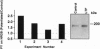Abstract
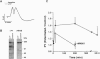
Long-term potentiation (LTP) is a form of synaptic memory that may subserve developmental and behavioral plasticity. An intensively investigated form of LTP is dependent upon N-methyl-D-aspartate (NMDA) receptors and can be elicited in the dentate gyrus and hippocampal CA1. Induction of this type of LTP is triggered by influx of Ca2+ through activated NMDA receptors, but the downstream mechanisms of induction, and even more so of LTP maintenance, remain controversial. It has been reported that the function of NMDA receptor channel can be regulated by protein tyrosine kinases and protein phosphatases and that inhibition of protein tyrosine kinases impairs induction of LTP. Herein we report that LTP in the dentate gyrus is specifically correlated with tyrosine phosphorylation of the NMDA receptor subunit 2B in an NMDA receptor-dependent manner. The effect is observed with a delay of several minutes after LTP induction and persists in vivo for several hours. The potential relevance of this post-translational modification to mechanisms of LTP and circuit plasticity is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Saito H. Tyrosine kinase inhibitors, herbimycin A and lavendustin A, block formation of long-term potentiation in the dentate gyrus in vivo. Brain Res. 1993 Sep 3;621(1):167–170. doi: 10.1016/0006-8993(93)90315-e. [DOI] [PubMed] [Google Scholar]
- Aniksztejn L., Ben-Ari Y. Expression of LTP by AMPA and/or NMDA receptors is determined by the extent of NMDA receptors activation during the tetanus. J Neurophysiol. 1995 Dec;74(6):2349–2357. doi: 10.1152/jn.1995.74.6.2349. [DOI] [PubMed] [Google Scholar]
- Asztely Fredrik, Wigström Holger, Gustafsson Bengt. The Relative Contribution of NMDA Receptor Channels in the Expression of Long-term Potentiation in the Hippocampal CA1 Region. Eur J Neurosci. 1992;4(8):681–690. doi: 10.1111/j.1460-9568.1992.tb00177.x. [DOI] [PubMed] [Google Scholar]
- Bashir Z. I., Alford S., Davies S. N., Randall A. D., Collingridge G. L. Long-term potentiation of NMDA receptor-mediated synaptic transmission in the hippocampus. Nature. 1991 Jan 10;349(6305):156–158. doi: 10.1038/349156a0. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993 Jan 7;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Burgard E. C., Sarvey J. M. Long-lasting potentiation and epileptiform activity produced by GABAB receptor activation in the dentate gyrus of rat hippocampal slice. J Neurosci. 1991 May;11(5):1198–1209. doi: 10.1523/JNEUROSCI.11-05-01198.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chain D. G., Hegde A. N., Yamamoto N., Liu-Marsh B., Schwartz J. H. Persistent activation of cAMP-dependent protein kinase by regulated proteolysis suggests a neuron-specific function of the ubiquitin system in Aplysia. J Neurosci. 1995 Nov;15(11):7592–7603. doi: 10.1523/JNEUROSCI.15-11-07592.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair M. C., Malenka R. C. A critical period for long-term potentiation at thalamocortical synapses. Nature. 1995 May 25;375(6529):325–328. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H., Otto T. LTP and memory: can we enhance the connection? Trends Neurosci. 1993 May;16(5):163–164. doi: 10.1016/0166-2236(93)90140-h. [DOI] [PubMed] [Google Scholar]
- Errington M. L., Lynch M. A., Bliss T. V. Long-term potentiation in the dentate gyrus: induction and increased glutamate release are blocked by D(-)aminophosphonovalerate. Neuroscience. 1987 Jan;20(1):279–284. doi: 10.1016/0306-4522(87)90019-4. [DOI] [PubMed] [Google Scholar]
- Grant S. G., O'Dell T. J., Karl K. A., Stein P. L., Soriano P., Kandel E. R. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992 Dec 18;258(5090):1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- Ishii T., Moriyoshi K., Sugihara H., Sakurada K., Kadotani H., Yokoi M., Akazawa C., Shigemoto R., Mizuno N., Masu M. Molecular characterization of the family of the N-methyl-D-aspartate receptor subunits. J Biol Chem. 1993 Feb 5;268(4):2836–2843. [PubMed] [Google Scholar]
- Izquierdo I. Pharmacological evidence for a role of long-term potentiation in memory. FASEB J. 1994 Nov;8(14):1139–1145. [PubMed] [Google Scholar]
- Kauer J. A., Malenka R. C., Nicoll R. A. A persistent postsynaptic modification mediates long-term potentiation in the hippocampus. Neuron. 1988 Dec;1(10):911–917. doi: 10.1016/0896-6273(88)90148-1. [DOI] [PubMed] [Google Scholar]
- Kirkwood A., Lee H. K., Bear M. F. Co-regulation of long-term potentiation and experience-dependent synaptic plasticity in visual cortex by age and experience. Nature. 1995 May 25;375(6529):328–331. doi: 10.1038/375328a0. [DOI] [PubMed] [Google Scholar]
- Kornau H. C., Schenker L. T., Kennedy M. B., Seeburg P. H. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995 Sep 22;269(5231):1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lev S., Moreno H., Martinez R., Canoll P., Peles E., Musacchio J. M., Plowman G. D., Rudy B., Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature. 1995 Aug 31;376(6543):737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- Lisman J. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9574–9578. doi: 10.1073/pnas.86.23.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H., Segal M. The inositol 1,4,5-trisphosphate pathway mediates cholinergic potentiation of rat hippocampal neuronal responses to NMDA. J Physiol. 1992 Feb;447:513–533. doi: 10.1113/jphysiol.1992.sp019015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon I. S., Apperson M. L., Kennedy M. B. The major tyrosine-phosphorylated protein in the postsynaptic density fraction is N-methyl-D-aspartate receptor subunit 2B. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3954–3958. doi: 10.1073/pnas.91.9.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H., Mishina M. Structure and function of the NMDA receptor channel. Neuropharmacology. 1995 Oct;34(10):1219–1237. doi: 10.1016/0028-3908(95)00109-j. [DOI] [PubMed] [Google Scholar]
- Morris R. G. Synaptic plasticity and learning: selective impairment of learning rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. J Neurosci. 1989 Sep;9(9):3040–3057. doi: 10.1523/JNEUROSCI.09-09-03040.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D., Joly M., Lynch G. Contributions of quisqualate and NMDA receptors to the induction and expression of LTP. Science. 1988 Dec 23;242(4886):1694–1697. doi: 10.1126/science.2904701. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Malenka R. C. Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature. 1995 Sep 14;377(6545):115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- O'Dell T. J., Kandel E. R., Grant S. G. Long-term potentiation in the hippocampus is blocked by tyrosine kinase inhibitors. Nature. 1991 Oct 10;353(6344):558–560. doi: 10.1038/353558a0. [DOI] [PubMed] [Google Scholar]
- Richter-Levin G., Segal M. The effects of serotonin depletion and raphe grafts on hippocampal electrophysiology and behavior. J Neurosci. 1991 Jun;11(6):1585–1596. doi: 10.1523/JNEUROSCI.11-06-01585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum K., Schul R., Meiri N., Hadari Y. R., Zick Y., Dudai Y. Modulation of protein tyrosine phosphorylation in rat insular cortex after conditioned taste aversion training. Proc Natl Acad Sci U S A. 1995 Feb 14;92(4):1157–1161. doi: 10.1073/pnas.92.4.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostas J. A., Brent V. A., Voss K., Errington M. L., Bliss T. V., Gurd J. W. Enhanced tyrosine phosphorylation of the 2B subunit of the N-methyl-D-aspartate receptor in long-term potentiation. Proc Natl Acad Sci U S A. 1996 Sep 17;93(19):10452–10456. doi: 10.1073/pnas.93.19.10452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J. H., Greenberg S. M. Molecular mechanisms for memory: second-messenger induced modifications of protein kinases in nerve cells. Annu Rev Neurosci. 1987;10:459–476. doi: 10.1146/annurev.ne.10.030187.002331. [DOI] [PubMed] [Google Scholar]
- Stelzer A., Simon G., Kovacs G., Rai R. Synaptic disinhibition during maintenance of long-term potentiation in the CA1 hippocampal subfield. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3058–3062. doi: 10.1073/pnas.91.8.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Okumura-Noji K. NMDA receptor subunits epsilon 1 (NR2A) and epsilon 2 (NR2B) are substrates for Fyn in the postsynaptic density fraction isolated from the rat brain. Biochem Biophys Res Commun. 1995 Nov 13;216(2):582–588. doi: 10.1006/bbrc.1995.2662. [DOI] [PubMed] [Google Scholar]
- Thomas K. L., Laroche S., Errington M. L., Bliss T. V., Hunt S. P. Spatial and temporal changes in signal transduction pathways during LTP. Neuron. 1994 Sep;13(3):737–745. doi: 10.1016/0896-6273(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Wang Y. T., Salter M. W. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature. 1994 May 19;369(6477):233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- Wyllie D. J., Nicoll R. A. A role for protein kinases and phosphatases in the Ca(2+)-induced enhancement of hippocampal AMPA receptor-mediated synaptic responses. Neuron. 1994 Sep;13(3):635–643. doi: 10.1016/0896-6273(94)90031-0. [DOI] [PubMed] [Google Scholar]