Abstract
Diets replete with n-3 poly-unsaturated fatty acids (n-3 PUFAs) are known to have therapeutic potential for the heart, although a specifically defined duration of n-3 PUFA diet required to achieve these effects remains unknown, as does their mechanism of action. This study was undertaken to establish whether adaptations in mitochondrial function and stress tolerance in the heart is evident following a short- (3 weeks) and long-term (14 weeks) dietary intervention of n-3 PUFAs, and to identify novel mechanisms by which these adaptations occur. Mitochondrial respiration (mO2), H2O2 emission (mH2O2) and Ca2+ retention capacity (mCa2+) were assessed in mouse hearts following dietary intervention. Mice fed n-3 PUFA’s for 14 weeks showed significantly lower mH2O2 and greater mCa2+ compared to all other groups. However, no significant differences were observed after 3 weeks of n-3 PUFA diet, or in mice fed a high fat diet devoid of n-3 PUFAs for 14 weeks. Interestingly, at 14 weeks n-3 PUFA mice had significantly greater glutathione reductase activity, reflected by a substantially higher GSH/GSSG ratio. Levels of protein adducts of 4-hydroxyhexenal, an aldehyde formed from peroxidation of n-3 PUFAs, were significantly elevated in n-3 PUFA fed mice, even at 3 weeks. These findings demonstrate distinct time-dependent effects of n-3 PUFAs on mitochondrial function and stress tolerance in the heart. In addition, they are first to provide direct evidence that increases in non-enzymatic lipid oxidation products precede these mitochondrial and redox-mediated adaptations, thereby revealing a novel mechanism for n-3 PUFA action in heart.
Keywords: n-3 PUFAs, mitochondria, glutathione, permeability transition pore, aldehydes, lipid peroxidation
INTRODUCTION
Considerable interest in the relationship between dietary fatty acids and cardiac function has arisen from a number of recent reports showing that low-glycemic/high-fat diets preserve contractile force in experimental models of heart failure [1–3]. Notably, some of the most dramatic effects have been demonstrated by inclusion of n-3 poly-unsaturated fatty acids (PUFAs) to the diet [4, 5], findings that have been mirrored and compounded in importance recently by clinical studies showing that dietary supplementation with low-to-moderate doses of fish oil n-3 PUFA docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) improve left ventricular function [6] and decrease hospitalization rates and overall mortality [7] in patients with heart failure. However, despite the potential benefits and improved cardiac performance brought about by n-3 PUFAs in these studies, the proper dosage and duration of intake required to achieve these effects remain controversial, and their mechanisms remain obscure.
Mitochondria are of paramount importance in the heart for a multiplicity of reasons in addition to ATP production. Due to their role as critical loci of necrotic and apoptotic cell death programs within the cardiomyocyte, mitochondria have repeatedly been implicated to play significant roles in cardiomyocyte cell death, a singular feature of heart failure [8, 9]. Much focus has also been directed at cardiolipin, the phospholipid that is exclusive and crucial to mitochondrial inner-membrane functionality and integrity, as its decrease in concentration and peroxidation have been associated with increased cell death and mitochondrial dysfunction [10–12]. It has recently been shown that dietary supplementation with fish oil, particularly DHA, increases mitochondrial tolerance to Ca2+ overload (i.e. decreased necrotic susceptibility), and alters the cardiolipin composition of the mitochondrial inner-membrane [13, 14].
Aside from the direct effects of the n-3 PUFAs on mitochondria and other targets in the heart, indirect mechanisms may also be at work in these systems and must be considered. The high degree of unsaturation in all PUFAs makes them particularly vulnerable to lipid peroxidation, and cardiomyocytes have a number of adaptive processes with which to prevent lipid peroxidation and counteract the products formed from these reactions [15–17]. Indeed, the α,β-unsaturated hydroxyalkenal 4-hydroxynonenal (HNE) is a well-known and widely studied product of n-6 PUFA peroxidation. As a result of its chemical stability, lipophilic structure and electrophilic reactivity, HNE readily reacts with proteins and phospholipids to trigger alterations in cellular processes and enzymatic activities [18]. In contrast to HNE, far less is known about 4-hydroxyhexenal (HHE), the aldehyde formed from non-enzymatic peroxidation of n-3 PUFAs [19].
Since n-3 PUFA treatment in the heart has been repeatedly shown to cause increased tolerance to stress, we speculated that this may be due to a low-level, sub-toxic stress imposed by n-3 PUFAs on the heart which may induce protective adaptations over time (i.e. hormesis). The present study was undertaken to establish whether adaptations in mitochondrial function and improved tolerance to stress in the heart is evident following a short- (3 weeks) and long-term (14 weeks) dietary intervention of n-3 PUFAs and to determine the mechanism by which these adaptations occur. We provide evidence that long-term dietary intervention with n-3 PUFAs causes a substantial decrease in mitochondrial reactive oxygen species (ROS) emission, combined with improved tolerance to Ca2+ overload. Intriguingly, these alterations are accompanied by increased activity of key enzymes in the glutathione redox-balance system. We also provide completely novel data regarding the formation of HHE through dietary n-3 PUFAs, and how HHE-protein adduct formation in heart precedes these mitochondria and redox-mediated adaptations during the course of the diet.
EXPERIMENTAL
Animals, reagents and diet
Chemicals used in this study were all obtained from Sigma-Aldrich, with the exception of Amplex Red and Calcium Green 5-N, which were purchased from Invitrogen. Mice used for this study were male C57BL/6 (Charles River), aged 4–8 weeks at onset of the diet (Harlan-Teklad, Madison, WI). The composition of these diets was previously described in detail [20]. Briefly, the standard chow diet (Std Chow) consisted of 5% fat by weight and n-3 PUFA diet consisted of 20% fat by weight, representing ~13% and ~42% total kcal/day from fat, respectively. The sources of fat in these diets are of mixed composition: of the 13% kcal/day in Std Chow, 2% is saturated fatty acids (SFA), 3% mono-unsaturated fatty acids (MUFA), and 8% mixed PUFAs. Of the 42% in n-3 PUFA diet, 8% is SFA, 9% MUFA, and 24% PUFA. The PUFA composition breakdown consists of 2% kcal/day from DHA, 3% EPA, 11% linolenic acid, and ~25% mixed n-6 PUFAs. An additional set of mice were placed on a 42% kcal/day high-fat diet completely devoid of n-3 PUFA’s, to serve as a high fat control (HFC) diet. Of the 42% kcal/day in HFC, 11% was SFA, 23% hydrogenated fatty acids (vegetable shortening), and 7% n-6 PUFA’s. Mice were fed ad libitum for 3 weeks or 14 weeks as indicated, at which time they were euthanized (in fed state) and hearts removed, rinsed in saline and a portion of left ventricle (LV) used for mitochondrial experiments. The remaining LV and right ventricle (RV) were snap-frozen in liquid N2 for biochemistry.
Preparation of permeabilized cardiac myofibers
This technique has been described by our group [21] and others [22] in detail. For this study, small portions (<30 mg) of LV tissue were dissected and placed in ice-cold buffer X, containing (in mM) 50 MES, 7.23 K2EGTA, 2.77 CaK2EGTA, 20 imidazole, 0.5 DTT, 20 taurine, 5.7 ATP, 14.3 PCr, and 6.56 MgCl2-6 H2O (pH 7.1, 290 mOsm). The muscle was trimmed of connective and vascular tissue. Five or six small muscle bundles (~4–6 mm in length, 1.0–2.5 mg wet weight) were prepared from each animal. These bundles were gently separated along their longitudinal axis with a pair of needle-tipped forceps under magnification (MX6 Stereoscope, Leica Microsystems, Inc., Wetzlar, DE). Cardiac fiber bundles were then treated with 50 μg/ml saponin in ice-cold buffer X, and incubated on a rotor for 30 min at 4°C. Following permeabilization, the fibers used for mitochondrial O2 (mO2) consumption experiments were placed in buffer Z containing (in mM) 105 K-MES, 30 KCl, 1 EGTA, 10 K2HPO4, and 5 MgCl2-6H2O, 0.005 glutamate, and 0.002 malate with 5.0 mg/ml BSA (pH 7.4, 290 mOsm). The fibers used for mitochondrial H2O2 (mH2O2) and Ca2+ uptake (mCa2+) were placed in ice-cold Buffer Y containing (in mM) 250 Sucrose, 10 Tris-HCl, 20 Tris base, 10 KH2PO4, 2 MgCl2·6H2O, and 0.5 mg/ml BSA). Permeabilized fibers remained in buffer Z on a rotator at 4°C until analysis (<100 min).
Measurement of mO2, mH2O2 and mCa2+ in permeabilized cardiac myofibers
All mitochondrial measurements were performed at 30°C. The Oroboros O2K Oxygraph system (Oroboros Instruments, Innsbruck, Austria) was used for all mO2 consumption measurements. The mH2O2 and mCa2+ measurements were obtained using a spectrofluorometer (Photon Technology Instruments, Birmingham, NJ), equipped with a thermo-jacketed cuvette chamber. The mO2 experiments were performed in Buffer Z. During the course of the experiments, substrates, nucleotides and respiratory inhibitors were provided as indicated in the Figure legends. Both mCa2+ and mH2O2 experiments in this study were all performed in Buffer Y with 100 μM ADP, 5 mM glucose and 1 U/ml hexokinase present to keep the mitochondria in a permanent, submaximal phosphorylating state. For mH2O2 measurements, Buffer Y contained 10 μM Amplex Red (Invitrogen, Carlsbad, CA), 1 U/ml horseradish peroxidase, 5 mM pyruvate, 2 mM malate, 5 mM Succinate, and mH2O2 emission was calculated as outlined in [21]. For mCa2+ measurements, Buffer Y contained 1 μM Calcium Green 5-N (Invitrogen) and 5 mM pyruvate, 2mM malate. At start of mCa2+ experiments, 1 μM Thapsigargin was added to inhibit SERCA, and 40 μM EGTA added to chelate residual Ca2+ and to establish Fmin. Pulses of 4 nmols Ca2+ (CaCl2) were sequentially added and Ca2+ uptake followed until mPTP opening as in [23]. At end of experiment, 1 mM CaCl2 was added to saturate the probe and establish Fmax. Changes in free Ca2+ in cuvette during mCa2+ uptake were then calculated using the known Kd for Calcium Green 5-N and the equations established by Tsien’s group for calculating free ion concentrations using ion-sensitive fluorophores [24]. At conclusion of all experiments, fibers were rinsed in ddH2O, lyophilized in a freeze-dryer (Labconco, Kansas City, MO) for > 2 hours and weighed on a micro-scale. Data are expressed as pmol·min−1·mg dry wt.−1 (mH2O2), or nmol·mg dry wt.−1 (mCa2+).
Glutathione (GSH/GSSG) and related enzyme activity measurements
Myocardial samples frozen in liquid N2 were homogenized in 10X (wt./vol) TEE buffer containing (in mM: 10 Tris base, 1 EDTA, 1 EGTA), and 0.5% Tween-20, using a glass grinder (Kimble Chase, Vineland, NJ), and protein samples prepared for glutathione measurements as described previously [25] using a modified Tietze method [26]. For measurements of GSSG, samples were prepared in presence of 1-methyl-2-vinylpyridium triflate. Glutathione reductase (GR) activity in myocardial tissue was determined in TEE buffer containing 1 mM GSSG and 0.5 mM NADPH, where activity was calculated from the linear decrease in NADPH absorbance with time [27]. Glutathione peroxidase (GPx) activity was determined in TEE buffer containing 1 mM GSH, 100 mU/ml glutathione reductase enzyme, 0.5 mM NADPH. The reaction is initiated with a nominal amount of tert-Butyl-Hydroperoxide and the activity of GPx was calculated from the linear decrease in NADPH absorbance with time [28].
Immunoblot analysis of myocardial protein
Samples of myocardium were homogenized in 10X (wt./vol) TEE buffer, to which Complete™ Protease inhibitor cocktail (Roche, Mannheim, Germany) was added. Protein was then separated using SDS-PAGE, transferred to PVDF membrane and subjected to immunoblot using Mito OxPhos antibody cocktail™ (Mitosciences, Eugene, OR), which recognizes discrete subunits of mitochondrial OxPhos complexes I (~20 kDa), II (~30 kDa), III (~39 kDa), IV (~45 kDa), V (~50 kDa),in the electron transport system, and anti-GAPDH (Advanced Immunochemical, Long Beach, CA). For immunoblot analysis of aldehyde protein adducts, monoclonal antibodies to HHE-adducts (Genox, Baltimore, MD) and HNE-adducts (Percipio Biosciences, Inc, Foster City, CA) were used. Densitometry was performed using Image J software [29]. Quantification of total HHE- and HNE-adduct levels were normalized to GAPDH for each group.
Statistics
Data are presented as means ± SEM. Data were normally distributed which allowed for the use of parametric statistics. Therefore, interval variables between groups were compared using two-tailed student’s t-test or 1-way ANOVA with post-hoc Dunnett’s t-test for significance (GraphPad Prism, La Jolla, CA), as appropriate. Differences between groups was considered statistically significant for P<0.05.
RESULTS
The body weights were identical between Std Chow and n-3 PUFA groups at 3 weeks, but by 14 weeks, animals had gained more weight on the n-3 PUFA diet than Std Chow (Table 1). Cardiac mass was not significantly affected by diet (not shown). In a previous report using these same cohorts of mice, we demonstrated that levels of n-3 PUFAs in tissues were substantially increased as a result of this diet [20], so we have not reported them here.
Table 1.
| Diet | Duration (wks) | Terminal Body Wt. (g) | Wt. Gain (g) |
|---|---|---|---|
| Std Chow | 3 | 22.8 ± 0.8 | 7.0 ± 0.8 |
| n-3 PUFA | 3 | 23.5 ± 0.5 | 6.9 ± 1.2 |
|
| |||
| Std Chow | 14 | 31.6 ± 1.5 | 15.8 ± 2.1 |
| n-3 PUFA | 14 | 39.1 ± 0.8 * | 22.6 ± 1.6 * |
| HFC | 14 | 35.9 ± 1.3 | 19.8 ± 0.9 |
Data shown are mean ± S.E.M. in N = 6;
P<0.05
Time-dependent effects of n-3 PUFA diet on mO2, mH2O2 and mCa2+ in mouse heart
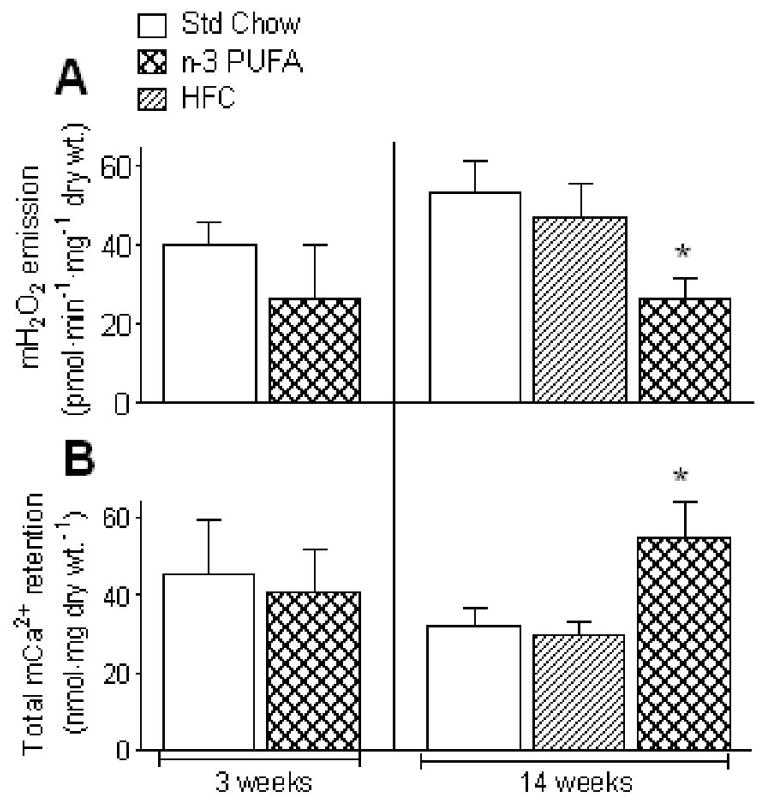
To keep cardiac mitochondria permanently in a submaximal phosphorylating state (most physiological), we used 100 μM ADP + glucose/hexokinase in our mH2O2 and mCa2+ experiments [23]. Mitochondria in permeabilized cardiac myofibers from mice fed n-3 PUFA diet for 3 weeks showed no significant differences in mH2O2 when supported by pyruvate, malate and succinate, or mCa2+ when supported by pyruvate, malate (Fig. 1A+B, left panel). However, under identical experimental conditions, cardiac mitochondria from mice fed n-3 PUFA diet for 14 weeks showed significant changes reflected by decreased rates of mH2O2 (Fig. 1A, right panel) and increased mCa2+ retention capacity (Fig. 1B, right panel). Both basal and maximal ADP-stimulated rates of mO2 supported by pyruvate, malate, glutamate and succinate in permeabilized cardiac myofibers from mice fed n-3 PUFA diet for both 3- and 14-weeks were not significantly increased, though directionality towards an increase was observed (Table 2). Basal and maximal ADP-stimulated mO2 supported by palmitoyl-L-carnitine and malate were also unchanged. Importantly, no significant differences were observed in respiratory control ratio (RCR), an indicator of mitochondrial coupling, between Std Chow and n-3 PUFA at either 3- or 14-weeks (Table 2), regardless of whether pyruvate/malate or palmitoyl-L-carnitine were used. Further, immunoblot analysis of complexes I – V in mitochondrial electron transport system from Std Chow and n-3 PUFA diet for 3- and 14-weeks showed no differences in protein content (not shown). To establish that the differences observed in cardiac mH2O2 and mCa2+ after 14 weeks of n-3 PUFA diet were due to n-3 PUFA and not simply a reflection of a high fat diet, an additional set of mice fed a high fat ‘control’ diet (HFC) comprised of a mixture of fatty acids was studied under identical experimental conditions. Mice fed HFC diet for 14 weeks were found to have no significant differences, compared to Std Chow, in any parameter of cardiac mitochondrial function measured (Fig. 1 + Table 1).
Figure 1.
Dietary n-3 PUFA intake causes time-dependent adaptations in mitochondrial function in mouse heart, resulting in decreased mH2O2 emission (A) and increased tolerance to mCa2+ overload (B) by 14 weeks. Shown are mean ± S.E.M. in N = 7 for 3 week diet and N = 5 for 14 week diet; * P<0.05 vs. Std Chow
Table 2.
| Diet | Duration (wks) | Respiratory Substrates/Conditions
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| P/M | P/MADP | P/M/G/SADP | P/M/G/SADP + Rot | RCR | PC/M | PC/MADP | RCR | ||
| Std Chow | 3 | 190.5 ± 13.6 | 819.1 ± 70.3 | 1437 ± 122.5 | 947.5 ± 72.1 | 5.0 ± 0.5 | 127 ± 19.1 | 506.3 ± 67.6 | 3.8 ± 0.3 |
| n-3 PUFA | 3 | 215.7 ± 11.6 | 1097 ± 123.9 | 2044 ± 271.8 | 1546 ± 169.6 | 5.1 ± 0.3 | 153 ± 19.5 | 611.7 ± 82.3 | 4.1 ± 0.5 |
|
| |||||||||
| Std Chow | 14 | 240.2 ± 36.7 | 1302 ± 200.7 | 1671 ± 259.3 | 969.4 ± 119.2 | 5.4 ± 0.5 | 164.6 ± 19.9 | 625.3 ± 93.6 | 3.6 ± 0.6 |
| n-3 PUFA | 14 | 347.6 ± 63.3 | 2248 ± 530.8 | 3301 ± 736.3 | 1832 ± 337.7 | 6.3 ± 0.6 | 205.5 ± 39.6 | 606.4 ± 103.9 | 3.1 ± 0.3 |
| HFC | 14 | 402.5 ± 152.6 | 2537 ± 732.8 | 3134 ± 759.8 | 1966 ± 444.2 | 6.2 ± 0.7 | 261.2 ± 57.2 | 856.9 ± 168.7 | 3.3 ± 0.4 |
Data shown are mean ± S.E.M. in N = 7 for 3 week n-3 PUFA diet and N = 5 for 14 week n-3 PUFA diet. High Fat Control diet (HFC). Rates of respiration are in presence of 5 mM pyruvate/2 mM malate (P/M); P/M + 5 mM ADP (P/MADP); P/MADP + 5 mM glutamate, 5 mM succinate (P/M/G/SADP); P/M/G/SADP + 1 μM Rotenone (P/M/G/SADP + Rot); 100 μM palmitoyl-L-carnitine/2 mM malate (PC/M); PC/M + 5 mM ADP (PC/MADP); Respiratory Control Ratio (RCR)
Effects of n-3 PUFA diet on cardiac glutathione and its related enzymes
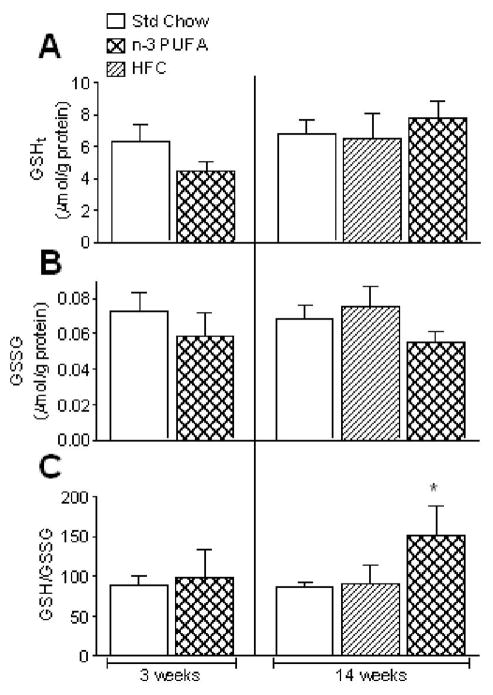
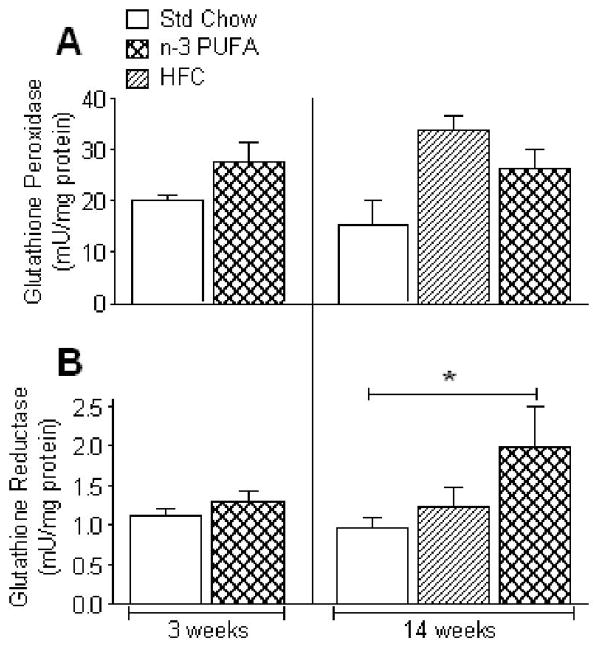
We speculated that the decreased mH2O2 and increased mCa2+ tolerance observed in the n-3 PUFA fed mouse hearts might be due to increased antioxidant capacity (e.g. glutathione) in the tissue, as other groups have shown that the rates of mH2O2 efflux and opening of mitochondrial permeability transition pore (mPTP) can be greatly affected by glutathione status [30–32]. No significant differences were observed in levels of total (GSHt) or oxidized glutathione (GSSG) following 3- or 14-weeks of n-3 PUFA diet, or 14-weeks of HFC diet (Fig. 2A+B). However, cardiac GSH/GSSG ratio was significantly higher in the 14-week n-3 PUFA mice compared to Std Chow (Fig. 2C). To determine whether the differences in GSH/GSSG ratio in n-3 PUFA group at 14 weeks were due to altered activity of the enzymes responsible for maintaining this ratio, we measured activities of glutathione peroxidase (GPx) and reductase (GR) in cardiac tissue from these mice. As shown in Fig. 3A, no significant differences were observed in cardiac GPx activity at either 3- or 14 weeks, but cardiac GR activity was nearly 2-fold greater in mice fed n-3 PUFA diet for 14 weeks, though unchanged at 3 weeks (Fig. 3B). Both GPx and GR activity remained similar in cardiac tissue from mice fed HFC diet for 14 weeks, as compared to Std Chow (Fig. 3).
Figure 2.
Time-dependent effects of dietary n-3 PUFAs on levels of total glutathione GSHt (A), oxidized glutathione GSSG (B) and GSH/GSSG ratio (C) in mouse hearts. Data are mean ± S.E.M. in N = 7 for 3 week diet and N = 6 for 14 week diet; * P<0.05 vs. Std Chow
Figure 3.
Rates of glutathione peroxidase (A) and glutathione reductase (B) activity in mouse heart after dietary n-3 PUFA treatment for 3 and 14 weeks. Data are mean ± S.E.M. in N = 7 for 3 week diet and N = 6 for 14 week diet; * P<0.05 vs. Std Chow
Aldehyde stress in myocardium of mice fed n-3 PUFA diet
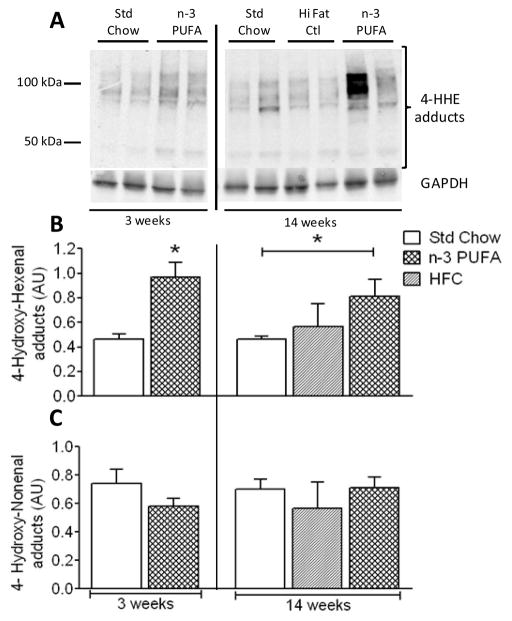
Since aldehydes have been shown to trigger compensatory increases in glutathione synthesis in heart [17, 33, 34], we sought to ascertain whether the increases in GR and GSH/GSSG after 14 weeks of n-3 PUFA diet were a result of increased aldehyde stress throughout the course of the diet. Interestingly, the levels of HHE-adduct formation were already substantially higher in hearts of n-3 PUFA mice at 3 weeks compared to Std Chow. At 14 weeks, levels of HHE-adducts in n-3 PUFA mice were still significantly greater than Std Chow, and no significant differences existed between Std Chow and HFC diet for 14 weeks (Fig. 4A + B). Unlike HHE, however, levels of HNE- adducts did not significantly change under any of the dietary treatments examined (Fig. 4C).
Figure 4.
Immunoblots of protein adducts with 4-hydroxyhexenal, a n-3 PUFA-derived aldehyde, in mouse heart following n-3 PUFA diet are shown in representative blots (A) and quantified by densitometry (B). Shown in (C) are quantified levels of protein adducts with 4-hydroxynonenal, a n-6 PUFA-derived aldehyde. Data are mean ± S.E.M. in N = 6 for 3 week diet and N = 4 for 14 week diet; * P<0.05 vs. Std Chow
DISCUSSION
Therapeutic application of dietary n-3 PUFAs in cardiac disease remains controversial despite years of experimental and clinical work in this area. The origin of much of this controversy can be attributed to the wide disparity in approaches used by investigators with respect to source of n-3 PUFA (fish vs. flaxseed oil) and dose required to achieve the observed effects. Even less is known about the time-dependent kinetics of these effects. In this context, the novelty of the study presented here is that 1) hearts from mice fed n-3 PUFA diet have decreased mitochondrial H2O2 emission and increased Ca2+-retention capacity; 2) these mitochondrial adaptations are dependent upon time; 3) these mitochondrial adaptations are accompanied by increased glutathione redox status and increased activity of GR; and 4) accumulation of HHE, a lesser known and under-studied aldehyde produced exclusively from lipid peroxidation of n-3 PUFAs, precedes these adaptations in mitochondrial function and glutathione redox chemistry during the course of the diet. These results show a unique and important chronological sequence of biochemical events which may explain, at least in part, a mechanistic basis of n-3 PUFA action in heart, and establish a role for non-enzymatic lipid oxidation products of n-3 PUFA’s in this process.
Increased capacity of cardiac mitochondria to buffer Ca2+ would be desirous for a variety of reasons, ranging from prevention of arrhythmia [35], increased activation of mitochondrial dehydrogenases and ATP production [36], and decreased susceptibility to Ca2+-induced opening of mPTP and cell death [37, 38]. In the present study, a number of factors may explain the observed increase in mitochondrial Ca2+-retention capacity in hearts of n-3 PUFA mice, consistent with reports from other labs. Similar studies using in vitro preparations of isolated cardiac mitochondria from fish oil-treated rats showed increased Ca2+-retention capacity [13, 14] before mPTP opening, and global ischemia/reperfusion injury in ex vivo rat hearts show that hearts from fish oil-fed animals have increased recovery of contractile force and decreased infarct sizes compared with chow-fed or n-6 PUFA-fed animals [39, 40]. The authors of these studies have largely focused on how n-3 PUFAs may be altering mitochondrial phospholipid fatty acid composition, particularly in cardiolipin, and the impact that this remodeling may have on the biophysical/biochemical interactions between cardiolipin and key proteins involved in mitochondrial Ca2+ uptake and retention capacity.
An invariable link in all of these previous studies on n-3 PUFA treatment is that the levels of n-6 PUFAs, particularly arachidonic acid (AA), were shown to decrease in cell membranes following n-3 PUFA treatment, and levels of DHA increased. Importantly, the characteristic decrease in AA and increase in DHA following fish oil treatment has been shown to be most pronounced in mitochondrial membranes [41], reflected even by alterations in cardiolipin composition [13]. While no studies to date have shown specifically how altering cardiolipin composition may affect mitochondrial Ca2+ uptake and retention capacity, there is good evidence that decreased AA levels may be important in this process given that AA has been shown to trigger increased susceptibility to Ca2+-induced mitochondrial swelling and cell death [42, 43]. Given these previous findings, it is plausible that the increased mitochondrial Ca2+ retention capacity and delayed mPTP observed in the present study is a result of mitochondrial phospholipid remodeling, although these parameters were not measured here. However, even if these parameters had been measured in this study, it would not have added much to the field given that these effects are already well-established, and at present no clear biophysical/biochemical mechanism exists as to how changing the composition of mitochondrial fatty acids and/or cardiolipin would change the structure/function of mitochondrial inner-membrane proteins and enzymes.
We decided to direct our investigation towards other potential novel mediators of Ca2+-induced mPTP to see if additional pathways and/or factors were also changing in response to n-3 PUFA diet. Given the substantial decrease in mitochondrial H2O2 emission in hearts from n-3 PUFA diet, another interesting and novel finding in this study, we decided to focus on glutathione and activity of its related enzymes. Our rationale for this was predicated on previous studies showing that oxidative stress and lipid peroxidation specifically alter key components of mPTP such as adenine nucleotide translocase [44, 45], cyclophilin D [46], and cardiolipin [47, 48]. If levels of glutathione were increased, or activities of glutathione-related enzymes GPx or GR were increased following n-3 PUFA diet, then theoretically the reactive thiols within these mPTP components would be preserved in a reduced state for longer periods of time, thereby decreasing the mPTP sensitivity to Ca2+. We found that hearts from n-3 PUFA fed mice at 14 weeks had significantly higher GSH/GSSG ratio, due in part to the significantly increased activity of GR. This increase in GR activity following n-3 PUFA treatment is an unprecedented finding and would partly explain the changes in mitochondrial H2O2 emission and Ca2+ retention seen in hearts of these animals [49]. Whether these adaptations in GSH/GSSG and GR activity in cardiac homogenate, as it was assessed in this study, is reflective of these levels in mitochondria is not completely known. However, because mitochondria cannot synthesize their own glutathione pools and must rely on cytosolic stores for their own supply [50], and because mitochondrial matrix represents such a large proportion of the total volume of mouse heart, it is reasonable to conclude that the values obtained in homogenate do reflect, to a large extent, that of the mitochondria.
In consideration of the mechanism by which n-3 PUFA diet may be inducing these adaptations in the heart, it becomes necessary to consider the chemical nature of PUFAs. Because of their structure and chemical composition, PUFA’s are highly susceptible to electrophilic attack and peroxidation, particularly at the methylene group located between the C=C bonds. Peroxidation of PUFAs is a process that can proceed through both enzymatic and spontaneous, non-enzymatic pathways. Enzymatic peroxidation of PUFAs, controlled largely by cyclo-oxygenases (COX) and lipoxygenases (LOX), result in production of eicosanoids. These pathways have been a well-known and intensely studied component of the systemic immune and inflammatory response for decades [51]. Non-enzymatic oxidation of PUFAs results in formation of a number of bioactive intermediates, and these intermediates, if left unchecked, ultimately form chemically stable and highly electrophilic aldehydes called 4-hydroxyalkenals [52]. The aldehyde formed in this manner that has received the most scrutiny from investigators in HNE, a nine-carbon aldehyde formed from peroxidation and degradation of n-6 PUFAs such as AA and linoleic acid [18]. Like many electrophiles, HNE exerts bi-directionality in its effect on cells and tissues. At low, sub-toxic levels, HNE is cardioprotective and induces up-regulation of many enzymes responsible for ROS de-toxification and cell survival, but is cytotoxic at high levels [53, 54]. Non-enzymatic peroxidation of n-3 PUFAs such as DHA, EPA and α-linolenic acid results in formation of HHE, a 6-carbon aldehyde with many similarities to HNE but slightly less electrophilic [19]. In contrast to HNE, almost nothing is known about HHE and its role in cell signaling and cytotoxicity.
Both HNE and HHE covalently react with proteins to form adducts. In this study, we have demonstrated for the first time that levels of HHE-adducts are increased in hearts of mice fed n-3 PUFA diet. Conversely, no significant changes in HNE-adducts were observed. These findings are significant because they unlock a potential clue as to the underlying signals driving the increased GSH/GSSG, GR, and mitochondrial adaptations at 14 weeks. One possibility is that chronic increases in lipid peroxidation products from n-3 PUFAs, including HHE, up-regulate the expression and activity of glutathione-related enzymes through an NF-E2-Related Factor 2 (Nrf2)-dependent mechanism. Indeed, both HNE [15] and HHE [34] have been shown to activate Nrf2 in cardiovascular cell types. Alternatively, an elegant and comprehensive study by Fukuda’s group has recently outlined a novel signaling pathway whereby aldehyde stress in the heart increases glutathione synthesis and enzyme activity through eukaryotic initiation factor (eIF) 2α-activating transcription factor 4 (Atf4) pathway [17]. Whether such pathways explain the data presented in this study remains to be determined, though it is interesting to note that increases in HHE-adducts are already present at 3 weeks, preceding the up-regulation of GSH/GSSG, GR and mitochondrial adaptations in chronological order (Fig. 4). This supports a causal role for HHE in this process.
To conclude, the findings of this study represent a completely novel paradigm regarding n-3 PUFA action in heart, in that it outlines a time-dependent framework of adaptive processes that occur in myocardium at the mitochondrial and tissue level during the course of n-3 PUFA treatment. Furthermore, it illustrates a role for n-3 PUFA lipid peroxidation products as potential mediators of n-3 PUFA cardioprotection, and lays a foundation for future work into the role of non-enzymatic lipid peroxidation of PUFAs in cardiac health and disease.
Acknowledgments
The authors would like to thank Benjamin Drew Rockett for his assistance in feeding the mice.
FUNDING
This study was supported in part by National Institutes of Health Grants R21 HL098780 to E.J.A. and R15AT006122-01o S.R.S.
Abbreviations used
- PUFA
poly-unsaturated fatty acids
- EPA
eicosapentaenoic acid
- DHA
docosahexaenoic acid
- ADP
adenosine diphosphate
- ATP
adenosine triphosphate
- AA
arachidonic acid
- HNE
4-hydroxynonenal
- HHE
4-hydroxyhexenal
- mPTP
mitochondrial permeability transition pore
References
- 1.Berthiaume JM, Bray MS, McElfresh TA, Chen X, Azam S, Young ME, Hoit BD, Chandler MP. The myocardial contractile response to physiological stress improves with high saturated fat feeding in heart failure. Am J Physiol Heart Circ Physiol. 2010;299:H410–421. doi: 10.1152/ajpheart.00270.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duda MK, O’Shea KM, Lei B, Barrows BR, Azimzadeh AM, McElfresh TE, Hoit BD, Kop WJ, Stanley WC. Low-carbohydrate/high-fat diet attenuates pressure overload-induced ventricular remodeling and dysfunction. J Card Fail. 2008;14:327–335. doi: 10.1016/j.cardfail.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rennison JH, McElfresh TA, Chen X, Anand VR, Hoit BD, Hoppel CL, Chandler MP. Prolonged exposure to high dietary lipids is not associated with lipotoxicity in heart failure. J Mol Cell Cardiol. 2009;46:883–890. doi: 10.1016/j.yjmcc.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duda MK, O’Shea KM, Lei B, Barrows BR, Azimzadeh AM, McElfresh TE, Hoit BD, Kop WJ, Stanley WC. Dietary supplementation with omega-3 PUFA increases adiponectin and attenuates ventricular remodeling and dysfunction with pressure overload. Cardiovasc Res. 2007;76:303–310. doi: 10.1016/j.cardiores.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duda MK, O’Shea KM, Tintinu A, Xu W, Khairallah RJ, Barrows BR, Chess DJ, Azimzadeh AM, Harris WS, Sharov VG, Sabbah HN, Stanley WC. Fish oil, but not flaxseed oil, decreases inflammation and prevents pressure overload-induced cardiac dysfunction. Cardiovasc Res. 2009;81:319–327. doi: 10.1093/cvr/cvn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nodari S, Triggiani M, Campia U, Manerba A, Milesi G, Cesana BM, Gheorghiade M, Dei Cas L. Effects of n-3 Polyunsaturated Fatty Acids on Left Ventricular Function and Functional Capacity in Patients With Dilated Cardiomyopathy. J Am Coll Cardiol. 2011;57:870–879. doi: 10.1016/j.jacc.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 7.Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 8.Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, Jaleel N, Chua BH, Hewett TE, Robbins J, Houser SR, Molkentin JD. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest. 2007;117:2431–2444. doi: 10.1172/JCI31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moorjani N, Westaby S, Narula J, Catarino PA, Brittin R, Kemp TJ, Narula N, Sugden PH. Effects of left ventricular volume overload on mitochondrial and death-receptor-mediated apoptotic pathways in the transition to heart failure. Am J Cardiol. 2009;103:1261–1268. doi: 10.1016/j.amjcard.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, Borisenko GG. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 11.Saini-Chohan HK, Holmes MG, Chicco AJ, Taylor WA, Moore RL, McCune SA, Hickson-Bick DL, Hatch GM, Sparagna GC. Cardiolipin biosynthesis and remodeling enzymes are altered during development of heart failure. J Lipid Res. 2009;50:1600–1608. doi: 10.1194/jlr.M800561-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sparagna GC, Chicco AJ, Murphy RC, Bristow MR, Johnson CA, Rees ML, Maxey ML, McCune SA, Moore RL. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J Lipid Res. 2007;48:1559–1570. doi: 10.1194/jlr.M600551-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Khairallah RJ, Sparagna GC, Khanna N, O’Shea KM, Hecker PA, Kristian T, Fiskum G, Des Rosiers C, Polster BM, Stanley WC. Dietary supplementation with docosahexaenoic acid, but not eicosapentaenoic acid, dramatically alters cardiac mitochondrial phospholipid fatty acid composition and prevents permeability transition. Biochim Biophys Acta. 2010;1797:1555–1562. doi: 10.1016/j.bbabio.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Shea KM, Khairallah RJ, Sparagna GC, Xu W, Hecker PA, Robillard-Frayne I, Des Rosiers C, Kristian T, Murphy RC, Fiskum G, Stanley WC. Dietary omega-3 fatty acids alter cardiac mitochondrial phospholipid composition and delay Ca2+-induced permeability transition. J Mol Cell Cardiol. 2009;47:819–827. doi: 10.1016/j.yjmcc.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Sano M, Shinmura K, Tamaki K, Katsumata Y, Matsuhashi T, Morizane S, Ito H, Hishiki T, Endo J, Zhou H, Yuasa S, Kaneda R, Suematsu M, Fukuda K. 4-hydroxy-2-nonenal protects against cardiac ischemia-reperfusion injury via the Nrf2-dependent pathway. J Mol Cell Cardiol. 2010;49:576–586. doi: 10.1016/j.yjmcc.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Hill BG, Dranka BP, Zou L, Chatham JC, Darley-Usmar VM. Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem J. 2009;424:99–107. doi: 10.1042/BJ20090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Endo J, Sano M, Katayama T, Hishiki T, Shinmura K, Morizane S, Matsuhashi T, Katsumata Y, Zhang Y, Ito H, Nagahata Y, Marchitti S, Nishimaki K, Wolf AM, Nakanishi H, Hattori F, Vasiliou V, Adachi T, Ohsawa I, Taguchi R, Hirabayashi Y, Ohta S, Suematsu M, Ogawa S, Fukuda K. Metabolic remodeling induced by mitochondrial aldehyde stress stimulates tolerance to oxidative stress in the heart. Circ Res. 2009;105:1118–1127. doi: 10.1161/CIRCRESAHA.109.206607. [DOI] [PubMed] [Google Scholar]
- 18.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 19.Van Kuijk FJ, Holte LL, Dratz EA. 4-Hydroxyhexenal: a lipid peroxidation product derived from oxidized docosahexaenoic acid. Biochim Biophys Acta. 1990;1043:116–118. doi: 10.1016/0005-2760(90)90118-h. [DOI] [PubMed] [Google Scholar]
- 20.Rockett BD, Salameh M, Carraway K, Morrison K, Shaikh SR. n-3 PUFA improves fatty acid composition, prevents palmitate-induced apoptosis, and differentially modifies B cell cytokine secretion in vitro and ex vivo. J Lipid Res. 2010;51:1284–1297. doi: 10.1194/jlr.M000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson EJ, Yamazaki H, Neufer PD. Induction of endogenous uncoupling protein 3 suppresses mitochondrial oxidant emission during fatty acid-supported respiration. J Biol Chem. 2007;282:31257–31266. doi: 10.1074/jbc.M706129200. [DOI] [PubMed] [Google Scholar]
- 22.Saks VA, Veksler VI, Kuznetsov AV, Kay L, Sikk P, Tiivel T, Tranqui L, Olivares J, Winkler K, Wiedemann F, Kunz WS. Permeabilized cell and skinned fiber techniques in studies of mitochondrial function in vivo. Mol Cell Biochem. 1998;184:81–100. [PubMed] [Google Scholar]
- 23.Anderson EJ, Rodriguez E, Anderson CA, Thayne K, Chitwood WR, Kypson AP. Increased propensity for cell death in diabetic human heart is mediated by mitochondrial-dependent pathways. Am J Physiol Heart Circ Physiol. 2011;300:H118–124. doi: 10.1152/ajpheart.00932.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsien RY. Fluorescent indicators of ion concentrations. Methods Cell Biol. 1989;30:127–156. doi: 10.1016/s0091-679x(08)60978-4. [DOI] [PubMed] [Google Scholar]
- 25.Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009 doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 27.Carlberg I, Mannervik B. Glutathione reductase. Methods Enzymol. 1985;113:484–490. doi: 10.1016/s0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- 28.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 29.Rasband WS. ImageJ. U. S. National Institutes of Health; Bethesda, Maryland, USA: 1997–2009. http://rsb.info.nih.gov/ij/ [Google Scholar]
- 30.Aon MA, Cortassa S, Maack C, O’Rourke B. Sequential opening of mitochondrial ion channels as a function of glutathione redox thiol status. J Biol Chem. 2007;282:21889–21900. doi: 10.1074/jbc.M702841200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Treberg JR, Quinlan CL, Brand MD. Hydrogen peroxide efflux from muscle mitochondria underestimates matrix superoxide production--a correction using glutathione depletion. FEBS J. 2010;277:2766–2778. doi: 10.1111/j.1742-4658.2010.07693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colell A, Garcia-Ruiz C, Miranda M, Ardite E, Mari M, Morales A, Corrales F, Kaplowitz N, Fernandez-Checa JC. Selective glutathione depletion of mitochondria by ethanol sensitizes hepatocytes to tumor necrosis factor. Gastroenterology. 1998;115:1541–1551. doi: 10.1016/s0016-5085(98)70034-4. [DOI] [PubMed] [Google Scholar]
- 33.Uchida K, Shiraishi M, Naito Y, Torii Y, Nakamura Y, Osawa T. Activation of stress signaling pathways by the end product of lipid peroxidation. 4-hydroxy-2-nonenal is a potential inducer of intracellular peroxide production. J Biol Chem. 1999;274:2234–2242. doi: 10.1074/jbc.274.4.2234. [DOI] [PubMed] [Google Scholar]
- 34.Ishikado A, Nishio Y, Morino K, Ugi S, Kondo H, Makino T, Kashiwagi A, Maegawa H. Low concentration of 4-hydroxy hexenal increases heme oxygenase-1 expression through activation of Nrf2 and antioxidative activity in vascular endothelial cells. Biochem Biophys Res Commun. 2010;402:99–104. doi: 10.1016/j.bbrc.2010.09.124. [DOI] [PubMed] [Google Scholar]
- 35.Xie LH, Weiss JN. Arrhythmogenic consequences of intracellular calcium waves. Am J Physiol Heart Circ Physiol. 2009;297:H997–H1002. doi: 10.1152/ajpheart.00390.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heineman FW, Balaban RS. Control of mitochondrial respiration in the heart in vivo. Annu Rev Physiol. 1990;52:523–542. doi: 10.1146/annurev.ph.52.030190.002515. [DOI] [PubMed] [Google Scholar]
- 37.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovasc Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 38.Bernardi P, Rasola A. Calcium and cell death: the mitochondrial connection. Subcell Biochem. 2007;45:481–506. doi: 10.1007/978-1-4020-6191-2_18. [DOI] [PubMed] [Google Scholar]
- 39.Pepe S, Tsuchiya N, Lakatta EG, Hansford RG. PUFA and aging modulate cardiac mitochondrial membrane lipid composition and Ca2+ activation of PDH. Am J Physiol. 1999;276:H149–158. doi: 10.1152/ajpheart.1999.276.1.H149. [DOI] [PubMed] [Google Scholar]
- 40.Pepe S, McLennan PL. Cardiac membrane fatty acid composition modulates myocardial oxygen consumption and postischemic recovery of contractile function. Circulation. 2002;105:2303–2308. doi: 10.1161/01.cir.0000015604.88808.74. [DOI] [PubMed] [Google Scholar]
- 41.Stillwell W, Jenski LJ, Crump FT, Ehringer W. Effect of docosahexaenoic acid on mouse mitochondrial membrane properties. Lipids. 1997;32:497–506. doi: 10.1007/s11745-997-0064-6. [DOI] [PubMed] [Google Scholar]
- 42.Di Paola M, Zaccagnino P, Oliveros-Celis C, Lorusso M. Arachidonic acid induces specific membrane permeability increase in heart mitochondria. FEBS Lett. 2006;580:775–781. doi: 10.1016/j.febslet.2005.12.090. [DOI] [PubMed] [Google Scholar]
- 43.Scorrano L, Penzo D, Petronilli V, Pagano F, Bernardi P. Arachidonic acid causes cell death through the mitochondrial permeability transition. Implications for tumor necrosis factor-alpha aopototic signaling. J Biol Chem. 2001;276:12035–12040. doi: 10.1074/jbc.M010603200. [DOI] [PubMed] [Google Scholar]
- 44.Giron-Calle J, Zwizinski CW, Schmid HH. Peroxidative damage to cardiac mitochondria. II. Immunological analysis of modified adenine nucleotide translocase. Arch Biochem Biophys. 1994;315:1–7. doi: 10.1006/abbi.1994.1463. [DOI] [PubMed] [Google Scholar]
- 45.Costantini P, Belzacq AS, Vieira HL, Larochette N, de Pablo MA, Zamzami N, Susin SA, Brenner C, Kroemer G. Oxidation of a critical thiol residue of the adenine nucleotide translocator enforces Bcl-2-independent permeability transition pore opening and apoptosis. Oncogene. 2000;19:307–314. doi: 10.1038/sj.onc.1203299. [DOI] [PubMed] [Google Scholar]
- 46.Linard D, Kandlbinder A, Degand H, Morsomme P, Dietz KJ, Knoops B. Redox characterization of human cyclophilin D: identification of a new mammalian mitochondrial redox sensor? Arch Biochem Biophys. 2009;491:39–45. doi: 10.1016/j.abb.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Petrosillo G, Casanova G, Matera M, Ruggiero FM, Paradies G. Synergistic effect of Ca2+ and peroxidized cardiolipin in the induction of permeability transition and cytochrome c release in rat heart mitochondria. Ital J Biochem. 2007;56:307–309. [PubMed] [Google Scholar]
- 48.Petrosillo G, Casanova G, Matera M, Ruggiero FM, Paradies G. Interaction of peroxidized cardiolipin with rat-heart mitochondrial membranes: induction of permeability transition and cytochrome c release. FEBS Lett. 2006;580:6311–6316. doi: 10.1016/j.febslet.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 49.Rigobello MP, Folda A, Scutari G, Bindoli A. The modulation of thiol redox state affects the production and metabolism of hydrogen peroxide by heart mitochondria. Arch Biochem Biophys. 2005;441:112–122. doi: 10.1016/j.abb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 50.Griffith OW, Meister A. Origin and turnover of mitochondrial glutathione. Proc Natl Acad Sci U S A. 1985;82:4668–4672. doi: 10.1073/pnas.82.14.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hwang D. Fatty acids and immune responses--a new perspective in searching for clues to mechanism. Annu Rev Nutr. 2000;20:431–456. doi: 10.1146/annurev.nutr.20.1.431. [DOI] [PubMed] [Google Scholar]
- 52.Riahi Y, Cohen G, Shamni O, Sasson S. Signaling and cytotoxic functions of 4-hydroxyalkenals. Am J Physiol Endocrinol Metab. 2010;299:E879–886. doi: 10.1152/ajpendo.00508.2010. [DOI] [PubMed] [Google Scholar]
- 53.Sano M. Cardioprotection by hormetic responses to aldehyde. Circ J. 2010;74:1787–1793. doi: 10.1253/circj.cj-10-0716. [DOI] [PubMed] [Google Scholar]
- 54.Poli G, Schaur RJ, Siems WG, Leonarduzzi G. 4-hydroxynonenal: a membrane lipid oxidation product of medicinal interest. Med Res Rev. 2008;28:569–631. doi: 10.1002/med.20117. [DOI] [PubMed] [Google Scholar]