Abstract
Significance
There is no effective drug treatment for fibrosis (i.e., pathological scarring). Identifying the fundamental mechanisms responsible for normal and pathological connective tissue deposition is likely to yield novel insights into how to control fibrotic conditions.
Recent Advances
An increasing body of evidence suggests a link between mechanical tension and the development of scar tissue. Integrins are the cell surface receptors that mediate interactions between the cell and the surrounding extracellular matrix (ECM). Recent evidence has suggested that, in fibroblasts, the integrin β1–subunit plays an essential role in mechanosignaling and in dermal homeostasis, repair, and fibrosis. The mechanism underlying these activities of integrin β1 appears to involve its ability to (1) mediate activation of latent transforming growth factor beta-1 via ECM contraction and (2) modulate collagen production via a focal adhesion kinase/rac1/nicotinamide adenine dinucleotide phosphate oxidase (NOX)/reactive oxygen species (ROS) pathway. Moreover, the integrin β1–binding protein CCN2, a secreted matricellular protein located within the cellular microenvironment, is required for dermal fibrogenesis.
Critical Issues
Mechanical tension is a key feature underlying the development of scar tissue. The mechanosignaling sensor integrin β1 is an essential, central mediator of dermal fibrosis, wound healing, and homeostasis.
Future Directions
Drugs targeting the molecular mechanism underlying integrin β1–mediated signaling may represent a novel therapeutic approach for treating fibroproliferative disorders. Clinical trials directly testing this hypothesis are warranted.

Andrew Leask, BSc, PhD
Scope and Significance
Fibroproliferative disorders, caused by excessive deposition of scar tissue by fibroblasts resident within connective tissue, are characterized by progressive deterioration of the normal structure and function of organs. Chronic fibrotic diseases can result in organ failure and, in the worst cases, death. Affected organs include the liver, kidney, lung, heart, pancreas, and skin. Fibroproliferative disorders of the skin include systemic sclerosis, Dupuytren's contracture, hypertrophic scars, and keloids. Mechanobiology, namely, how organisms sense mechanical signals and transduce them into biological responses, is being increasingly appreciated to play a critical role in fibroproliferative disorders.1,2 For example, keloids tend to appear on areas where the body suffers local mechanical tension, such as on the shoulders and upper body.1 Thus, mechanotransductive signals present in the local microenvironment are expected to play a major role in the pathogenesis of fibrotic disorders.
Not only are certain areas of the body more subject to increased local mechanical forces, but the skin also becomes under increased local mechanical tension, for example, in response to tissue injury, or within scar tissue. Indeed, a shared feature of scars is their markedly elevated stiffness compared with the relatively compliant tissue texture of healthy tissue. For example, the force present in muscle has been estimated to be ∼10 kPa; in tissues in that are macroscopically soft, such as the liver, fat, and brain, forces have been estimated to range between 0.1 and 1 kPa, whereas the dermis of healthy human skin is estimated to range between 1 and 5 kPa.3 In contrast, fibrotic tissue is substantially less compliant and exhibits substantially greater forces of 20–100 kPa, similar to that of collagen-dense tendon.3
Within the connective tissue of wounded or fibrotic skin is a specialized form of fibroblasts termed myofibroblasts, which share characteristics of smooth muscle cells, as they contain highly contractile stress fibers consisting of the protein alpha–smooth muscle actin (α-SMA).4 The presence of the myofibroblast is what distinguished clinically defined scar tissue from neighboring healthy tissue.5,6 The myofibroblasts themselves inherently exhibit enhanced mechanotransductive abilities as evidenced by their elevated abilities to adhere to and contract the extracellular matrix (ECM), and hence directly contribute to the enhanced tensile strength of scar tissue.6–8 Intriguingly, the appearance of the myofibroblasts within connective tissue seems to depend entirely on mechanical loading. For example, the potent fibrogenic cytokine transforming growth factor beta-1 (TGF-β1) can only upregulate α-SMA in fibroblasts grown on stiff, but not on compliant, collagen.9 Furthermore, TGF-β1 cannot induce myofibroblast formation in focal adhesion kinase (FAK)-deficient fibroblasts, or in fibroblasts treated with the FAK/src inhibitor PP2.10 Finally, α-SMA is only capable of being incorporated into stress fibers in cells subjected to significant mechanical loading.11,12 Collectively, these data strongly suggest that extracellular mechanical forces regulate how dermal fibroblasts contribute to connective tissue deposition and remodeling in skin homeostasis, wound repair, and fibrosis. This review discusses recent data suggesting that integrin β1 plays an essential role in these processes.
Translational and Clinical Relevance
The overall concept that local variances in mechanotranduction are important for dermal homeostasis and fibroproliferative conditions is supported by recent clinical data obtained using humans. For example, although it is generally considered that resting fibroblasts in healthy connective tissue are stress shielded (due to the highly compliant surrounding ECM), a recent report has indicated that keloids tend to occur on highly mobile sites in the body that inherently possess elevated skin stretching and hence are normally subject to comparatively high, local tension compared to the surrounding tissue.13 As a result, understanding how fibroblasts sense and transduce local mechanical signals from the microenvironment is likely to lead to novel therapeutic approaches that prevent, reduce, or even reverse the development and/or progression of these conditions and is therefore likely to be of future clinical benefit.
Basic Science Perspective
Cells sense extracellular mechanical forces through the cytoskeleton, which is connected to the surrounding ECM through cell surface structures known as focal adhesions. Thus, distant molecules within the ECM, cytoplasm, and nucleus are mechanically coupled. In the case of myofibroblasts, the connective tissue cells responsible for wound repair and fibrosis, α-SMA–containing stress fibrers, span the length of the cell and are connected to cell surface ECM receptors called integrins, which are localized to focal adhesions.3,4 Integrins, responsible for sensing mechanical stress, are heterodimers comprised of α- and β-subunits; the main integrins that actively participate in fibroblast proliferation, collagen contraction, and myofibroblast differentiation include α1β1, α2β1, and α11β1.14–16 Although other integrins, notably αvβ5, appear to also participate in myofibroblast differentiation,17,18 these observations suggest that, in fibroblasts, integrin β1 may play a central role in mechanotransduction and fibrogenic responses in the dermis. This review discusses the data underlying this hypothesis.
Discussion of Findings and Relevant Literature
Integrin β1 is an essential mechanotransduction receptor in dermal fibroblasts
As discussed above, the key distinguishing feature of fibrotic tissue is the presence of the myofibroblasts, which contains α-SMA stress fibers. These stress fibers are attached to the ECM through specialized cell surface structures called focal adhesions, which contain clusters of cell surface ECM receptors called integrins. The precise origin of myofibroblasts in skin connective tissue is unclear; however, it is likely that myofibroblasts arise, at least in part, via differentiation of local, resident fibroblasts in response to growth factors and mechanical tension.4 Normally, fibroblasts resident in connective tissue are shielded from stress by the surrounding, compliant ECM; yet, upon injury, this stress shielding is lost, and a series of events is initiated in which fibroblasts possess progressively stronger attachments to the ECM.19 This increase in mechanical loading results in activation of latent TGF-β through a complex mechanism involving integrins, focal contacts, and actin-based ECM contraction.20 In resting tissue, TGF-β, together with its latency-associated peptide (LAP), is bound to latent TGF-β1–binding protein-1, which is a part of the ECM.20 Cell force, via integrins (at a force estimated to be ∼40 pN), is exerted on LAP, leading to the unfolding of two LAP domains: the α1 helix and the latency lasso that normally keep TGF-β1 associated with LAP.20 Integrin β1 knockout dermal fibroblasts are less able to adhere to and contract the ECM.21 In mice harboring a fibroblast-specific deletion of integrin β1, activation of latent TGF-β is impaired; delayed wound healing results, which is rescued by addition of active TGF-β.21
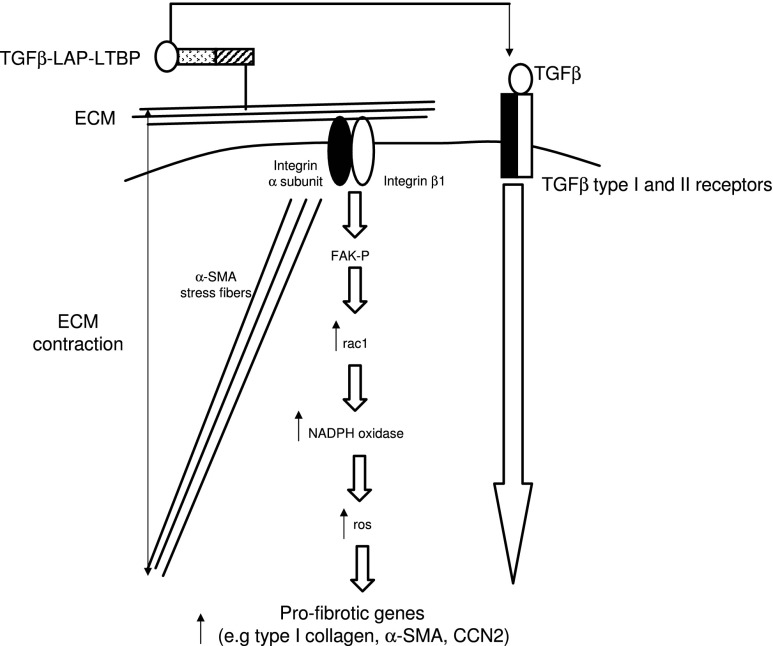
Not only does integrin β1 appear to be involved with activation of latent TGF-β during the wound healing process in mice, but integrin β1 also appears to directly mediate collagen and α-SMA production in skin. Fibroblast-specific integrin β1 knockout mice not only are resistant to bleomycin-induced skin fibrosis, but also possess a progressive thinning of the dermis.22,23 Relative to wild-type control dermal fibroblasts, integrin β1–deficient dermal fibroblasts express reduced levels of collagen type I and α-SMA mRNAs.23 Integrin β1 knockout dermal fibroblasts also show diminished rac1 activation and reactive oxygen species (ROS) generation.23 Reintroduction of active rac1 into integrin β1 knockout fibroblasts restores ROS generation, and addition of H2O2 into integrin β1 knockout fibroblasts restores collagen type I and α-SMA mRNA expression.23 rac1 is known to promote ROS generation via NADPH oxidases (NOXes); NOX inhibition reduces collagen type I and α-SMA mRNA expression in wild-type dermal fibroblasts.23 Phosphorylation of FAK is reduced in integrin β1 knockout fibroblasts and in wild-type fibroblasts treated with a neutralizing anti-integrin β1 antibody.24 Moreover, FAK is required for the expression of collagen type I and α-SMA mRNAs.24 Collectively, these data suggest that integrin β1 can directly control the expression of ECM genes in fibroblasts via a FAK/rac1/NOX/ROS-dependent mechanism (Fig. 1).
Figure 1.
Mechancosignaling pathways mediating dermal connective tissue homeostasis, repair, and fibrosis. In differentiating myofibroblasts, ECM contraction via FA mediated by α-SMA results in the liberation of active TGFβ from LAP. In fibroblasts, integrin β1 contributes to basal collagen and α-SMA expression via a ROS-dependent mechanism. α-SMA, alpha–smooth muscle actin; ECM, extracellular matrix; FA, focal adhesion; FAK, focal adhesion kinase; LAP, latency-associated peptide; NOX, nicotinamide adenine dinucleotide phosphate oxidase; ROS, reactive oxygen species; TGF-β, transforming growth factor beta.
Specific preclinical in vivo data illustrating the role of the signaling pathway operating downstream of integrin β1 in tissue repair and fibrosis have recently been generated. For example, mice harboring fibroblast-specific deletions for FAK and rac1 are resistant to bleomycin-induced skin fibrosis.25,26 Moreover, mice harboring a fibroblast-specific knockout for rac1 show delayed wound closure related to a relative failure to generate ROS.27 Dermal fibroblasts taken from lesional (scarred) areas of patients with the autoimmune connective tissue disease scleroderma show a persistent fibrotic phenotype, including the overproduction of type I collagen and the enhanced ability to contract the ECM. However, when these cells are treated with either FAK or rac inhibitors, this fibrotic phenotype is reversed.24,28 It is important to note that both FAK and rac seem to be important for the expression of type I collagen in normal fibroblasts, as well as the behavior of normal fibroblasts.24,28 Similarly, the antioxidant epigallocatechin-3-gallate suppresses collagen production and contraction by normal and fibrotic sclerodermal fibroblasts.29 These mechanistic observations are interesting as the involvement of NOX/ROS in fibrosis has been recently recognized (for reviews, see Samarkoon et al. and Hecker et al.).30,31). The individual NOX genes responsible for skin tissue repair and fibrosis are unclear. However, NOX4 is upregulated in patients with idiopathic pulmonary fibrosis, and NOX4-produced ROS are critical for models of lung injury and the ability of TGF-β to induce lung myofibroblast differentiation.32,33 These results are consistent with the general hypothesis that mechanosignaling via the integrin β1 subunit is required for dermal homeostasis, wound healing, and fibrotic responses in vivo.
The matricellular integrin β1-binding protein CCN2: a more specific antifibrotic target?
It is clear that, however, the loss of integrin β1 or rac expression and FAK or ROS inhibition affect normal physiology in that basal expression of type I collagen (for example) in healthy fibroblasts is impaired by loss of integrin β1 expression or FAK inhibition (as discussed above).23,24,26–28 This notion leads to the hypothesis that identifying a more selective target for antifibrotic drug intervention that is nonetheless involved with the profibrotic action of integrin β1 would be important.
In this regard, the emerging awareness of the involvement of matricellular proteins in mechanosignaling may be especially useful. The term matricellular protein was first introduced by Bornstein in 1995 to describe a group of ECM proteins that plays minimal roles in matrix structural integrity, but regulates a multitude of cellular responses.34–36 Matricellular proteins are secreted, nonstructural ECM proteins with regulatory functions exerted through different mechanisms, including as the direct binding to other matrix proteins, triggering of their specific surface receptors, and binding to growth factors and cytokines modulating their activity. Abundantly expressed during development, their level is generally low in steady-state condition in adult tissues, but they are readily upregulated in wound healing and tissue remodeling.37–39 The overall function of matricellular proteins is to act in the cellular microenvironment to modulate cellular processes such as cell adhesion and migration, ECM deposition, cell survival, and proliferation. However, the actual in vivo role of each matricellular protein appears to vary depending based on the particular context and the microenvironment that accompanies tissue homeostasis or repair activities. As such, they appear to be ideally suited to contribute to tissue plasticity by acting as adaptor molecules within the cellular microenvironment enabling a rapid response to changing conditions.
Take-Home Messages.
Mechanotransduction plays an essential role in dermal homeostasis, wound healing, and fibrosis.
Integrins are heterodimers, and in fibroblasts, the integrin β1 subunit is a essential mechanosensory receptor necessary for fibroblasts to maximally sense mechanical tension and acts to promote connective tissue maintenance and deposition via a FAK/rac/NADPH oxidase/ROS-dependent mechanism and through the activation of latent TGF-β.
Clinical studies in which drugs targeting the mechanism underlying integrin β1–mediated mechanotransduction are warranted in conditions of dermal scarring and connective tissue disease.
In this scenario, the matricellular protein CCN2 (formerly known as connective tissue growth factor [CTGF]) is largely absent from normal skin, but is selectively upregulated in the dermis during wound healing and fibrosis.37 As such, CCN2 has long been hypothesized to play an essential, specific role in these processes. CCN2 is a member of the CCN family of matricellular proteins, which contains six members.37 The CCN acronym is derived from the first three members of the family identified, namely, CYR61, CTGF, and nephroblastoma overexpressed (NOV); mammalian members of the family have been renamed CCN1–6 of their discovery to reflect their role as matriceullular proteins, as opposed to growth factors.40 The CCN proteins possess a common modular structure, with an N-terminal secretory peptide preceding four conserved domains with sequence homologies to insulin-like growth factor-binding protein, von Willebrand factor type C repeat, thrombospondin type I repeat, and a carboxyl-terminal domain that contains a cysteine knot motif.37,40 The activities of the CCN proteins are largely attributed to the ability of members of the family to bind specfic integrins and heparan sulfate proteoglycans in a cell type- and context-dependent manner. For example, in the context of fibroblasts, CCN2 supports cell adhesion by binding integrin subunits containing integrin β1.41,42 CCN2 also promotes integrin β1–mediated adhesion in other systems.43 In cutaneous wound healing and fibrosis, Ccn2 is highly expressed in myofibroblasts of the granulation tissue.44,45 In vitro and in vivo, CCN2 enhances and alters profibrotic, adhesive signaling responses to ECM components, and growth factors.42,46,47 Although dispensible for development of connective tissue in skin, CCN2 is required for bleomycin-induced skin fibrogenesis.48,49 CCN2 appears to be required for the appearance of myofibroblasts in conditions of fibrogenesis.49 CCN2 appears to act not on affecting myofibroblast differentiation of resident fibroblasts, but may act to recruit myofibroblasts (possibly pericytes or mesenchymal precursor cells).49 CCN2 appears not to mediate normal tissue repair (Liu and Leask, unpublished observations). Thus, CCN2 may prove in the future to be an exquisitely suitable target to affect mechanotransduction in the fibrotic milieu.
Caution, Clincal Remarks, and Recommendations
Integrin β1 is an essential sensor of mechanotransduction, and signaling pathways through which integrin β1 promotes this activity have been elucidated. The basic principles that mechanotransduction appears to be important for fibrosis in humans, and that integrin β1 is an essential mechanotransduction signaling mediator have been suggested by preclinical observations using patients and cells derived from patients. Accordingly, pilot studies using compounds targeting the mechanism through which integrin β1 in patients are warranted. Since integrin β1 has been shown to act via FAK, rac, NADPH oxidase, and ROS, studies using inhibitors of these pathways might be used in patients to see if fibrosis can be attenuated or reversed. Drugs that block these pathways that might be evaluated in clinical trials to block scarring include clinical-stage FAK inhibitors VS-6063 (formerly PF-04554878; Verastem) or PF-00562271 (Pfizer), NADPH oxidase inhibitors such as GKT137831 (Genkyotex) or antioxidants such as N-acetylcysteine (NAC; an over-the-counter herbal supplement), the selective rac GTPase inhibitor, PD3766 (P2D Bioscience), and the Akt inhibitor GSK690693 (Glaxo Smith Kline). Most of these drugs are being evaluated in, or being considered for, clinical trials for cancer;50–55 however, some of these, notably NAC, have also been tested in lung fibrosis and have shown to have positive effects.56,57 Moreover, it should be noted that the dual NOX1/NOX4 inhibitor GKT137831 reduced liver fibrosis in mice, does not exert toxic effects in animal models, and is also well tolerated in phase I clinical trials.58,59 The similarity between fibrosis and cancers is perhaps not surprising, since the oncogenic capacity derives communication between cells in the stroma as well as from the tumor cells themselves, mediated by alterations in ECM-mediated tensegrity.60,61 Indeed, in both fibrosis and cancers, it is believed that the ECM microenvironment may be responsible for regulating cellular behavior, and that altering the microenvironment can orchestrate cellular plasticity offering a way reprogramming diseased cells back to a healthy fate.1,61,62 Similarly, anti-CCN strategies are being considered for cancer;63 drugs targeting CCN2 action are currently being pursued in hypertropic scars and in lung fibrosis include RXI-109 (RXi Pharmaceuticals), EXC 001 (Pfizer), and FG-3019 (Fibrogen).
Abbreviations and Acronyms
- α-SMA
alpha–smooth muscle actin
- ECM
extracellular matrix
- FA
focal adhesion
- FAK
focal adhesion kinase
- LAP
latency-associated peptide
- NAC
N-acetylcysteine
- NOX
nicotinamide adenine dinucleotide phosphate oxidase
- ROS
reactive oxygen species
- TGF-β
transforming growth factor beta
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the author listed. No ghostwriters were used to write this article.
About the Author
Andrew Leask is an Associate Professor at the University of Western Ontario.
References
- 1.Huang C. Ogawa R. Fibroproliferative disorders and their mechanobiology. Connect Tissue Res. 2012;53:187. doi: 10.3109/03008207.2011.642035. [DOI] [PubMed] [Google Scholar]
- 2.Ingber DE. Mechanobiology and diseases of mechanotransduction. Ann Med. 2003;35:564. doi: 10.1080/07853890310016333. [DOI] [PubMed] [Google Scholar]
- 3.Hinz B. Tissue stiffness, latent TGF-beta1 activation, and mechanical signal transduction: implications for the pathogenesis and treatment of fibrosis. Curr Rheumatol Rep. 2009;11:120. doi: 10.1007/s11926-009-0017-1. [DOI] [PubMed] [Google Scholar]
- 4.Liu S. Kapoor M. Denton CP. Abraham DJ. Leask A. Loss of beta1 integrin in mouse fibroblasts results in resistance to skin scleroderma in a mouse model. Arthritis Rheum. 2009;60:2817. doi: 10.1002/art.24801. [DOI] [PubMed] [Google Scholar]
- 5.Rajkumar VS. Howell K. Csiszar K. Denton CP. Black CM. Abraham DJ. Shared expression of phenotypic markers in systemic sclerosis indicates a convergence of pericytes and fibroblasts to a myofibroblast lineage in fibrosis. Arthritis Res Ther. 2005;7:R1113. doi: 10.1186/ar1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y. Shi-Wen X. van Beek J. Kennedy L. McLeod M. Renzoni EA. Bou-Gharios G. Wilcox-Adelman S. Goetinck PF. Eastwood M. Black CM. Abraham DJ. Leask A. Matrix contraction by dermal fibroblasts requires transforming growth factor-beta/activin-linked kinase 5, heparan sulfate-containing proteoglycans, and MEK/ERK: insights into pathological scarring in chronic fibrotic disease. Am J Pathol. 2005;167:1699. doi: 10.1016/s0002-9440(10)61252-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vedrenne N. Coulomb B. Danigo A. Bonté F. Desmoulière A. The complex dialogue between (myo)fibroblasts and the extracellular matrix during skin repair processes and ageing. Pathol Biol (Paris) 2012;60:20. doi: 10.1016/j.patbio.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Leask A. Possible strategies for anti-fibrotic drug intervention in scleroderma. J Cell Commun Signal. 2011;5:125. doi: 10.1007/s12079-011-0122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arora PD. Narani N. McCulloch CA. The compliance of collagen gels regulates transforming growth factor-beta induction of alpha-smooth muscle actin in fibroblasts. Am J Pathol. 1999;154:871. doi: 10.1016/s0002-9440(10)65334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu S. Xu SW. Kennedy L. Pala D. Chen Y. Eastwood M. Carter DE. Black CM. Abraham DJ. Leask A. FAK is required for TGFbeta-induced JNK phosphorylation in fibroblasts: implications for acquisition of a matrix-remodeling phenotype. Mol Biol Cell. 2007;18:2169. doi: 10.1091/mbc.E06-12-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goffin JM. Pittet P. Csucs G. Lussi JW. Meister JJ. Hinz B. Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. J Cell Biol. 2006;172:259. doi: 10.1083/jcb.200506179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinz B. Masters and servants of the force: the role of matrix adhesions in myofi broblast force perception and transmission. Eur J Cell Biol. 2006;85:175. doi: 10.1016/j.ejcb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa R. Okai K. Tokumura F. Mori K. Ohmori Y. Huang C. Hyakusoku H. Akaishi S. The relationship between skin stretching/contraction and pathologic scarring: the important role of mechanical forces in keloid generation. Wound Repair Regen. 2012;20:149. doi: 10.1111/j.1524-475X.2012.00766.x. [DOI] [PubMed] [Google Scholar]
- 14.Pozzi A. Wary KK. Giancotti FG. Gardner HA. Integrin alpha1beta1 mediates a unique collagen-dependent proliferation pathway in vivo. J Cell Biol. 1998;142:587. doi: 10.1083/jcb.142.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schiro JA. Chan BM. Roswit WT. Kassner PD. Pentland AP. Hemler ME. Eisen AZ. Kupper TS. Integrin alpha 2 beta 1 (VLA-2) mediates reorganization and contraction of collagen matrices by human cells. Cell. 1991;67:403. doi: 10.1016/0092-8674(91)90191-z. [DOI] [PubMed] [Google Scholar]
- 16.Carracedo S. Lu N. Popova SN. Jonsson R. Eckes B. Gullberg D. The fibroblast integrin alpha11beta1 is induced in a mechanosensitive manner involving activin A and regulates myofibroblast differentiation. J Biol Chem. 2010;285:10434. doi: 10.1074/jbc.M109.078766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y. Hagood JS. Lu B. Merryman WD. Murphy-Ullrich JE. Thy-1-integrin alphav beta5 interactions inhibit lung fibroblast contraction-induced latent transforming growth factor-beta1 activation and myofibroblast differentiation. J Biol Chem. 2010;285:22382. doi: 10.1074/jbc.M110.126227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asano Y. Ihn H. Yamane K. Jinnin M. Tamaki K. Increased expression of integrin alphavbeta5 induces the myofibroblastic differentiation of dermal fibroblasts. Am J Pathol. 2006;168:499. doi: 10.2353/ajpath.2006.041306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinz B. Gabbiani G. Mechanisms of force generation and transmission by myofibroblasts. Curr Opin Biotechnol. 2003;14:538. doi: 10.1016/j.copbio.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Buscemi L. Ramonet D. Klingberg F. Formey A. Smith-Clerc J. Meister JJ. Hinz B. The single-molecule mechanics of the latent TGF-β1 complex. Curr Biol. 2011;21:2046. doi: 10.1016/j.cub.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 21.Liu S. Xu SW. Blumbach K. Eastwood M. Denton CP. Eckes B. Krieg T. Abraham DJ. Leask A. Expression of integrin beta1 by fibroblasts is required for tissue repair in vivo. J Cell Sci. 2010;123(Pt 21):3674. doi: 10.1242/jcs.070672. [DOI] [PubMed] [Google Scholar]
- 22.Liu S. Kapoor M. Denton CP. Abraham DJ. Leask A. Loss of beta1 integrin in mouse fibroblasts results in resistance to skin scleroderma in a mouse model. Arthritis Rheum. 2009;60:2817. doi: 10.1002/art.24801. [DOI] [PubMed] [Google Scholar]
- 23.Liu S. Leask A. Integrin β1 is required for dermal homeostasis. J Invest Dermatol. 2013;133:899. doi: 10.1038/jid.2012.438. [DOI] [PubMed] [Google Scholar]
- 24.Shi-Wen X. Thompson K. Khan K. Liu S. Murphy-Marshman H. Baron M. Denton CP. Leask A. Abraham DJ. Focal adhesion kinase and reactive oxygen species contribute to the persistent fibrotic phenotype of lesional scleroderma fibroblasts. Rheumatology (Oxford) 2012;51:2146. doi: 10.1093/rheumatology/kes234. [DOI] [PubMed] [Google Scholar]
- 25.Liu S. Kapoor M. Shi-wen X. Kennedy L. Denton CP. Glogauer M. Abraham DJ. Leask A. Role of Rac1 in a bleomycin-induced scleroderma model using fibroblast-specific Rac1-knockout mice. Arthritis Rheum. 2008;58:2189. doi: 10.1002/art.23595. [DOI] [PubMed] [Google Scholar]
- 26.Wong VW. Rustad KC. Akaishi S. Sorkin M. Glotzbach JP. Januszyk M. Nelson ER. Levi K. Paterno J. Vial IN. Kuang AA. Longaker MT. Gurtner GC. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med. 2011;18:148. doi: 10.1038/nm.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu S. Kapoor M. Leask A. Rac1 expression by fibroblasts is required for tissue repair in vivo. Am J Pathol. 2009;174:1847. doi: 10.2353/ajpath.2009.080779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu SW. Liu S. Eastwood M. Sonnylal S. Denton CP. Abraham DJ. Leask A. Rac inhibition reverses the phenotype of fibrotic fibroblasts. PLoS One. 2009;4:e7438. doi: 10.1371/journal.pone.0007438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dooley A. Shi-Wen X. Aden N. Tranah T. Desai N. Denton CP. Abraham DJ. Bruckdorfer R. Modulation of collagen type I, fibronectin and dermal fibroblast function and activity, in systemic sclerosis by the antioxidant epigallocatechin-3-gallate. Rheumatology (Oxford) 2010;49:2024. doi: 10.1093/rheumatology/keq208. [DOI] [PubMed] [Google Scholar]
- 30.Samarakoon R. Overstreet JM. Higgins PJ. TGF-β signaling in tissue fibrosis: Redox controls, target genes and therapeutic opportunities. Cell Signal. 2012;25:264. doi: 10.1016/j.cellsig.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hecker L. Cheng J. Thannickal VJ. Targeting NOX enzymes in pulmonary fibrosis. Cell Mol Life Sci. 2012;69:2365. doi: 10.1007/s00018-012-1012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hecker L. Vittal R. Jones T. Jagirdar R. Luckhardt TR. Horowitz JC. Pennathur S. Martinez FJ. Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15:1077. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amara N. Goven D. Prost F. Muloway R. Crestani B. Boczkowski J. NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFbeta1-induced fibroblast differentiation into myofibroblasts. Thorax. 2010;65:733. doi: 10.1136/thx.2009.113456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol. 1995;130:503. doi: 10.1083/jcb.130.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiodoni C. Colombo MP. Sangaletti S. Matricellular proteins: from homeostasis to inflammation, cancer, and metastasis. Cancer Metastasis Rev. 2010;29:295. doi: 10.1007/s10555-010-9221-8. [DOI] [PubMed] [Google Scholar]
- 36.Kyriakides TR. Bornstein P. Matricellular proteins as modulators of wound healing and the foreign body response. Thromb Haemost. 2003;90:986. doi: 10.1160/TH03-06-0399. [DOI] [PubMed] [Google Scholar]
- 37.Leask A. Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119(Pt 23):4803. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- 38.Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol Rev. 2012;92:635. doi: 10.1152/physrev.00008.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norris RA. Moreno-Rodriguez R. Hoffman S. Markwald RR. The many facets of the matricellular protein periostin during cardiac development, remodeling, and pathophysiology. J Cell Commun Signal. 2009;3:275. doi: 10.1007/s12079-009-0063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brigstock DR. Goldschmeding R. Katsube KI. Lam SC. Lau LF. Lyons K. Naus C. Perbal B. Riser B. Takigawa M. Yeger H. Proposal for a unified CCN nomenclature. Mol Pathol. 2003;56:127. doi: 10.1136/mp.56.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leu SJ. Liu Y. Chen N. Chen CC. Lam SC. Lau LF. Identification of a novel integrin alpha 6 beta 1 binding site in the angiogenic inducer CCN1 (CYR61) J Biol Chem. 2003;278:33801. doi: 10.1074/jbc.M305862200. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y. Abraham DJ. Shi-Wen X. Pearson JD. Black CM. Lyons KM. Leask A. CCN2 (connective tissue growth factor) promotes fibroblast adhesion to fibronectin. Mol Biol Cell. 2004;15:5635. doi: 10.1091/mbc.E04-06-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao R. Brigstock DR. Connective tissue growth factor (CCN2) in rat pancreatic stellate cell function: integrin alpha5beta1 as a novel CCN2 receptor. Gastroenterology. 2005;129:1019. doi: 10.1053/j.gastro.2005.06.067. [DOI] [PubMed] [Google Scholar]
- 44.Kapoor M. Liu S. Huh K. Parapuram S. Kennedy L. Leask A. Connective tissue growth factor promoter activity in normal and wounded skin. Fibrogenesis Tissue Repair. 2008;1:3. doi: 10.1186/1755-1536-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu S. Taghavi R. Leask A. Connective tissue growth factor is induced in bleomycin-induced skin scleroderma. J Cell Commun Signal. 2010;4:25. doi: 10.1007/s12079-009-0081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mori T. Kawara S. Shinozaki M. Hayashi N. Kakinuma T. Igarashi A. Takigawa M. Nakanishi T. Takehara K. Role and interaction of connective tissue growth factor with transforming growth factor-beta in persistent fibrosis: a mouse fibrosis model. J Cell Physiol. 1999;181:153. doi: 10.1002/(SICI)1097-4652(199910)181:1<153::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 47.Wang Q. Usinger W. Nichols B. Gray J. Xu L. Seeley TW. Brenner M. Guo G. Zhang W. Oliver N. Lin A. Yeowell D. Cooperative interaction of CTGF and TGF-β in animal models of fibrotic disease. Fibrogenesis Tissue Repair. 2011;4:4. doi: 10.1186/1755-1536-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu S. Leask A. CCN2 is not required for skin development. J Cell Commun Signal. 2011;5:179. doi: 10.1007/s12079-011-0129-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu S. Shi-wen X. Abraham DJ. Leask A. CCN2 is required for bleomycin-induced skin fibrosis in mice. Arthritis Rheum. 2011;63:239. doi: 10.1002/art.30074. [DOI] [PubMed] [Google Scholar]
- 50.Lechertier T. Hodivala-Dilke K. Focal adhesion kinase and tumour angiogenesis. J Pathol. 2012;226:404. doi: 10.1002/path.3018. [DOI] [PubMed] [Google Scholar]
- 51.van Nimwegen MJ. van de Water B. Focal adhesion kinase: a potential target in cancer therapy. Biochem Pharmacol. 2007;73:597. doi: 10.1016/j.bcp.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 52.Bonner MY. Arbiser JL. Targeting NADPH oxidases for the treatment of cancer and inflammation. Cell Mol Life Sci. 2012;69:2435. doi: 10.1007/s00018-012-1017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Firuzi O. Miri R. Tavakkoli M. Saso L. Antioxidant therapy: current status and future prospects. Curr Med Chem. 2011;18:3871. doi: 10.2174/092986711803414368. [DOI] [PubMed] [Google Scholar]
- 54.Katz E. Sims AH. Sproul D. Caldwell H. Dixon MJ. Meehan RR. Harrison DJ. Targeting of Rac GTPases blocks the spread of intact human breast cancer. Oncotarget. 2012;3:608. doi: 10.18632/oncotarget.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hers I. Vincent EE. Tavaré JM. Akt signalling in health and disease. Cell Signal. 2011;23:1515. doi: 10.1016/j.cellsig.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 56.Behr J. Demedts M. Buhl R. Costabel U. Dekhuijzen RP. Jansen HM. MacNee W. Thomeer M. Wallaert B. Laurent F. Nicholson AG. Verbeken EK. Verschakelen J. Flower CD. Petruzzelli S. De Vuyst P. van den Bosch JM. Rodriguez-Becerra E. Lankhorst I. Sardina M. Boissard G IFIGENIA Study Group. Lung function in idiopathic pulmonary fibrosis—extended analyses of the IFIGENIA trial. Respir Res. 2009;10:101. doi: 10.1186/1465-9921-10-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Demedts M. Behr J. Buhl R. Costabel U. Dekhuijzen R. Jansen HM. MacNee W. Thomeer M. Wallaert B. Laurent F. Nicholson AG. Verbeken EK. Verschakelen J. Flower CD. Capron F. Petruzzelli S. De Vuyst P. van den Bosch JM. Rodriguez-Becerra E. Corvasce G. Lankhorst I. Sardina M. Montanari M IFIGENIA Study Group. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353:2229. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 58.Jiang JX. Chen X. Serizawa N. Szyndralewiez C. Page P. Schröder K. Brandes RP. Devaraj S. Török NJ. Liver fibrosis and hepatocyte apoptosis are attenuated by GKT137831, a novel NOX4/NOX1 inhibitor in vivo. Free Radic Biol Med. 2012;53:289. doi: 10.1016/j.freeradbiomed.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Genkyotex: Genkyotex's NOX inhibitor GKT137831 phase I data presented at Kidney Week 2012. www.genkyotex.com/genkyotex/index.cfm/news-events. [May 2;2013 ]. www.genkyotex.com/genkyotex/index.cfm/news-events
- 60.Noguera R. Nieto OA. Tadeo I. Fariñas F. Alvaro T. Extracellular matrix, biotensegrity and tumor microenvironment. An update and overview. Histol Histopathol. 2012;27:693. doi: 10.14670/HH-27.693. [DOI] [PubMed] [Google Scholar]
- 61.Jun JI. Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov. 2011;10:945. doi: 10.1038/nrd3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Quail DF. Taylor MJ. Postovit LM. Microenvironmental regulation of cancer stem cell phenotypes. Curr Stem Cell Res Ther. 2012;7:197. doi: 10.2174/157488812799859838. [DOI] [PubMed] [Google Scholar]
- 63.Dhar A. Ray A. The CCN family proteins in carcinogenesis. Exp Oncol. 2010;32:2. [PubMed] [Google Scholar]