p=0.003). No differences were observed between the two groups with respect to other characteristics, including age, clinical T Stage, Gleason score, radiation dose, duration of neoadjuvant ADT, type of neoadjuvant ADT, total duration of ADT, time from pre-RT PSA measurement to start of RT and follow-up time (p>0.05).
Table 1.
Comparison of patient, tumor and treatment characteristics between patients with a pre-RT PSA <0.5 ng/ml and ≥0.5 ng/ml.
| All patients N=196 |
Pre-RT PSA <0.5 N=81 |
Pre-RT PSA ≥0.5 N=115 |
p value | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Characteristics | N (%) | N (% column) | % row | N (% column) | % row | |
| Age | 0.173 | |||||
| Median | 67.3 | 68.1 | 67.0 | |||
| Range | 49.1–86.6 | 51.7–84.8 | 49.1–86.6 | |||
| Clinical T Stage | 0.182 | |||||
| T1 | 30 (15.3) | 15 (18.5) | 50.0 | 15 (13.0) | 50.0 | |
| T2 | 72 (36.7) | 23 (28.4) | 31.9 | 49 (42.6) | 68.1 | |
| T3 | 87 (44.4) | 40 (49.4) | 46.0 | 47 (40.9) | 54.0 | |
| T4 | 1 (0.5) | 1 (1.2) | 100 | 0 (0) | 0 | |
| Tx | 6 (3.1) | 2 (2.5) | 33.3 | 4 (3.5) | 66.7 | |
| Gleason Score | 0.105 | |||||
| 5–6 | 15 (7.7) | 7 (8.6) | 46.7 | 8 (7.0) | 53.3 | |
| 7 | 57 (29.1) | 17 (21.0) | 29.8 | 40 (34.8) | 70.2 | |
| 8–10 | 124 (63.2) | 57 (70.4) | 46.0 | 67 (58.3) | 54.0 | |
| iPSA (ng/ml) – cont. | <0.001 | |||||
| Median | 19.4 | 9.8 | 25.7 | |||
| Range | 1.3–281.0 | 1.8–85.3 | 1.3–281.0 | |||
| iPSA (ng/ml) – cat. | <0.001 | |||||
| ≤10 | 60 (30.6) | 41 (50.6) | 68.3 | 19 (16.5) | 31.7 | |
| 10.1–20 | 41 (20.9) | 20 (24.7) | 48.8 | 21 (18.3) | 51.2 | |
| >20 | 95 (48.5) | 20 (24.7) | 21.0 | 75 (65.2) | 79.0 | |
| Radiation dose (Gy) | 0.297 | |||||
| Median | 75.6 | 75.6 | 75.6 | |||
| Range | 63.0–79.2 | 63.0–79.2 | 66.0–79.2 | |||
| Duration of neoadjuvant ADT (mo) | 0.481 | |||||
| Median | 2.9 | 2.9 | 2.9 | |||
| Range | 1.6–11.5 | 1.9–10.1 | 1.6–11.5 | |||
| Total duration of ADT (yrs) | 0.556 | |||||
| Median | 2 | 2 | 2 | |||
| Range | 0.3–17.6 | 0.4–14.8 | 0.3–17.6 | |||
| Type of neoadjuvant ADT | 0.432 | |||||
| LH/RH monotherapy/orchiectomy | 137 (69.9) | 54 (66.7) | 39.4 | 83 (72.2) | 60.6 | |
| Total androgen blockade | 59 (30.1) | 27 (33.3) | 45.8 | 32 (27.8) | 54.2 | |
| Race | 0.003 | |||||
| African-American | 27 (13.8) | 4 (4.9) | 14.8 | 23 (20.0) | 85.2 | |
| Others | 169 (86.2) | 77 (95.1) | 45.6 | 92 (80.0) | 54.4 | |
| Time from pre-RT PSA measurement to start of RT (days) | 0.923 | |||||
| Median | 10 | 9 | 10 | |||
| Range | −3 – 163 | −3 – 84 | −3 – 163 | |||
| Follow-up (yrs) | 0.252 | |||||
| Median | 7.0 | 7.6 | 6.5 | |||
| Range | 0.5–18.1 | 2.6–16.2 | 0.5–18.1 | |||
Abbreviations: pre-RT PSA = pre-radiation therapy prostate specific antigen, iPSA = pre-treatment prostate specific antigen, ADT = androgen deprivation therapy.
Table 2 summarizes the impact of patient, tumor, and treatment characteristics on FFS. Univariate analysis indicated that pre-RT PSA ≥0.5 ng/ml (hazard ratio [HR] 1.92, 95% confidence interval [CI] 1.21–3.05, p=0.006, Figure 1), iPSA >20 ng/ml (HR 2.18, 95% CI 1.22–3.89, p=0.008) and total duration of ADT (1–2 years: HR 2.37, 95% CI 1.17–4.78, p=0.016) were associated with increased risk of failure. In a multivariate model, pre-RT PSA ≥0.5 (HR=1.84, 95% CI 1.09–3.08, p=0.021) and total duration of ADT (1–2 years: HR 2.51, 95% CI 1.21–5.20, p=0.013) remained significantly associated with increased risk of failure. Importantly, the duration of neoadjuvant ADT did not predict for FFS, indicating that the biochemical response, rather than the duration of neoadjuvant ADT, was the critical determinant.
Table 2.
Failure-free survival based on patient, tumor and treatment characteristics#.
| Characteristics | N (events) | Univariate analysis | Multivariate analysis* | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| p-value | HR | 95% CI | p-value | HR | 95% CI | ||
|
| |||||||
| Pre-RT PSA (ng/ml) | |||||||
| <0.5 | 81 (26) | 1.00 | 1.00 | ||||
| ≥0.5 | 115 (59) | 0.006 | 1.92 | 1.21 – 3.05 | 0.021 | 1.84 | 1.09–3.08 |
| Clinical T Stage | |||||||
| T1–T2a | 55 (14) | 1.00 | 1.00 | ||||
| T2b–T2c | 47 (24) | 0.095 | 1.76 | 0.91 – 3.42 | 0.077 | 1.83 | 0.94–3.58 |
| T3–T4 | 88 (46) | 0.40 | 1.30 | 0.71 – 2.38 | 0.30 | 1.39 | 0.74–2.60 |
| Gleason Score | |||||||
| 5–6 | 15 (6) | 1.00 | |||||
| 7 | 57 (25) | 0.33 | 1.56 | 0.64 – 3.83 | |||
| 8–10 | 124 (54) | 0.33 | 1.53 | 0.66 – 3.57 | |||
| iPSA (ng/ml) | |||||||
| ≤10 | 60 (15) | 1.00 | 1.00 | ||||
| 10.1–20 | 41 (20) | 0.068 | 1.87 | 0.95 – 3.65 | 0.51 | 1.28 | 0.61–2.68 |
| >20 | 95 (50) | 0.008 | 2.18 | 1.22 – 3.89 | 0.19 | 1.54 | 0.80–2.96 |
| iPSA (ng/ml) | 196 (85) | 0.17 | 1.00 | 0.99 – 1.01 | |||
| Duration of neoadjuvant ADT (mo) | 194 (85) | 0.14 | 1.09 | 0.97 – 1.22 | 0.087 | 1.10 | 0.99–1.23 |
| Total duration of ADT (yrs) | |||||||
| <1 | 39 (11) | 1.00 | 1.00 | ||||
| 1–2 | 60 (28) | 0.016 | 2.37 | 1.17 – 4.78 | 0.013 | 2.51 | 1.21–5.20 |
| >2 | 95 (46) | 0.15 | 1.62 | 0.84 – 3.14 | 0.19 | 1.58 | 0.79–3.16 |
| Type of neoadjuvant ADT | |||||||
| LH/RH monotherapy/orchiectomy | 137 (65) | ||||||
| Total androgen blockade | 59 (20) | 0.40 | 0.81 | 0.49 – 1.34 | |||
| Age | 196 (85) | 0.11 | 0.98 | 0.95 – 1.01 | 0.49 | 0.99 | 0.96–1.02 |
| Race | |||||||
| Others | 169 (73) | 1.00 | |||||
| African-American | 27 (12) | 0.59 | 1.19 | 0.64 – 2.19 | |||
| Radiation dose (Gy) | 196 (85) | 0.40 | 0.97 | 0.91 – 1.04 | |||
Abbreviations: pre-RT PSA = pre- radiation therapy prostate specific antigen, iPSA = pre-treatment prostate specific antigen, ADT = androgen deprivation therapy, HR = hazard ration, 95% CI = 95% confidence interval.
Because death is included as an event in the definition of FFS, Cox proportional hazards regression was performed to model the impact of patient, tumor and treatment characteristics on the FFS.
After univariate analyses, factors that reached a p-value of 0.25 or less were included in a multivariate model.
Figure 1.
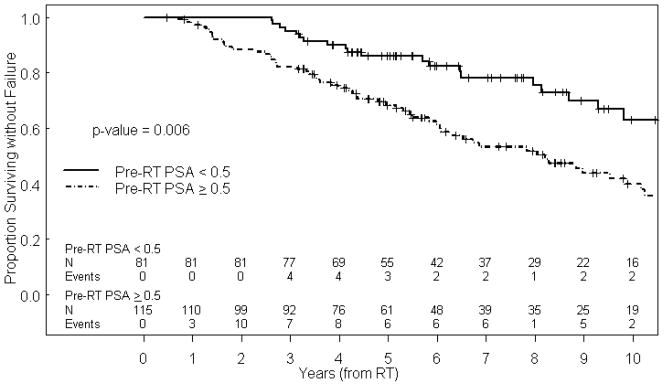
Kaplan-Meier estimate of FFS based on pre-RT PSA.
pre-RT PSA = pre-radiotherapy prostate specific antigen, RT = radiation therapy.
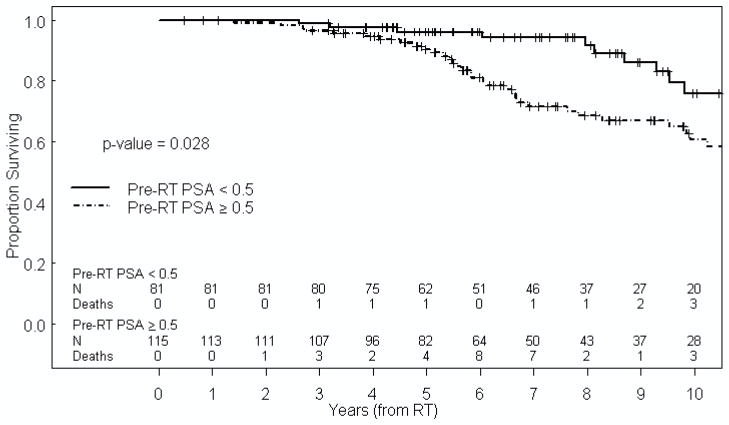
Table 3 summarizes the impact of patient, tumor, and treatment characteristics on OS. Only pre-RT PSA ≥0.5 ng/ml was found to be significantly associated with increased risk of all-cause mortality on both univariate and multivariate analysis (HR 1.95, 95% CI 1.04–3.64, p=0.037, Figure 2). While on univariate analysis African-American men were found to have a worse OS (HR 1.95, 95% CI 1.01–3.79, p=0.048), this factor was not found to be statistically significant when included in a multivariate model. Importantly, the duration of neoadjuvant ADT did not predict for OS, again indicating that the biochemical response, rather than the duration of neoadjuvant ADT, was the critical determinant. In addition to FFS and OS, the relationship of pre-RT PSA level and TDM (HR 4.82, 95% CI 1.48–15.8, p=0.009) and PCSM (HR 4.52, 95% CI 1.08–18.9, p=0.039) were also found to be statistically significant on Fine and Gray multivariate competing risk analyses.
Table 3.
Overall survival based on patient, tumor and treatment characteristics.
| Characteristics | N (events) | Univariate analysis | Multivariate analysis* | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| p-value | HR | 95% CI | p-value | HR | 95% CI | ||
|
| |||||||
| Pre-RT PSA (ng/ml) | |||||||
| <0.5 | 81 (15) | 1.00 | 1.00 | ||||
| ≥0.5 | 115 (39) | 0.028 | 1.95 | 1.07 – 3.54 | 0.037 | 1.95 | 1.04–3.64 |
| Clinical T Stage | |||||||
| T1–T2a | 55 (10) | 1.00 | |||||
| T2b–T2c | 47 (12) | 0.60 | 0.79 | 0.34 – 1.86 | |||
| T3–T4 | 88 (32) | 0.63 | 0.83 | 0.40 – 1.73 | |||
| Gleason Score | |||||||
| 5–6 | 15 (4) | 1.00 | 1.00 | ||||
| 7 | 57 (17) | 0.25 | 1.92 | 0.64 – 5.77 | 0.37 | 1.69 | 0.54–5.25 |
| 8–10 | 124 (33) | 0.39 | 1.59 | 0.56 – 4.51 | 0.63 | 1.30 | 0.44–3.86 |
| iPSA (ng/ml) | |||||||
| ≤10 | 60 (11) | 1.00 | |||||
| 10.1–20 | 41 (10) | 0.96 | 0.98 | 0.41 – 2.32 | |||
| >20 | 95 (33) | 0.36 | 1.38 | 0.69 – 2.75 | |||
| iPSA (ng/ml) | 196 (54) | 0.80 | 1.00 | 0.99 – 1.01 | |||
| Duration of neoadjuvant ADT (mo) | 194 (54) | 0.96 | 1.00 | 0.85 – 1.18 | |||
| Total duration of ADT (yrs) | |||||||
| <1 | 39 ( 6) | 1.00 | 1.00 | ||||
| 1–2 | 60 (14) | 0.17 | 1.95 | 0.75 – 5.10 | 0.093 | 2.31 | 0.87–6.13 |
| >2 | 95 (34) | 0.12 | 1.98 | 0.83 – 4.72 | 0.14 | 2.01 | 0.80–5.02 |
| Type of neoadjuvant ADT | |||||||
| LH/RH monotherapy/orchiectomy | 137 (43) | ||||||
| Total androgen blockade | 59 (11) | 0.41 | 0.76 | 0.39 – 1.47 | |||
| Age | 196 (54) | 0.74 | 1.01 | 0.97 – 1.05 | |||
| Race | |||||||
| Others | 169 (43) | 1.00 | 1.00 | ||||
| African-American | 27 (11) | 0.048 | 1.95 | 1.01 – 3.79 | 0.22 | 1.57 | 0.76–3.28 |
| Radiation dose (Gy) | 196 (54) | 0.82 | 1.01 | 0.93 – 1.09 | |||
Abbreviations: pre-RT PSA = pre- radiation therapy prostate specific antigen, iPSA = pre-treatment prostate specific antigen, ADT = androgen deprivation therapy, HR = hazard ration, 95% CI = 95% confidence interval.
After univariate analyses, factors that reached a p-value of 0.25 or less were included in a multivariate model.
Figure 2.
Kaplan-Meier estimate of OS based on pre-RT PSA.
pre-RT PSA = pre-radiotherapy prostate specific antigen, RT = radiation therapy.
Table 4 summarizes the patient, tumor and treatment characteristics associated with achieving a pre-RT PSA <0.5 ng/ml. Multivariate analysis revealed that patients with higher iPSA (10.1–20 ng/ml: OR=0.39, 95% CI 0.17–0.90, p=0.027; >20 ng/ml: OR=0.13, 95% CI 0.06–0.27, p<0.001) and of African-American race (OR 0.25, 95% CI 0.08–0.82, p=0.022) were significantly less likely to reach a pre-RT PSA <0.5 ng/ml after neoadjuvant ADT. The iPSA was significantly higher in African-American men compared with other men, however, there was no interaction found between iPSA and race with respect to pre-RT PSA (iPSA 10.1–20 ng/ml and African-American race: pinteraction=0.991; iPSA >20 ng/ml and African-American race: pinteraction=0.466; iPSA as a continuous variable and African-American race: pinteraction=0.173). Importantly, the duration of neoadjuvant ADT did not predict for having a pre-RT PSA <0.5 ng/ml, suggesting the existence of populations of responders and non-responders to the neoadjuvant portion of ADT, independent of its duration.
Table 4.
Patient, tumor and treatment characteristics associated with pre-RT PSA <0.5 ng/ml.
| Characteristics | Univariate analysis | Multivariate analysis* | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| p-value | OR | 95% CI | p-value | OR | 95% CI | |
| Clinical T Stage | ||||||
| T1–T2a | 1.00 | |||||
| T2b–T2c | 0.87 | 0.94 | 0.43 – 2.06 | NS | ||
| T3–T4 | 0.28 | 1.44 | 0.74 – 2.81 | NS | ||
| Gleason Score | ||||||
| 5–7 | 1.00 | |||||
| 8–10 | 0.085 | 1.70 | 0.93 – 3.11 | NS | ||
| iPSA (ng/ml) | ||||||
| ≤10 | 1.00 | 1.00 | ||||
| 10.1–20 | 0.050 | 0.44 | 0.20 – 1.00 | 0.027 | 0.39 | 0.17 – 0.90 |
| >20 | <0.001 | 0.12 | 0.06 – 0.26 | <0.001 | 0.13 | 0.06 – 0.27 |
| iPSA (ng/ml) | <0.001 | 0.96 | 0.94 – 0.98 | NS | ||
| Duration of neoadjuvant ADT (mo) | 0.78 | 0.98 | 0.84 – 1.14 | NS | ||
| Type of neoadjuvant ADT | ||||||
| LH/RH monotherapy/orchiectomy | 1.00 | |||||
| Total androgen blockade | 0.41 | 1.30 | 0.70 – 2.40 | NS | ||
| Age | 0.19 | 1.03 | 0.99 – 1.07 | NS | ||
| Testosterone level | 0.012 | 0.95 | 0.92 – 0.99 | / | ||
| Race | ||||||
| Others | 1.00 | 1.00 | ||||
| African-American | 0.005 | 0.21 | 0.07 – 0.63 | 0.022 | 0.25 | 0.08 – 0.82 |
Abbreviations: pre-RT PSA = pre-radiation therapy prostate specific antigen, iPSA = pre-treatment prostate specific antigen, ADT = androgen deprivation therapy, OR = odds ration, 95% CI = 95% confidence interval, NS = not statistically significant.
After univariate analyses, multivariate analysis followed by backward elimination was performed. Testosterone level was not considered in the multivariate model, because only 62 (31.6%) patients had data on testosterone levels.
In a subset, multivariate analysis restricted to only those patients who received ≥73.8 Gy of RT and patients who had pre-RT PSA measured within 60 days before the start of RT (N=133), pre-RT PSA ≥0.5 ng/ml was significantly associated with increased risk of failure (HR 2.26, 95% CI 1.22–4.19, p=0.010), worse OS (HR 2.35, 95% CI 1.02–5.45, p=0.046) and shorter TDM (HR 8.03, 95% CI 1.04–61.95, p=0.046). We could not fit the regression model to estimate the risk of death from prostate cancer, since there were no deaths from prostate cancer among patients with pre-RT PSA <0.5 ng/ml.
DISCUSSION
The importance of long-term ADT in conjunction with conventional-dose RT for high-risk prostate cancer patients has been well documented (1–5). The mechanistic basis for the observed benefit of combined modality treatment however is not well understood at present. Animal studies have shown that RT is most effective when tumor reduction in response to neoadjuvant ADT is maximal (20). This would agree with the increasing clinical evidence that biochemical response to neoadjuvant ADT, as measured by PSA immediately before RT, rather than duration, is the critical factor determining the benefit of combined treatment (10–14). However, it is difficult to exclude the possibility that ADT is also having an impact on micrometastatic disease and the pre-RT PSA is a biomarker of the effectiveness of this mechanism.
The present study demonstrates improved FFS, TDM, PCSM and importantly OS in patients who achieve a pre-RT PSA <0.5 ng/ml, compared with those with pre-RT PSA ≥0.5 ng/ml. This observation provides strong clinical evidence that, independent of the duration of neoadjuvant ADT or the iPSA, a critical factor responsible for predicting FFS, TDM, PCSM, and OS in response to RT and ADT is the biological effectiveness of neoadjuvant ADT as measured by pre-RT PSA level. A data driven cut-point analysis identified a PSA level of 0.5 ng/ml as the optimal discriminator of FFS, TDM, PCSM, and OS. One interpretation is that achieving a pre-RT PSA <0.5 ng/ml represents the degree of androgen signaling suppression that is both necessary and sufficient to achieve optimal local response to dose-escalated RT as well as maximal effect on micrometastatic disease.
Of note, the majority of prior studies investigating the prognostic value of pre-RT PSA response to neoadjuvant ADT were conducted during an era when neoadjuvant ADT was typically followed by conventional-dose (≤70 Gy) RT. Since then, several randomized trials have demonstrated that dose escalation (≥74 Gy) provides significant improvement in both local control and freedom from biochemical relapse, particularly in intermediate- and high-risk prostate cancer patients (6,7). Therefore, the earlier studies cannot be directly extrapolated to the contemporary era of dose-escalated RT combined with long-term ADT. Our study is the first to demonstrate that pre-RT PSA has a strong prognostic impact on FFS, TDM, PCSM, and OS in men with high-risk prostate cancer treated in the contemporary dose-escalated RT era with long-term ADT. Our data suggests that patients failing to achieve a pre-RT PSA <0.5 ng/ml are good candidates for clinical trials testing more potent androgen depleting agents such as abiraterone or MDV3100 in combination with radiation. These hypotheses remain to be tested in the setting of prospective randomized trials.
Patients with an iPSA >10 ng/ml and African-American men were found to be significantly less likely to reach pre-RT PSA <0.5 ng/ml after neoadjuvant ADT. The association of higher iPSA and pre-RT PSA ≥0.5 ng/ml is straightforward and in agreement with previous reports (10, 21). On the other hand, the association of African-American race with pre-RT PSA ≥0.5 ng/ml has not been previously reported and may be one of the reasons why African-American men tend to have worse outcomes and consistently higher mortality rates for prostate cancer. In contrast to our results Fowler and colleagues reported no significant racial difference in PSA nadir after gonadal androgen withdrawal (20). However, the majority of the patients had clinically advanced or metastatic disease at the time of initiation of androgen withdrawal. When focusing on the patients with early prostate cancer (T1b–T2), PSA nadirs were significantly greater in African-American men than in white men prior to adjustment for iPSA (20). In our study, iPSA was also significantly higher in African-American men compared with other men, however, there was no interaction found between these two factors and both proved to be jointly prognostic for pre-RT PSA ≥0.5 ng/ml in multivariate analysis.
In addition to the inherent limitations of a retrospective design, several other potential limitations to our study exist. Despite the fact that the duration of neoadjuvant ADT was not shown to significantly predict for pre-RT PSA level or any of the outcome measures, the patients in this study received neither a uniform duration nor type of ADT prior to assessment of pre-RT PSA, potentially altering the true cut-point separating poor responders from good responders. However, the median duration of neoadjuvant ADT in both groups was 2.9 months and type of neoadjuvant ADT did not differ significantly between both groups. We note at least one other report in the literature that has identified a PSA nadir level of 0.5 ng/ml in response to three months of neoadjuvant ADT as a predictor of outcome (11). An additional limitation is the lack of uniform testosterone measurements, which might have shed light on a potential mechanistic basis underlying the differences in pre-RT PSA observed in this study. It is possible that inadequate androgen suppression in a subset of men accounted for elevated pre-RT PSA levels. Alternatively, other castrate-resistant mechanisms of androgen receptor pathway activation may be at play. These hypotheses remain to be tested in the prospective setting.
CONCLUSION
In conclusion, our results demonstrate that for men with clinically localized, non-metastatic high-risk prostate cancer receiving long-term ADT and dose-escalated RT, attaining a pre-RT PSA <0.5 ng/ml after neoadjuvant ADT predicts for improved FFS, TDM, PCSM, and OS. This provides clinical evidence that the degree of suppression of the AR signaling axis is a significant determinant of outcome in men treated with dose-escalated RT. The ability to identify men at increased risk of death from prostate cancer based on pre-RT PSA levels should spur the design of new clinical trials testing more potent novel anti-androgen therapies in combination with radiation to improve outcome for men who are poor responders to current standard of care androgen deprivation regimens.
SUMMARY.
PSA response to neoadjuvant androgen deprivation therapy (ADT) prior to radiotherapy (pre-RT PSA) is a significant independent predictor of failure free survival, time to distant metastases, prostate cancer-specific survival, and overall survival in men with high-risk prostate cancer treated with dose-escalated RT and long-term ADT. The pre-RT PSA level therefore potentially identifies men who are candidates for clinical trials investigating novel therapies (e.g. abiraterone and MDV3100) in combination with RT.
Acknowledgments
This work was supported in part by the Cancer Center Support Grant (NCI Grant P30 CA016672).
Footnotes
Conflict of Interest Notification: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pilepich MV, Winter K, John MJ, et al. Phase III radiation therapy oncology group (RTOG) trial 86-10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2001;50:1243–1252. doi: 10.1016/s0360-3016(01)01579-6. [DOI] [PubMed] [Google Scholar]
- 2.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomized trial. Lancet. 2002;360:103–108. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 3.Hanks GE, Pajak TF, Porter A, et al. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: The Radiation Therapy Oncology Group protocol 92-02. J Clin Oncol. 2003;21:3972–3978. doi: 10.1200/JCO.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 4.D’Amico AV, Manola J, Loffredo M, et al. 6-Month androgen suppression plus radiation therapy vs. radiation therapy alone for patients with clinically localized prostate cancer: A randomized controlled trial. JAMA. 2004;292:821–827. doi: 10.1001/jama.292.7.821. [DOI] [PubMed] [Google Scholar]
- 5.Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to radiotherapy in prostate carcinoma – long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys. 2005;61:1285–1290. doi: 10.1016/j.ijrobp.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 6.Dearnaley DP, Sydes MR, Graham JD, et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomized controlled trial. Lancet Oncol. 2007;8:475–487. doi: 10.1016/S1470-2045(07)70143-2. [DOI] [PubMed] [Google Scholar]
- 7.Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from proton radiation oncology group/american college of radiology 95-09. J Clin Oncol. 2010;28:1106–1111. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 9.Crook J, Ludgate C, Malone S, et al. Final report of multicenter Canadian phase III randomized trial of 3 versus 8 months of neoadjuvant androgen deprivation therapy before conventional-dose radiotherapy for clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2009;73:327–333. doi: 10.1016/j.ijrobp.2008.04.075. [DOI] [PubMed] [Google Scholar]
- 10.Alexander A, Crook J, Jones S, et al. Is biochemical response more important than duration of neoadjuvant hormone therapy before radiotherapy for clinically localized prostate cancer? An analysis of the 3- versus 8-month randomized trial. Int J Radiat Oncol Biol Phys. 2010;76:23–30. doi: 10.1016/j.ijrobp.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 11.Zelefsky MJ, Lyass O, Fuks Z, et al. Predictors of improved outcome for patients with localized prostate cancer treated with neoadjuvant androgen ablation therapy and three-dimensional conformal radiotherapy. J Clin Oncol. 1998;16:3380–3385. doi: 10.1200/JCO.1998.16.10.3380. [DOI] [PubMed] [Google Scholar]
- 12.Ludgate C, Bishop DC, Pai H, et al. Neoadjuvant hormone therapy and external-beam radiation for localized high-risk prostate cancer: the importance of PSA nadir before radioation. Int J Radiat Oncol Biol Phys. 2005;62:1309–1315. doi: 10.1016/j.ijrobp.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell DM, McAleese J, Park RM, et al. Failure to achieve a PSA level ≤1 ng/ml after neoadjuvant LHRHa therapy predicts for lower biochemical control rate and overall survival in localized prostate cancer treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2007;69:1467–1471. doi: 10.1016/j.ijrobp.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Heymann JJ, Benson MC, O’Toole KM, et al. Phase II study of neoadjuvant androgen deprivation followed by external-beam radiotherapy with 9 months of androgen deprivation for intermediate- to high-risk localized prostate cancer. J Clin Oncol. 2007;25:77–84. doi: 10.1200/JCO.2005.05.0419. [DOI] [PubMed] [Google Scholar]
- 15.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 16.Williams BA, Mandrekar JN, Mandrekar SJ, et al. Finding optimal cut points for continuous covariates with binary and time-to-event outcomes. Department of Health Sciences Research, Mayo Clinic; Rochester, MN: 2006. Technical Report Series #79. [Google Scholar]
- 17.Marubini E, Valsecchi MG. Analysing survival data from clinical trials and observational studies. Chichester, UK: John Wiley & Sons; 1995. [Google Scholar]
- 18.Choudhury JB. Nonparametric confidence interval estimation for competing risk analysis: application to contraceptive data. Stat Med. 2002;21:1129–1140. doi: 10.1002/sim.1070. [DOI] [PubMed] [Google Scholar]
- 19.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 20.Zietman AL, Prince EA, Nakfoor BM, et al. Androgen deprivation and radiation therapy: Sequencing studies using the Shionogi in vivo tumor system. Int J Radiat Oncol Biol Phys. 1997;38:1067–70. doi: 10.1016/s0360-3016(97)00309-x. [DOI] [PubMed] [Google Scholar]
- 21.Fowler JE, Bigler SA, Renfroe DL, et al. Prostate specific antigen in black and white men after hormonal therapies for prostate cancer. J Urol. 1997;158:150–154. doi: 10.1097/00005392-199707000-00047. [DOI] [PubMed] [Google Scholar]