Abstract
Significance
The Golgi apparatus is essential for protein processing, sorting, and transport. Processing includes carbohydrate modifications and proteolytic cleavage, and transport can involve secretion from the cell or relocation to a specific cellular compartment. Rapid and synchronized reorientation of the Golgi in migrating cells is thought to facilitate polarized secretion, providing membrane and secreted products to the proximal plasma membrane. This function is a fundamental process in cell motility. Whether the Golgi structure and positioning is functionally required for directed secretion and polarity in cell migration responses, such as wound healing, is yet to be elucidated.
Recent Advances
: Exciting recent analysis examined the effects of perturbed Golgi positioning without disruption of microtubular or actin cytoskeleton assembly or protein secretion, in the context of cellular polarity and directional migration in wound repair. This was achieved by Yadav et al. (2009) through depletion of Golgin-160 or GMAP210 (Golgi microtubule associated protein of 210 kDa), which resulted in fragmentation and dispersal of Golgi without altering secretion kinetics. As a consequence, the direction of secretion, cell polarization, and cell migration in response to wounding were severely impaired. Thus, in response to a scratch wound, cell polarity requires peri-centrosomal positioning of the Golgi apparatus, implying that after initiation by a polarity cue there is a dependence on the Golgi's directed secretion to maintain the polarized state that facilitates cell migration.
Critical Issues
Golgi peri-centrosomal positioning can now be included among the growing list of cellular processes and signaling pathways that are critical for establishment of cellular polarity in response to external stimuli—a key feature of wound repair.
Future Directions
A complete understanding of the function of Golgi components in motility merits attractive avenues for future investigations that will ultimately bring regulators of Golgi into the clinic whereby treatment of skin-related disorders will greatly benefit.

Stephen M. Jane, MBBS, PhD, FRACP, FRCPA
Scope
Chronic wounds, severe skin damage, and burns have been successfully treated with grafting from a healthy donor area onto the damaged area of the skin. Although normal physiological skin repair is an efficient process, its acceleration or enhancement through the activity of exogenous factors remains the holy grail. Golgi secretion is required for almost every cellular process including wound healing; however, the relationship between Golgi positioning and directed secretion in cell polarity and motility is still unknown. This mechanism is also relevant for other morphogenetic processes that require epidermal migration such as gastrulation, and neural tube closure.1 Mechanistic insights into Golgi biology are expected to decipher the essential regulators that affect directional cell migration, and these in turn may provide new therapeutic avenues in the vexing field of wound repair.
Background
Camillo Golgi (1844–1926) was an Italian histologist who shared the 1906 Nobel Prize in Physiology and Medicine for his discovery of the organelle in animal cells that bears his name. The existence of the Golgi apparatus was debated for decades (many scientists believed that it only represented a staining artifact), until confirmed in the mid-1950s by the use of the electron microscope. Since then, the Golgi apparatus has been extensively studied. It is the organelle from which proteins are sorted and shipped to their intended destinations by their placement into one of at least three different types of vesicles, namely, exocytotic, secretory, or lysosomal vesicles. Furthermore, an anterograde secretion pathway has been shown to supply plasma membrane components through vesicle fusion and protein expression.2 Secretory cargo exits the Golgi in pleomorphic membrane bounded vesicles in microtubule-dependent exocytosis necessary for the domain-specific fusion of post-Golgi vesicles with the plasma membrane.3 Golgi membranes are largely confined to a region surrounding the centrosome or microtubule-organizing center (MTOC), which is critical for the cellular positioning of the Golgi apparatus.4 Diverse physiological processes including embryonic development, chemotaxis, immune function, inflammatory responses, and wound healing require cellular polarity and motility.5–7 In response to a scratch wound, the planar cell polarity requires alignment of the Golgi apparatus within the axis of secretion.7 The leading edge relies on this constant feeding, resulting in growth and extension at the wound margin.8,9
Relevant Basic Science Context
Cells migrate directionally in response to a variety of signals, including gradients of chemokines, growth factors, or extracellular matrix molecules. The wounding stimulus triggers the secretion of molecules and factors, for example fibroblast growth factors,10 which act as a positive regulator and signal transducer in wound repair. These molecular signals become the driving force in transmission of activity to the cytoskeleton and provide the link between membrane receptor signaling and the regulation of the cytoskeleton.11 The plasticity of the cytoskeleton dictates modifications of the cytoplasmic networks of actin microfilaments, intermediate filaments, and microtubules and the generation of intracellular movement of cell components.12 Coordinated interactions between filamentous actin and microtubules are fundamental for maintaining the cell shape, mitotic spindle orientation, motility, growth cone guidance, and wound healing. The outcome is dependent upon the cell type and can range from the polymerization and/or reorganization of actin to the polarized capture and stabilization of microtubules and their MTOC.13 Extracellular stimuli were shown to induce repositioning of both the MTOC and the Golgi apparatus yielding a striking alignment with respect to the stimulus.4 Reorganization of the cytoskeleton causes the nucleus to move to the back of the cell, the centrosome and MTOC together with Golgi to orient facing the leading edge. The processing and trafficking of macromolecules by the Golgi apparatus becomes primarily designated to the wound site. Simultaneously, centrosome-directed polarized dynamics are also required for the generation of cell polarity and cannot be achieved without Golgi remodeling and reorientation. Thus, the cascade of signaling events converges on the driving force of Golgi and directs its secretion toward the wound margin. Recently, Yadav et al. have shown that disruption of the topographical orientation of the Golgi apparatus does not affect the secretion rates. However, the delivery of specific proteins, which are required for cells to polarize, migrate, and heal the wound, is no longer supplied to the leading edge.9
Experimental Model or Material: Advantages and Limitations
The majority of experimental models in Yadav's findings address the dynamic of Golgi network by disrupting its structure and the direction of secretion in vitro.9 Inhibition of the Golgi apparatus was achieved using pharmacological treatment (Brefeldin A)14 or by knocking-down structural proteins such as Golgin-160 or GMAP210 by the small interfering RNAs (siRNA) technique. siRNA knock-down of either Golgin-160 or GMAP210 was specific and did not affect the expression levels of other golgins. In addition, the siRNA neither affected the cytoskeletal structure nor the secretion kinetics. Interestingly, the expression of a full-length Golgin-160 in the siRNA knockdown cells reversed the siRNA effect and confirmed a direct role for Golgin-160 in the establishment of Golgi positioning. Thus, it was relatively easy to impair the wound healing in knock-down cells, which offered a controlled environment to test specific cellular and molecular hypotheses. However, it remains much more complex to optimize and enhance this process. Therefore, it would be ideal to expand the investigation of Golgi positioning to in vivo studies. The translation of these findings into conditional knock-out mice (that can be induced in the epidermis using specific Cre strains) will be pertinent for better understanding of morphogenetic processes that require polarized migration. Assessment of wound repair in mice with an epidermal deletion of Golgin-160 or GMAP210 will provide a more stringent assessment of the role of these factors and of Golgi positioning in cell migration.
Discussion of Findings and Relevant Literature
Two distinct mechanisms underpin the coordinated movement of epithelial sheets during healing: (i) closure occurs by protrusion of filopodia and lamellipodia at the wound edge, with cells crawling toward the opposing wound margin, a typical scenario in adult skin, (ii) actomyosin cables are organized into a purse string at the wound margin that ultimately pulls the edges together, as observed in embryonic epidermal wound repair. Both processes require activation of signaling pathways assembled in a coordinating cascade and that orients Golgi toward the driving edge. Golgi reorientation is a hallmark of cell polarization and part of a feedback loop that sustains the polarized state in wound closure (Fig. 1). The structural and dynamic changes of Golgi orient vesicular trafficking to maintain cell polarity and directed migration to the wound. The research of Golgi secretion has been confounded by the lack of methods for understanding the mechanism of macromolecular trafficking through and within the Golgi. Mechanisms that control Golgi positioning and secretion, aiming to specifically and reversibly activate the secretion of targeted proteins from this organelle, will have a major impact in the process of wound healing. Recently, Yadav et al. discovered that in the absence of specific golgins (Golgin-160 and GMAP210), peri-centrosomal positioning of the Golgi apparatus was altered.9 Golgin-160 is a peripheral Golgi protein localized to the Golgi cistern distinct from the trans-Golgi network. It is involved in the structure and dynamics of the Golgi apparatus.15 GMAP210, a cis-Golgi microtubule binding protein, interacts with γ-tubulin containing complexes. It displays microtubule anchoring and membrane fusion activities, thus contributing to the assembly and maintenance of the Golgi ribbon around the centrosome. Knockdown of the golgin GMAP210 has been shown to disperse Golgi,16 and in an siRNA screen, Yadav et al. identified that GMAP210, as well as Golgin-160, knockdown significantly fragmented and dispersed the Golgi apparatus without disassembly of the microtubule or actin cytoskeletal systems. The two proteins appear to independently confer Golgi positioning and to impact on the polarized migration, and this argues strongly for a primary role of Golgi positioning in the wound response. These findings are relevant for a recent study which shows the importance of conventional Golgi assembly and secretion in a genetic disorder and offers a potential therapeutic strategy for the treatment of disease related to Golgi secretion.17 The peri-centrosomal positioning of the Golgi apparatus could be a byproduct of a strategy using microtubules plus end-directed motility to orient delivery of post-Golgi membrane carriers over large distances and to specific cellular sites.16 The remodeling and reorientation of Golgi was shown in another study to be regulated by phosphorylation of Golgi reassembly stacking protein 65 (GRASP65) by the extracellular signal-regulated kinase, causing loss of GRASP65 oligomerization and cisternal unstacking.18 Transmission electron microscopy analysis revealed that the dispersed Golgi ribbon membranes in Golgin-160 and GMAP210 knockdown cells were dispersed ministacks similar to those present in cells treated with the inhibitor of microtubule polymerization Nocodazole. The presence of ministacks suggests maintenance of compartmentalization, and this was consistent with a fluorescence analysis showing that marker segregation was maintained in the Golgi membranes of the knockdown cells. The reassembled membranes failed to move inward and form a Golgi ribbon, but the rate of reticulum endoplasmic (ER) export, estimated using image analysis, was similar to controls. Although secretion persisted in these cells with normal kinetics, it was evenly distributed in response to wounding rather than directed to the wound edge. The strong defects were observed in cell polarization and cell migration in response to wounding demonstrating that Golgi positioning and the directed secretion are critical in maintaining cell polarity and migration.9
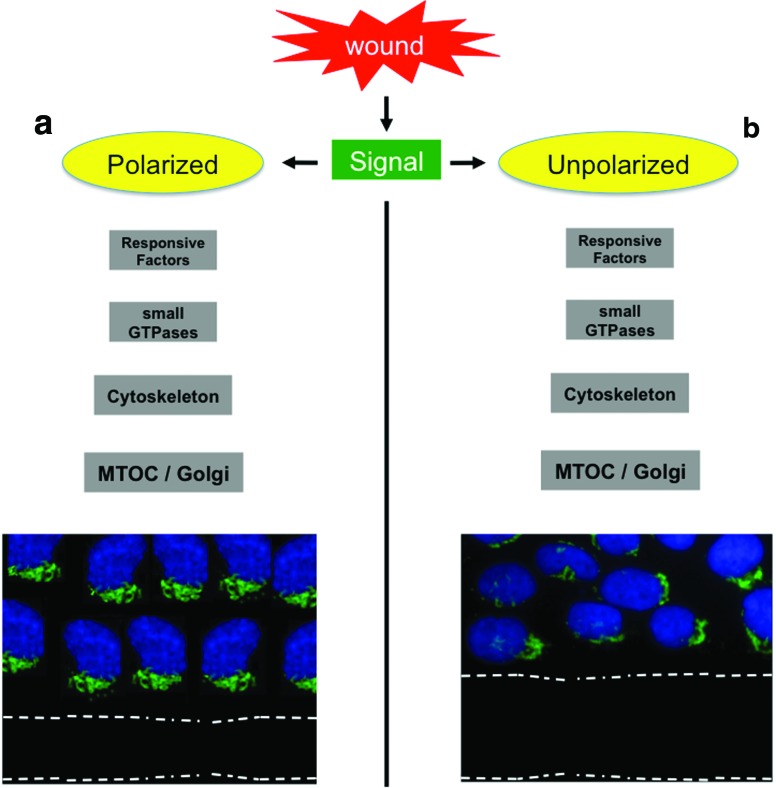
Figure 1.
Positioning of the Golgi apparatus in wound response. The wound scratch triggers a cascade of molecular signals. (a) The end of the physiological response converts on the Golgi network to achieve a peri-centrosomal positioning and a polarized status of secretion toward the wound edge. (b) Disruption of the coordinated molecular pathway impairs the polarized status of Golgi and disrupts the directional cell migration. MTOC, microtubule organizing center. Color images available online at www.liebertpub.com/wound
Innovation
The techniques used by Yadav et al. were elegant and described an accurate tracing of Golgi secretion. The Tet-on system was employed for inducible expression with the temperature-sensitive mutant vesicular stomatitis virus glycoprotein (VSVG)-green fluorescent protein (GFP) in knockdown cells. By incubating the cells at various temperatures in different stages, the authors managed to control and manipulate the ER to surface trafficking of VSVG-GFP in vitro. Importantly, the GFP allowed the visualization and quantification of the surface-VSVG at the indicated time points. Furthermore, Golgi orientation was quantitated by plotting the normalized intensity of GalNacT2-GFP fluorescence using best-fit circles and quadrants within a 180° radius in front of the nucleus and perpendicular to the wound edge. This measurement allowed an unbiased and precise observation to track the movement of Golgi objects in response to wounding.7,9 Indeed, this work highlights several methods to monitor the specific secretion of Golgi apparatus and paves the way to identify the micro-environmental signaling that orients and stabilizes Golgi, which may have a promising impact in wound healing studies and the associated complications.
Caution, Critical Remarks, and Recommendations
The authors conducted their experiments in vitro in a single cell line, HeLa, which is not epidermal and has abnormally rapid proliferation. This raises the question of how reliable or significant the findings in this study are for clinical outcome. It would be ideal to conduct similar experiments in different mammalian epidermal cells and in vivo to validate the role of Golgin-160 and GMAP210 in Golgi peri-centrosomal positioning. Analysis of different stimuli on wound healing in the context of Golgi inhibition will reveal how important the Golgi positioning is in this process. However, it is still arguable that these findings may not be consistent with in vivo physiology, putting into consideration that there are various environmental factors and cell types involved in wound healing, which may override or compensate for the lack of Golgi positioning. Besides, in the absence of either golgin, the positioning of Golgi apparatus was disrupted while the cytoskeleton remained intact. It is worth considering the status of the cytoskeleton architecture independently of Golgi, as the cytoskeleton is involved directly in defects in epidermal migration.12 A potentially important therapeutic opportunity presented by targeting Golgi will require further understanding the mechanisms that disrupt its normal function, and the effects of loss of expression or modification of Golgi components. Although an interesting advance is presented here, we are still a long way from understanding the implications of these findings for clinical wound care.
Future Developments of Interest
The future of Golgi research requires better understanding of its assembly and orientation within the cell and includes the controlled and reversible release of functional proteins from aggregates based on ligand-induced oligomerization.19,20 This will be necessary to distinguish anterograde from retrograde vesicular transport and to rapidly activate or inactivate the function of a targeted protein within the time frame of a single round of Golgi transport. An important future direction will aim to use gene replacement of mutated forms of the golgins after siRNA-mediated knockdown to determine which of the known activities are functionally required for Golgi membrane motility in living cells. Small peptides that can reproduce the effect of Golgi-secreted protein will be highly desirable for the treatment of Golgi-dependent diseases. Bioengineered molecules should provide extrinsic factors useful in reorientation of the Golgi network. Thus, future horizons will ultimately bring regulators of Golgi into the clinic, whereby treatment of skin-related disorders may greatly benefit.
Take-Home Messages.
Basic science advances
Depletion of either Golgi structural components Golgin-160 or GMAP210 impairs its positioning in the axis of migration. The secretion rates and the cytoskeleton networks were unaffected, which argues strongly for a primary role for Golgi positioning in wound response.9 Thus, Yadav et al. have elegantly shown that directional secretion of Golgi apparatus is a critical component of cell polarity and motility.
Clinical science advances
The positioning of Golgi apparatus is centered downstream of the wound healing signaling pathways. It is a hallmark of cell polarization in effective wound healing. Genetic skin disorders and open wounds,12 which are due to impaired upstream Golgi effectors, should benefit from targeting Golgi positioning and the directed secretion.
Relevance to clinical care
The positioning of the centrosome-Golgi complex within a 180° arc in the axis of migration is crucial for effective healing. Unmasking the mechanisms that polarize Golgi secretion will be central for wound healing enhancement and treatment of disorders that require directed secretion toward the wound edge.
Abbreviations and Acronyms
- ECM
extracellular matrix
- GFP
green fluoresecent protein
- GMAP210
Golgi microtubule associated protein of 210 kDa
- GRASP65
Golgi reassembly stacking protein 65
- MTOC
microtubule organizing center
- siRNA
small interfering ribonucleic acid
- VSVG
vesicular stomatitis virus glycoprotein
Acknowledgements and Funding Sources
S.M.J. is a Principal Research Fellow of the Australian National Health and Medical Research Council (NHMRC). Our laboratory is supported by Project Grants from the NHMRC, the Association for International Cancer Research, and The March of Dimes Foundation.
Author Disclosure and Ghostwriting
The authors declare no conflicts of interest. No ghostwriters were used in the writing of this article.
“I could feel my own wound but not those of others.”21
– Jean Nicolas Arthur Rimbaud
References
- 1.Merte J. Jensen D. Wright K, et al. Sec24b selectively sorts Vangl2 to regulate planar cell polarity during neural tube closure. Nat Cell Biol. 2010;12:41. doi: 10.1038/ncb2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prigozhina NL. Waterman-Storer CM. Protein kinase D-mediated anterograde membrane trafficking is required for fibroblast motility. Curr Biol. 2004;14:88. doi: 10.1016/j.cub.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Schmoranzer J. Kreitzer G. Simon SM. Migrating fibroblasts perform polarized, microtubule-dependent exocytosis towards the leading edge. J Cell Sci. 2003;116(Pt 22):4513. doi: 10.1242/jcs.00748. [DOI] [PubMed] [Google Scholar]
- 4.Kupfer A. Louvard D. Singer SJ. Polarization of the Golgi apparatus and the microtubule-organizing center in cultured fibroblasts at the edge of an experimental wound. Proc Natl Acad Sci USA. 1982;79:2603. doi: 10.1073/pnas.79.8.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ridley AJ. Schwartz MA. Burridge K, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 6.Jaffe AB. Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 7.Caddy J. Wilanowski T. Darido C, et al. Epidermal wound repair is regulated by the planar cell polarity signaling pathway. Dev Cell. 2010;19:138. doi: 10.1016/j.devcel.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mellor H. Cell motility: Golgi signalling shapes up to ship out. Curr Biol. 2004;14:R434. doi: 10.1016/j.cub.2004.05.038. [DOI] [PubMed] [Google Scholar]
- 9.Yadav S. Puri S. Linstedt AD. A primary role for Golgi positioning in directed secretion, cell polarity, and wound healing. Mol Biol Cell. 2009;20:1728. doi: 10.1091/mbc.E08-10-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurtner GC. Werner S. Barrandon Y. Longaker MT. Wound repair and regeneration. Nature. 2008;453:314. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 11.Kodama A. Lechler T. Fuchs E. Coordinating cytoskeletal tracks to polarize cellular movements. J Cell Biol. 2004;167:203. doi: 10.1083/jcb.200408047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuchs E. The cytoskeleton and disease: genetic disorders of intermediate filaments. Annu Rev Genet. 1996;30:1971. doi: 10.1146/annurev.genet.30.1.197. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe T. Noritake J. Kaibuchi K. Regulation of microtubules in cell migration. Trends Cell Biol. 2005;15:76. doi: 10.1016/j.tcb.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Bershadsky AD. Futerman AH. Disruption of the Golgi apparatus by brefeldin A blocks cell polarization and inhibits directed cell migration. Proc Natl Acad Sci USA. 1994;91:5686. doi: 10.1073/pnas.91.12.5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams D. Hicks SW. Machamer CE. Pessin JE. Golgin-160 is required for the Golgi membrane sorting of the insulin-responsive glucose transporter GLUT4 in adipocytes. Mol Biol Cell. 2006;17:5346. doi: 10.1091/mbc.E06-05-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rios RM. Sanchis A. Tassin AM. Fedriani C. Bornens M. GMAP-210 recruits gamma-tubulin complexes to cis-Golgi membranes and is required for Golgi ribbon formation. Cell. 2004;118:323. doi: 10.1016/j.cell.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Gee HY. Noh SH. Tang BL. Kim KH. Lee MG. Rescue of deltaF508-CFTR trafficking via a GRASP-dependent unconventional secretion pathway. Cell. 2011;146:746. doi: 10.1016/j.cell.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 18.Bisel B. Wang Y. Wei JH, et al. ERK regulates Golgi and centrosome orientation towards the leading edge through GRASP65. J Cell Biol. 2008;182:837. doi: 10.1083/jcb.200805045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spencer DM. Wandless TJ. Schreiber SL. Crabtree GR. Controlling signal transduction with synthetic ligands. Science. 1993;262:1019. doi: 10.1126/science.7694365. [DOI] [PubMed] [Google Scholar]
- 20.Rothman JE. The future of Golgi research. Mol Biol Cell. 2010;21:3776. doi: 10.1091/mbc.E10-05-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Starkie E. Arthur Rimbaud: A Biography. New York: New Directions; 1961. [Google Scholar]