Abstract
Background
Reported birth prevalences of congenital limb defects (CLD) vary between countries: from 13/10,000 in Finland for the period 1964–1977 to 30.4/10,000 births in Scotland from 1964–1968. Epidemiological studies permit the timely detection of trends in CLD and of associations with other birth defects. The aim of this study is to describe the birth prevalence of CLD in the northern Netherlands.
Methods
In a population-based, epidemiological study we investigated the birth prevalences of CLD for 1981–2010. Data were collected by the European Surveillance of Congenital Anomalies in the northern Netherlands (EUROCAT-NNL). We excluded malpositions, club foot, and dislocation/dysplasia of hips or knees. Trends were analysed for the 19-year period 1992–2010 using χ2 tests, as well as CLD association with anomalies affecting other organs.
Results
The birth prevalence of CLD was 21.1/10,000 births for 1981–2010. There was an overall decrease in non-syndromic limb defects (P = 0.023) caused by a decrease in the prevalence of non-syndromic syndactyly (P < 0.01) in 1992–2010. Of 1,048 children with CLD, 55% were males, 57% had isolated defects, 13% had multiple congenital anomalies (MCA), and 30% had a recognised syndrome. The upper:lower limb ratio was 2:1, and the left:right side ratio was 1.2:1. Cardiovascular and urinary tract anomalies were common in combination with CLD (37% and 25% of cases with MCA). Digestive-tract anomalies were significantly associated with CLD (P = 0.016).
Conclusions
The birth prevalence of CLD in the northern Netherlands was 21.1/10,000 births. The birth prevalence of non-syndromic syndactyly dropped from 5.2/10,000 to 1.1/10,000 in 1992–2010.
Keywords: Congenital limb deformities, Congenital abnormalities, Prevalence, Epidemiology
Background
Limb defects seen in childhood are mainly congenital and occur when a part of or the entire limb fails to form normally during pregnancy. Reduction defects may be disabling limb defects due to the failure of several elements to form properly [1]. Less disabling limb defects are polydactyly, defined as complete or partial supernumerary digits, and syndactyly, fusion of two or more digits [2]. Disruptive events appear to be the most common cause of congenital limb defects (CLD) [3]. During the gestational period, disruptive events, such as amniotic band or vascular disruptions, may cause amputation or hypoperfusion of the developing limbs [4]. Various CLD are due to prenatal exposure to different teratogens [5], the best-known example of which is thalidomide, which caused a wide range of CLD, especially intercalary reductions and preaxial defects, in the 1960s [6]. To prevent further tragedies, several international registries of congenital defects were established. The European Surveillance of Congenital Anomalies (EUROCAT) network of registries in thirty-seven countries and the International Clearinghouse for Birth Defects Surveillance and Research are two such registries that have the goal of monitoring birth defects [7,8]. Monitoring CLD birth prevalences (BP) permits estimates of how common CLD are in the general population, early detection of risk factors for CLD and its associations with other congenital anomalies, and comparison of standard data collections.
Complete epidemiological descriptions of all CLD in different countries are rather scarce. In Finland a BP of 13/10,000 births was found for the period 1964–1977, whereas in Scotland it was estimated at 30/10,000 for 1964–1968 [9,10]. More common reports in the literature are studies on specific types of CLD, especially of congenital reduction defects [11-22]. Reported BPs of reduction defects vary widely in time, and between countries. In Italy the prevalence was as low as 4.8/10,000, while in France it was 10.4/10,000 from 1979–1987 [13,23,24]. The BP/10,000 of CLD in the Netherlands from 1997–2007 was 9.9 for polydactyly, 7.0 for syndactyly and 1.4 for reduction defects [25].
There is no up-to-date, detailed information on CLD in the Dutch population available. Therefore, the aim of this study is to describe the epidemiology of CLD in a population-based study in the northern Netherlands for the period 1981–2010.
Methods
Data source
The CLD cases have been collected by EUROCAT in the northern Netherlands (EUROCAT-NNL) since 1981. Children or foetuses with one or more major congenital defects and whose mothers lived in the northern provinces (Groningen, Drenthe or Friesland) at the time of delivery were eligible for registration.
Cases were ascertained according to EUROCAT’s Central Registry guidelines [26]. EUROCAT-NNL registers foetuses irrespective of gestational age, spontaneous abortions, terminations of pregnancies (foetuses of ≤ 24 weeks’ gestation) following prenatal diagnosis because of a congenital malformation, stillbirths (foetuses of ≥ 24 weeks’ gestation), live births, and children diagnosed before 11 years of age.
Cases are reported by general practitioners, midwives and physicians [27]. Hospital registries are also actively and regularly searched by the EUROCAT-NNL personnel to find eligible children/pregnancies. Various sources including hospital files, obstetric and pathology records are searched for case assessment (type of malformation, chronic diseases and dates of screening procedures). When new information becomes available for an already registered case, the case is updated in the EUROCAT-NNL database until the child reaches the age of 11 years. The paediatric cardiology centre (part of the University Medical Centre Groningen) covers all births in the EUROCAT-NNL registration area and supplies systematic lists with cases and diagnostic details to the registry [28]. For all reported cases, results of genetic tests are downloaded from the genetics department, if these results are available. Abnormal karyotype reports are recorded from prenatal and postnatal samples [28].
Since 1992, parents or guardians are asked to give informed consent for registration of their child and for the use the data for research purposes. The response rate is 80%. Up to 1992, no parental approval was required to register cases.
This study was approved by the Medical Ethical Committee, University Medical Centre Groningen, the Netherlands (number M12.118639).
Classification
EUROCAT adopted the International Classification of Diseases (ICD-9), with modifications, from the British Paediatric Association for births up to 2001, and the tenth revision (ICD-10) from 2002 onwards [29,30]. The two ICD guides were used to code cases into clinical and anatomical types. There were three clinical types of CLD: (1) isolated CLD, if the case only had one or more limb defects but no other major congenital anomalies; (2) multiple congenital anomalies (MCA), if there was a limb defect combined with at least one major non-limb defect unrelated to a syndrome; or (3) CLD as part of a genetic disorder or syndrome (recognised conditions). There were four anatomical categories: polydactyly (ICD10-Q69 and ICD9-7550), syndactyly (ICD10-Q70 and ICD9-7551, 7550.4), reduction defects (transverse, longitudinal, intercalary and central) (ICD10 Q71-73 and ICD9 7552-7554), and “other CLD” (ICD10-Q74, ICD9-7555, 7556, 7558). Split hand (ICD9 7555.11-7555.14) and split foot (ICD9 7556.12-7556.15) were considered central reduction defects since they are coded as reduction defects in ICD10. Proximal femoral focal deficiency (ICD10-Q72.4, ICD9-7553.80) was classified as an intercalary reduction defect of the lower limb. The “other CLD” classification included limb anomalies like Sprengel’s and Madelung’s deformity, macrodactyly, radioulnar synostosis, hemihypertrophy, limb undergrowth, and arthrogryposis multiplex congenita.
Study population
All children and foetuses with CLD, diagnosed before or after birth, were included in this study. Children with only minor CLD, such as clinodactyly, camptodactyly, brachydactyly of the fourth and fifth fingers, trigger finger, syndactyly of the second and third toes, sandal gap, and short big toe were not included, because EUROCAT does not register minor CLD. We also excluded malpositions, club foot and dislocation/dysplasia of hips or knees (dislocation of patella) from our analysis because these musculoskeletal anomalies are common birth defects and their inclusion in the calculations of total birth prevalence for CLD would have given an inflated birth prevalence. The ICD-codes were thoroughly checked against the descriptions of CLD and rectified if necessary.
To gain insight into CLD trends, we included children with isolated CLD and MCA (non-syndromic CLD). Because the registration method changed in 1992 with the introduction of informed consent, the trend analysis was conducted for the period 1992–2010. Localisation of the CLD (left/right side) was only studied in live births with isolated CLD or MCA, because of possible lack of information on localisation in stillbirths and spontaneous abortions or termination of pregnancies. Syndromic CLD were excluded from the laterality analysis because they have characteristic patterns [31]. We determined the most frequent anomalies in other organ-systems that occurred in combination with CLD (MCA cases). A clinical geneticist reviewed the cases that were suspected of having monogenic or genetic causality based on the description of the CLD, associated anomalies, and/or family history.
Statistical analyses
BPs were determined by dividing the number of affected cases by the total number of births (live births, stillbirths, spontaneous abortions/termination of pregnancies) in the EUROCAT-NNL region. To visualise trends, a three-year moving average prevalence was calculated. The χ2 test for trend was used to analyse changes over time in BP and to determine whether a type of CLD was preferentially associated with a congenital anomaly affecting another organ system. Only MCA cases that had one type of CLD were included in this analysis. The association between the number of CLD and the number of anomalies in other organ-systems was tested for trend using the χ2 test. If χ2 assumptions (expected cell counts) were not met, the exact method was used.
Two-tailed values of P < 0.05 were considered statistically significant. PASW Statistics 18.0 for Windows (SPSS Inc., 2009, Chicago, IL, http://www.spss.com) was used for the analyses.
Results
Birth prevalence and study population
From 1981–2010, 1,048 cases with CLD were recorded among 497,751 births in the northern Netherlands, yielding a BP of 21.1/10,000 (Table 1). The prevalence for transverse reduction defects was 3.9/10,000 births and for longitudinal reduction defects 2.4/10,000 (equal rates for preaxial and postaxial: 1.3/10,000).
Table 1.
Total birth prevalence per type of congenital limb defects for the period 1981–2010 in the northern Netherlands
| CLD | Isolated CLD, n | Multiple congenital anomalies, n | CLD is part of a recognised condition, n | Total, n | Prevalence per 10,000 |
|---|---|---|---|---|---|
| Polydactyly |
291 |
45 |
83 |
419 |
8.4 |
| Upper limb |
228 |
38 |
63a |
329 |
6.6 |
| Preaxial |
73 |
13 |
20 |
106 |
2.1 |
| Postaxial |
141 |
21 |
39 |
201 |
4.0 |
| NOS |
14 |
4 |
6 |
24 |
0.5 |
| Lower limb |
75 |
9 |
32 |
116 |
2.3 |
| Preaxial |
12 |
1 |
8 |
21 |
0.4 |
| Postaxial |
59 |
7 |
19 |
85 |
1.7 |
| NOS |
4 |
1 |
5 |
10 |
0.2 |
| NOS |
4 |
0 |
2 |
6 |
0.1 |
| Upper and lower limb |
16 |
2 |
14 |
32 |
0.6 |
| Reduction defects |
180 |
42 |
120 |
342 |
6.9 |
| Upper limb |
128 |
28 |
94 |
250 |
5.0 |
| Transverse |
89 |
17 |
39 |
145 |
2.9 |
| Longitudinal |
21b |
9 |
51 |
81 |
1.6 |
| Preaxial |
12 |
7 |
33 |
52 |
1.0 |
| Postaxial |
10 |
2 |
18 |
30 |
0.6 |
| Intercalary |
3 |
1 |
5 |
9 |
0.2 |
| Central |
16 |
1 |
4 |
21 |
0.4 |
| Multiple |
1 |
0 |
5 |
6 |
0.1 |
| Lower limb |
66 |
25 |
58 |
149 |
3.0 |
| Transverse |
29 |
15 |
31 |
75 |
1.5 |
| Longitudinal |
23c |
5 |
24 |
52 |
1.0 |
| Preaxial |
4 |
3 |
9 |
16 |
0.3 |
| Postaxial |
21 |
2 |
16 |
39 |
0.8 |
| Intercalary |
7 |
5 |
10 |
22 |
0.4 |
| Central |
13 |
1 |
2 |
16 |
0.3 |
| Multiple |
7 |
2 |
8d |
17 |
0.3 |
| NOS |
1 |
1 |
0 |
2 |
0.04 |
| NOS |
0 |
0 |
1 |
1 |
0.02 |
| Upper and lower limb |
14 |
11 |
33 |
58 |
1.2 |
| Syndactyly |
126 |
29 |
77 |
232 |
4.7 |
| Upper limb |
77 |
17 |
46 |
140 |
2.8 |
| Lower limb |
59 |
12 |
46 |
117 |
2.4 |
| NOS |
0 |
1 |
0 |
1 |
0.02 |
| Upper and lower limb |
10 |
1 |
15 |
26 |
0.5 |
| Other CLDe |
41 |
34 |
88 |
163 |
3.3 |
| Upper limb |
18 |
14 |
66 |
98 |
2.0 |
| Lower limb |
25 |
22 |
53 |
100 |
2.0 |
| NOS |
0 |
1 |
0 |
1 |
0.02 |
| Upper and lower limb |
2 |
3 |
31 |
36 |
0.7 |
| Multiple CLDf |
38g |
14g |
49g |
101 |
2.0 |
| Total no. of cases |
598 |
135 |
315 |
1048 |
21.1 |
| Total no. of live births | 587 | 77 | 159 | 823 | 16.5 |
Total number of births for the period 1981–2010: n = 497,751.
Abbreviations: CLD–congenital limb defects, NOS–not otherwise specified, n–number of children with CLD.
aTwo children had preaxial and postaxial polydactyly of upper limbs.
bOne child had preaxial and postaxial longitudinal reduction defects of upper limbs.
cTwo children had preaxial and postaxial longitudinal reduction defects of lower limbs.
dOne child had transversal, longitudinal and intercalary reduction defects of lower limbs.
eThe category consisted of CLD like arthrogryposis (n = 40), hemihypertrophy (n = 25), contractures of elbows/knees/fingers (n = 19), undergrowth of limbs (n = 9), radio-ulnar synostosis (n = 5), macrodactyly (n = 4).
fCategory containing cases with several CLD included in the study.
gChildren in the group with isolated (n = 2), multiple congenital anomalies (n = 1), and CLD as part of a recognised condition (n = 4) had three types of CLD, whereas the rest of the children with multiple limb defects had two types of CLD.
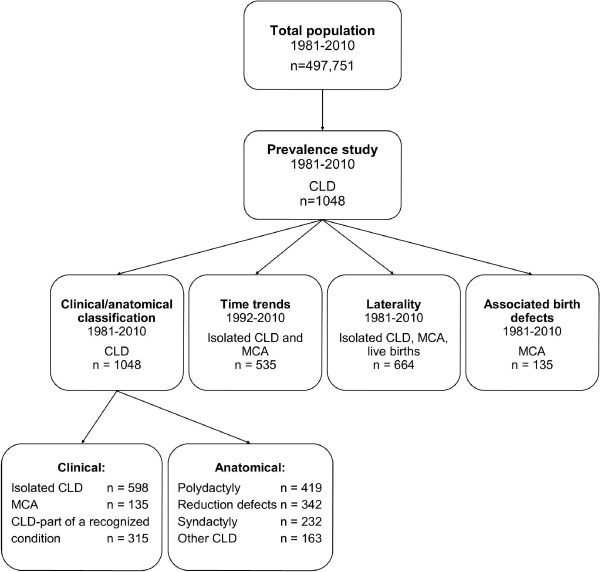
Of the 1,048 cases, 823 (79%) were live-born children; 181 (17%) were spontaneous abortions, stillbirths or infants who died shortly after birth; and 44 (4%) were termination of pregnancies. Of all 1,048 cases, 578 (55%) were males and 4 were of undetermined gender. More males (455/823; 55%) were also registered in the live births. An overview of the data is given in Figure 1.
Figure 1.
Overview of population included in the northern Netherlands study. Abbreviations and notations: CLD–congenital limb defects, MCA–multiple congenital anomalies, n–number of children with CLD.
Classification
There were 598 (57%) isolated CLD cases and 135 (13%) MCA cases (Table 1). The remaining 315 (30% of total) cases had a recognised condition, which included 96 chromosomal defects (31%), 103 monogenic defects (33%), 9 deletions (3%), and 107 other recognised conditions (34%) (Table 2). Trisomy 13 (n = 29; 30% of chromosomal defects) and trisomy 18 (n = 24; 25%) were found most often in the cases with chromosomal abnormalities. Cases with trisomy 13 most often had postaxial polydactyly of an upper limb (n = 19), while the monogenic abnormalities contained, for example, cases with arthrogryposis with a known gene (n = 12), Greig syndrome (n = 10), and Holt-Oram syndrome (n = 7). Other recognised conditions were mainly amniotic bands (n = 27, 25%), of which most of the cases had transverse reduction defects (14 upper limb, 10 lower limb) and syndactyly (9 upper limb, 4 lower limb), arthrogryposis (n = 14, 13%) and VATER/VACTERL association (vertebral defects, anal atresia, cardiac anomalies, tracheo-oesophageal fistula with oesophageal atresia, renal dysplasia, limb defects (n = 13, 12%)).
Table 2.
Description of the recognised conditions with congenital limb defects (CLD)
| Recognised condition | CLD, n | Type of CLD and the number of cases |
|---|---|---|
| Chromosomal |
96 |
|
| Trisomy 13, Patau |
29 |
Polydactyly: Preax. LL = 1, Postax. UL = 19 and LL = 6, NOS UL = 4 and LL = 3 |
| |
|
Syndactyly LL = 1 |
| Trisomy 18, Edwards |
24 |
Polydactyly: Preax. UL = 2, Postax. UL = 3 |
| |
|
Reduction: Transv. UL = 1 and LL = 1, Preax. UL = 7 (radius aplasia, thumb aplasia/hypoplasia) and LL = 1, Postax. UL = 2 |
| |
|
Syndactyly: UL = 2, LL = 5 |
| |
|
Other CLD: UL = 3, LL = 3 |
| Triploidy 69 |
11 |
Polydactyly Preax. UL = 1 |
| |
|
Syndactyly: UL = 7, LL = 8 |
| |
|
Other CLD LL = 1 (shortening toes) |
| Trisomy 21, Down |
6 |
Polydactyly Preax. LL = 1 |
| |
|
Syndactyly: UL = 2, LL = 3 |
| Trisomy 13, translocation |
3 |
Polydactyly: Postax. UL = 2 and LL = 1, NOS UL = 1 and LL = 1 |
| Unlisted chromosomal anomalya |
23 |
|
| Monogenenic |
103 |
|
| Arthrogryposis with a known gene |
12 |
Other CLD: UL = 12 and LL = 8 (joint contractions) |
| Greig syndrome |
10 |
Polydactyly: Preax. UL = 3 and LL = 7, Postax. UL = 4 and LL = 1 |
| |
|
Syndactyly UL = 2, LL = 6 |
| Holt-Oram syndrome |
7 |
Polydactyly Preax. UL = 3 (triphalangeal thumb) |
| |
|
Reduction Preax. UL = 4 (radius aplasia/dysplasia) |
| Apert syndrome |
5 |
Polydatyly Preax. UL = 1 |
| |
|
Syndactyly: UL = 4 (3 digits II-V, 1 all digits), LL = 3 (all digits) |
| |
|
Other CLD = 2 |
| Cornelia de Lange syndrome |
5 |
Reduction: Transv. UL = 4 (bilateral), Postax. LL = 1, Central UL = 1 (split hand) |
| |
|
Syndactyly LL = 1 |
| |
|
Other UL = 3 (all monodactyly) |
| Bardet-Biedl syndrome |
4 |
Polydactyly: Postax. UL = 3 (bilateral) and LL = 3 (2 bilateral) |
| |
|
Reduction Transv. LL = 1 |
| Thanatophoric dysplasia/dwarfism |
3 |
Reduction: Transv. UL = 1 and LL = 1, Intercalary UL = 2 and LL = 2 |
| Meckel-Gruber syndrome |
3 |
Polydactyly: Postax. UL = 2 (1 bilateral) and LL = 2 (bilateral) |
| |
|
Syndactyly: LL = 1 (bilateral) |
| Peters plus syndrome |
3 |
Reduction: Transv. UL = 2 (short UL) and LL = 1 (short LL), Intercalary LL = 1 (reduction of femur bilateral); |
| Unlisted monogenica anomaly |
51 |
|
| Deletions |
9 |
Polydactyly: Preax. UL = 1, Postax. UL = 1 |
| |
|
Reduction: Transv. UL = 1 and LL = 1, Intercalary UL = 1 and LL = 1, Central UL = 1 (split hand) and LL = 1 (split foot) |
| |
|
Syndactyly: UL = 1, LL = 3 |
| Other recognised conditions |
107 |
|
| Amniotic bands |
27 |
Reduction: Transv. UL = 14 and LL = 10, Preax. UL = 2 and LL = 6, Postax. UL = 5, Intercalary UL = 1 |
| |
|
Syndactyly: UL = 9, LL = 4 |
| |
|
Other CLD: UL = 4, LL = 5 (constriction bands) |
| Caudal regression syndrome |
4 |
Reduction: Transv. UL = 1 and LL = 1, Preax. UL = 1 (atresia radius and thumb), Postax. LL = 1 |
| |
|
Syndactyly UL = 1 |
| Acardiacus |
3 |
Reduction: Transv. UL = 1 and LL = 1, Postax. UL = 1 and LL = 1, NOS = 1 |
| |
|
Syndactyly LL = 1 |
| Femoral facial syndrome |
3 |
Reduction Intercalary = 1 (femoral hypoplasia) |
| |
|
Syndactyly LL = 2 |
| |
|
Other CLD = 1 (contractures elbows and knees) |
| Limb–body-wall complex |
6 |
Reduction: Transv. UL = 1 and LL = 3 (right side), Intercalary UL = 1, Postax. LL = 2 |
| |
|
Other CLD LL = 1 |
| Oculo-auriculo-vertebral spectrum |
4 |
Polydactyly Preax. UL = 1 |
| |
|
Reduction Transv. UL = 1 |
| |
|
Other CLD: UL = 1 (Sprengel deformity), LL = 1 (hemihypertophy) |
| VATER/VACTERL association |
13 |
Polydactyly: Preax. UL = 2, Postax. LL = 1 |
| |
|
Reduction: Transv. UL = 1 and LL = 1, Preax. UL = 8 (radius aplasia with or without thumb agenesis/hypoplasia), Central UL = 1 (split hand) |
| |
|
Other CLD LL = 1 (flexion-extension deformity) |
| Poland syndrome |
4 |
Reduction: Preax. UL = 2 (radius aplasia/dysplasia and thumb aplasia), Intercalary UL = 1 |
| |
|
Syndactyly UL = 3 |
| Foetal valproate syndrome |
3 |
Polydactyly Preax. UL = 1 |
| |
|
Reduction Preax. UL = 2 (radius aplasia) |
| |
|
Syndactyly UL = 1 |
| Arthrogryposis multiplex congenitab |
14 |
Other CLD: UL = 14 (joints contractures), LL = 11 (joints contractures) |
| Femur-Fibula-Ulna complex |
9 |
Reduction: Transv. LL = 1, Preax. LL = 1, Postax. UL = 5 (ulna hypoplasia, missing fingers) and LL = 8 (fibula aplasia, missing toes), Intercalary UL = 1 and LL = 4 (femur hypoplasia) |
| |
|
Syndactyly: UL = 3, LL = 2 |
| |
|
Other CLD UL = 1 |
| Klippel-Trenaunay-Weber syndrome |
5 |
Other CLD: Hypertrophy UL = 3 (entire upper limb = 2, macrodactyly = 1) and LL = 2 (entire lower limb) |
| Unlisted other recognised conditionsa |
12 |
|
| Total | 315 |
Abbreviations: CLD–congenital limb defects, UL–upper limb, LL–lower limb, Transv.–transversal, Preax.–preaxial, Postax.–postaxial, n–number of children with CLD.
aRecognised conditions occurring in less than 3 cases are not listed in the table.
bUnknown gene.
In this study, termination of pregnancy was performed in 44 cases. Isolated CLD occurred in 2 of the 44 cases (split hands and feet; mixed reduction defects of the lower limb), MCA in 15 (CLD with one or more other major non-CLD defects: CNS and neural tube defects (n = 5), urinary (n = 5), digestive system (n = 4), cardiovascular (n = 3), respiratory system (n = 3)), and recognized conditions in 27 (chromosomal (n = 14), other recognized condition (n = 7), and monogenic (n = 6)).
Trend analyses for the period 1992–2010
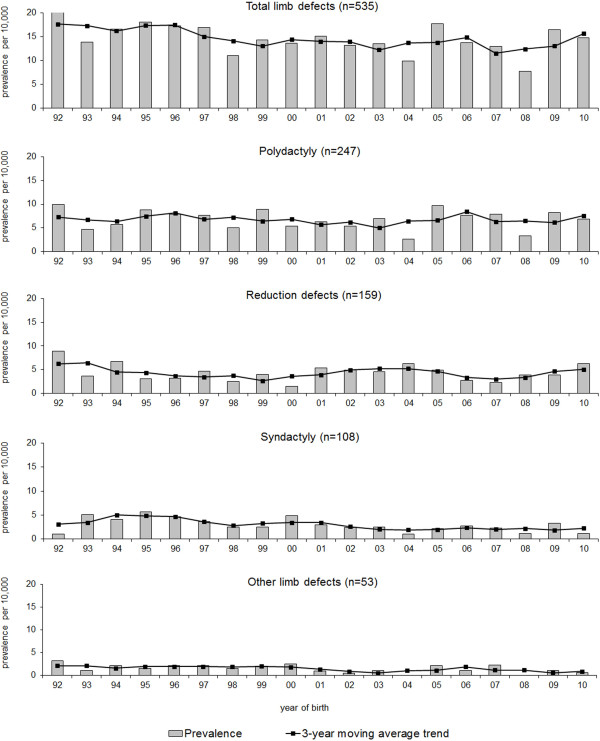
A significant decrease of the BP rate over time was found for non-syndromic CLD as a group and for non-syndromic syndactyly in particular (CLD: χ2 = 5.2, P = 0.023, syndactyly: χ2 = 6.8, P = 0.009) (Figure 2). The decrease in non-syndromic syndactyly from 5.2/10,000 to 1.1/10.000 births was also found to be responsible for the decrease of non-syndromic CLD as a total group (CLD syndactyly excluded: χ2 = 1.5, P = 0.215). A significant decrease was also noticed in the heterogeneous group of “other CLD” (χ2 = 4.8, P = 0.028). When we included recognised conditions in the analysis, no trend was identified for syndactyly (χ2 = 1.5, P = 0.218), but there was a significant decreasing trend present for CLD as a group (χ2 = 9.3, P = 0.002).
Figure 2.
Time trends for non-syndromic congenital limb defects (isolated and MCA) for the period 1992–2010. MCA–multiple congenital anomalies. Total limb defects: P for trend, 0.023; Polydactyly: P for trend, 0.574; Reduction defects: P for trend, 0.381; Syndactyly: P for trend, 0.009; Other congenital limb defects (CLD): P for trend, 0.028.
Localisation and laterality of limb defects
Upper limbs were more often affected than lower limbs (upper:lower = 2:1). Upper versus lower limb ratios were 3:1 in polydactyly, 1.5:1 in syndactyly, and 2:1 in reduction defects (Table 3). Limb defects were more often left-sided (left:right = 1.2:1).
Table 3.
Description of laterality in live-born children with a limb defect a
|
Right, |
Left, |
Bilateral, |
Total of sites,
b
|
|
|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | |
| Polydactyly (n = 322) |
90 (13.6) |
98 (14.8) |
118 (17.8) |
306 (46.1) |
| Upper limb |
65 (9.8) |
72 (10.8) |
95 (14.3) |
232 (34.9) |
| Lower limb |
25 (3.8) |
26 (3.9) |
23 (3.5) |
74 (11.1) |
| Syndactyly (n = 140) |
40 (6.0) |
49 (7.4) |
44 (6.6) |
133 (20.0) |
| Upper limb |
24 (3.6) |
29 (4.4) |
26 (3.9) |
79 (11.9) |
| Lower limb |
16 (2.4) |
20 (3.0) |
18 (2.7) |
54 (8.1) |
| Reduction defects (n = 193) |
78 (11.7) |
88 (13.3) |
34 (5.1) |
200 (30.1) |
| Upper limb |
52 (7.8) |
67 (10.1) |
14 (2.1) |
133 (20.0) |
| Transverse |
38 (5.7) |
49 (7.4) |
7 (1.1) |
94 (14.2) |
| Longitudinal |
8 (1.2) |
9 (1.4) |
6 (0.9) |
23 (3.5) |
| Intercalary |
0 |
2 (0.3) |
0 |
2 (0.3) |
| Central |
6 (0.9) |
7 (1.1) |
1 (0.2) |
14 (2.1) |
| Lower limb |
26 (3.9) |
21 (3.2) |
20 (3.0) |
67 (10.1) |
| Transverse |
10 (1.5) |
10 (1.5) |
10 (1.5) |
30 (4.5) |
| Longitudinal |
10 (1.5) |
9 (1.4) |
3 (0.5) |
22 (3.3) |
| Intercalary |
3 (0.5) |
1 (0.2) |
0 |
4 (0.6) |
| Central |
3 (0.5) |
1 (0.2) |
7 (1.1) |
11 (1.7) |
| Other CLD (n = 58) |
18 (2.7) |
32 (4.8) |
9 (1.4) |
59 (8.9) |
| Upper limb |
5 (0.8) |
14 (2.1) |
4 (0.6) |
23 (3.5) |
| Lower limb |
13 (2.0) |
18 (2.7) |
5 (0.8) |
36 (5.4) |
| Total CLD (n = 664) |
226 (34.0) |
267 (40.2) |
205 (30.9) |
698 (105.1) |
| Upper limb |
146 (22.0) |
182 (27.4) |
139 (20.9) |
467 (70.3) |
| Lower limb | 80 (12.0) | 85 (12.8) | 66 (9.9) | 231 (34.8) |
Abbreviations and notations: CLD–congenital limb defects, N–number of sites.
Percentages are calculated from the total number of children with isolated CLD and multiple congenital anomalies (n = 664). In 58 infants localisation was unknown.
aIncluded only live births with isolated and multiple congenital defects (including a limb defect) because of 1) lack of information on stillbirths and abortions, and 2) genetic abnormalities or syndromes have characteristic patterns [31].
bNumber of sites exceeds the number of children due to multiple CLD in some cases.
Associated anomalies
CLD were most common with cardiovascular anomalies (Table 4): 19 ventricular septal defects (of which 8 cases had polydactyly), 7 atrial septal defects, 6 tetralogy of Fallot, and 4 coarctation of the aorta. Urinary tract anomalies were also frequent: 12 bi- or unilateral renal agenesis (of which 7 cases had reduction defects), 5 hydronephrosis, and 5 cystic kidney. Anomalies of the central nervous system were found in fewer cases with CLD: 10 cases of hydrocephaly (of which 5 had syndactyly) and 4 of microcephaly. More males were affected in cases with genital anomalies (n = 17, uniformly spread between CLD) of which most were hypospadias (n = 14).
Table 4.
Anomalies in other organ systems occurring with congenital limb defects a
|
Anomalies |
Polydactyly |
Syndactyly |
Total reduction defects |
Other CLD |
Total CLD
c
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
(n = 45) |
(n = 29) |
(n = 42) |
(n = 34) |
(n = 135) |
|||||||
| Total b , n (%) | Preax | Postax | n (%) | Total b , n (%) | Transv | Preax | Postax | Intercal | Total, n (%) | n (%) | |
| CNS and neural tube defects |
6 (4.4) |
1 |
4 |
8 (5.9) |
10 (7.4) |
7 |
5 |
0 |
0 |
6 (4.4) |
27 (20.0) |
| Hydrocephaly |
1 (0.7) |
0 |
0 |
5 (3.7) |
3 (2.2) |
2 |
2 |
0 |
0 |
3 (2.2) |
10 (7.4) |
| Microcephaly |
0 |
0 |
0 |
1 (0.7) |
4 (3.0) |
3 |
2 |
0 |
0 |
0 |
4 (3.0) |
| Eye |
0 |
0 |
0 |
5 (3.7) |
2 (1.5) |
1 |
0 |
1 |
0 |
3 (2.2) |
9 (6.7) |
| Ear |
2 (1.5) |
1 |
1 |
1 (0.7) |
1 (0.7) |
1 |
0 |
0 |
0 |
1 (0.7) |
4 (3.0) |
| Cardiovascular |
19 (14.1) |
9 |
9 |
7 (5.2) |
16 (11.9) |
9 |
6 |
1 |
2 |
17 (12.6) |
50 (37.0) |
| Tetralogy of Fallot |
2 (1.5) |
2 |
0 |
0 |
2 (1.5) |
2 |
0 |
0 |
0 |
2 (1.5) |
6 (4.44) |
| Atrium septum defects |
1 (0.7) |
1 |
0 |
1 (0.7) |
3 (2.2) |
2 |
1 |
0 |
0 |
3 (2.2) |
7 (5.2) |
| Ventricular septum defects |
8 (5.9) |
4 |
3 |
4 (3.0) |
6 (4.4) |
2 |
3 |
1 |
2 |
6 (4.4 |
19 (14.1) |
| Coarctation aortae |
2 (1.5) |
0 |
2 |
0 |
1 (0.7) |
0 |
1 |
0 |
0 |
1 (0.7) |
4 (3.0) |
| Respiratory |
8 (5.9) |
2 |
4 |
3 (2.2) |
5 (3.7) |
2 |
3 |
0 |
0 |
6 (4.4) |
19 (14.1) |
| Choanal atresia |
3 (2.2) |
0 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
3 (2.2) |
| Lung hypoplasia |
1 (0.7) |
0 |
0 |
1 (0.7) |
0 |
0 |
0 |
0 |
0 |
4 (3.0) |
5 (3.7) |
| Clefts |
7 (5.2) |
2 |
4 |
5 (3.7) |
3 (2.2) |
1 |
2 |
0 |
0 |
2 (1.5) |
15 (11.1) |
| Cleft palate |
3 (2,2) |
0 |
3 |
3 (2.2) |
2 (1.5) |
1 |
1 |
0 |
0 |
1 (0.7) |
8 (5.9) |
| Cleft lip |
2 (1.5) |
0 |
1 |
0 |
1 (0.7) |
0 |
1 |
0 |
0 |
0 |
3 (2.2) |
| Digestive |
8 (5.9) |
1 |
6 |
3 (2.2) |
14 (10.4) |
8 |
5 |
1 |
0 |
3 (2.2) |
25 (18.5) |
| Malformations of oesophagus |
0 |
0 |
0 |
0 |
4 (3.0) |
2 |
0 |
0 |
0 |
0 |
4 (3.0) |
| Atresia/stenosis large intestine |
2 (1.5) |
0 |
2 |
0 |
5 (3.7) |
3 |
2 |
0 |
0 |
0 |
7 (5.2) |
| Anorectal atresia |
2 (1.5) |
0 |
2 |
0 |
5 (3.7) |
3 |
2 |
0 |
0 |
0 |
7 (5.2) |
| Genital |
6 (4.4) |
0 |
6 |
3 (2.2) |
10 (7.4) |
6 |
4 |
0 |
0 |
4 (3.0) |
23 (17.0) |
| Female |
1 (0.7) |
0 |
1 |
0 |
4 (3.0) |
1 |
2 |
0 |
0 |
0 |
5 (3.7) |
| Male |
5 (3.7) |
0 |
5 |
3 (2.2) |
5 (3.7) |
3 |
1 |
0 |
0 |
4 (3.0) |
17 (12.6) |
| Hypospadias |
4 (3.0) |
0 |
4 |
3 (2.2) |
3 (2.2) |
2 |
1 |
0 |
0 |
2 (1.5) |
12 (8.9) |
| Urinary |
6 (4.4) |
1 |
3 |
10 (7.4) |
13 (9.6) |
6 |
6 |
1 |
1 |
8 (5.9) |
34 (25.2) |
| Renal agenesis (uni/bilateral) |
3 (2.2) |
1 |
1 |
1 (0.7) |
7 (5.2) |
5 |
3 |
1 |
0 |
1 (0.7) |
12 (8.9) |
| Cystic kidney |
0 |
0 |
0 |
1 (0.7) |
3 (2.2) |
1 |
2 |
0 |
1 |
1 (0.7) |
5 (3.7) |
| Potter sequence |
0 |
0 |
0 |
1 (0.7) |
2 (1.5) |
1 |
2 |
0 |
0 |
1 (0.7) |
4 (3.0) |
| Hydronephrosis |
1 (0.7) |
0 |
0 |
2 (1.5) |
0 |
0 |
0 |
0 |
0 |
2 (1.5) |
5 (3.7) |
| Horseshoe kidney |
0 |
0 |
0 |
1 (0.7) |
1 (0.7) |
0 |
0 |
0 |
0 |
3 (2.2) |
4 (3.0) |
| Abdominal wall defects |
2 (1.5) |
0 |
1 |
0 |
1 (0.7) |
1 |
0 |
0 |
0 |
2 (1.5) |
5 (3.7) |
| Omphalocele |
1 (0.7) |
0 |
1 |
0 |
1 (0.7) |
1 |
0 |
0 |
0 |
2 (1.5) |
4 (3.0) |
| Other | 4 (3.0) | 2 | 2 | 3 (2.2) | 2 (1.5) | 2 | 0 | 0 | 0 | 4 (3.0) | 12 (8.9) |
Abbreviations and notations: CLD–congenital limb defects, CNS–central nervous system, Transv–transversal, Preax–preaxial, Postax–postaxial, Intercal–intercalary, n–number of children with CLD.
aOnly cases with multiple congenital anomalies (including a limb defect) were included because genetic abnormalities or syndromes have particular associations [17]. General categories and examples of anomalies occurring with CLD were given.
bNumbers do not always add up due to multiple CLD in some cases or due to lack of information on subcategories of CLD (e.g. preaxial, postaxial). Percentages are calculated from the total number of children with multiple congenital anomalies and CLD (n = 135).
cIn addition to one or several major non-limb defects, 24 cases (17.8%) also had a malposition, or a clubfoot, or hip dysplasia/dislocation (polydactyly n = 1, syndactyly n = 8, reduction defects n = 9, other CLD n = 12).
We only found a significant association between digestive anomalies and CLD (P = 0.016). Reduction defects were more likely to occur in combination with digestive tract anomalies, whereas syndactyly and “other CLD” were less likely to occur with such anomalies (Table 5). We found no significant association between the number of CLD and the number of anomalies in other organ-systems (χ2 = 3.4, df 1, linear-by-linear association P = 0.067).
Table 5.
Associated anomalies with congenital limb defects a
|
Anomalies |
|
Polydactyly |
Reduction defects |
Syndactyly |
Other CLD |
Total |
|
|
|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | χ 2 | P | ||
| Cardiovascular |
yes |
15 (45.5) |
9 (27.3) |
1 (3.0) |
8 (24.2) |
33 (100) |
1.98 |
0.160 |
| |
no |
25 (35.7) |
21 (30.0) |
14 (20.0) |
10 (14.3) |
70 (100) |
|
|
| Urinary anomalies |
yes |
5 (21.7) |
9 (39.1) |
5 (21.7) |
4 (17.4) |
23 (100) |
1.85 |
0.194# |
| |
no |
35 (43.8) |
21 (26.3) |
10 (12.5) |
14 (17.5) |
80 (100) |
|
|
| CNS and neural tube defects |
yes |
6 (31.6) |
7 (36.8) |
4 (21.1) |
2 (10.5) |
19 (100) |
1.63 |
0.246# |
| |
no |
34 (40.5) |
23 (27.4) |
11 (13.1) |
16 (19.0) |
84 (100) |
|
|
| Genital |
yes |
6 (31.6) |
8 (42.1) |
2 (10.5) |
3 (15.8) |
19 (100) |
1.09 |
0.354# |
| |
no |
34 (40.5) |
22 (26.2) |
13 (15.5) |
15 (17.9) |
84 (100) |
|
|
| Digestive |
yes |
7 (35.0) |
11 (55.0) |
1 (5.0) |
1 (5.0) |
20 (100) |
6.23 |
0.016#* |
| |
no |
33 (39.8) |
19 (22.9) |
14 (16.9) |
17 (20.5) |
83 (100) |
|
|
| Respiratory |
yes |
7 (50.0) |
4 (28.6) |
0 |
3 (21.4) |
14 (100) |
0.52 |
0.514# |
| |
no |
33 (37.1) |
26 (29.2) |
15 (16.9) |
15 (16.9) |
89 (100) |
|
|
| Clefts (palate, lip) |
yes |
6 (54.5) |
2 (18.2) |
2 (18.2) |
1 (9.1) |
11 (100) |
0.10 |
0.774# |
| |
no |
34 (37.0) |
28 (30.4) |
13 (14.1) |
17 (18.5) |
92 (100) |
|
|
| Eye |
yes |
0 |
1 (20.0) |
2 (40.0) |
2 (40.0) |
5 (100) |
0.10 |
0.838# |
| |
no |
40 (40.8) |
29 (29.6) |
13 (13.3) |
16 (16.3) |
98 (100) |
|
|
| Ear |
yes |
2 (66.7) |
1 (33.3) |
0 |
0 |
3 (100) |
0.03 |
1.000# |
| |
no |
38 (38.0) |
29 (29.0) |
15 (15.0) |
18 (18.0) |
100 (100) |
|
|
| Total cases per CLD type | 40 (38.8) | 30 (29.1) | 15 (14.6) | 18 (17.5) | 103 (100) | |||
Abbreviations and notations: CLD–congenital limb defects, n–number of children with CLD, CNS–central nervous system, χ2–test value, P–value showing the significance of association of anomalies with limb defect.
aOnly MCA cases with multiple congenital anomalies that had one type of CLD were included in this analysis; cases with a CLD and a malposition, or hip dysplasia/dislocation, or clubfoot were excluded from the analysis.
*Significant P value.
#Exact P values.
Discussion
We aimed to describe the epidemiology of CLD in the northern Netherlands. From 1981–2010, the birth prevalence of CLD was 21.1/10,000 births, which falls between the BPs found in two other European registries: Finland (13/10,000 for 1964–1977) and Scotland (30.4/10,000 for 1964–1968) [9,10]. However, there were differences in the inclusion criteria of all three registries.
More recent BPs for CLD are available on the official website of the EUROCAT network of over thirty-seven national registries [32]. These registries include live births, stillbirths and terminations of pregnancies, which allow their BPs for types of CLD to be compared with the Netherlands (NNL). Six registries have reported complete data for the period 1981–2010 (Dublin, Ireland; Odense, Denmark; Paris, France; Hainaut, Belgium; Emilia-Romagna and Tuscany, Italy).
Our BP for polydactyly (8.4/10,000) was close to the figures reported for Emilia-Romagna and Hainaut (8.14 and 8.55; Table 6), but much lower that the BP in Paris (13.8). We found a BP for syndactyly of 4.7/10,000, which is comparable to that reported for Emilia-Romagna (4.5) and Paris (5.1). Our BP for reduction defects (6.9) was close to that reported by Hainaut (7.3; Table 6).
Table 6.
Birth prevalences per 10,000 births in six EUROCAT registries for the period 1981-2010
| Registry | Polydactyly | Reduction defects | Syndactyly | Total of the three CLD |
|---|---|---|---|---|
| Ireland - Dublin |
7.6 |
5.7 |
4.3 |
17.6 |
| Denmark - Odense |
7.8 |
8.2 |
6.2 |
22.2 |
| France - Paris |
13.8 |
8.0 |
5.1 |
26.9 |
| Belgium - Hainaut |
8.6 |
7.3 |
7.6 |
23.4 |
| Italy - Emilia Romagna |
8.1 |
5.4 |
4.5 |
18.0 |
| Italy - Tuscany |
7.2 |
5.2 |
6.2 |
18.6 |
| Northern Netherlandsa |
8.8 |
8.6 |
5.8 |
23.2 |
| Northern Netherlandsb | 8.4 | 6.9 | 4.7 | 20.0 |
aBirth prevalences for the northern Netherlands on the EUROCAT website differ from the ones reported in this study due to thorough verification and corrections of miscoding.
bBirth prevalences in this study.
The total BP of each EUROCAT registry includes the club foot and hip dysplasia/dislocation, which hampers direct comparison with the total BP determined in our study (21.1/10,000). However, we can compare the summed BPs for polydactyly, syndactyly and reduction defects. Our calculated BP (20/10,000) is similar to those calculated for Emilia-Romagna (18.0), Tuscany (18.6), and Odense (22.2) (Table 6).
A literature review summarized the BPs of reduction defects in different countries and time periods [23]. BPs varied from 3.3 to 8.1 (in 1970) and to 5.0/10,000 in Canada (Alberta, 1966–1975), from 6.6 to 4.8/10,000 in USA (Atlanta, 1968–1993), and even to 10.4/10,000 births in France (1979–1987) [23]. The authors also mentioned that the BPs of reduction defects may have been underestimated in countries that excluded terminations of pregnancies from their registries. The latest advances in prenatal diagnosis are leading to more terminations because of CLD [23]. In our NNL study, the most common type of reduction defects were transverse (3.9/10,000). Some studies reported similar prevalences for this type: France had 4.3/10,000 births for 1979–1987, Italy 2.6/10,000 for 1978–1987, and there was a prevalence of 4.0/10,000 in six combined EUROCAT registries (Strasbourg, Belfast, Emilia Romagna, Odense, Groningen, Basque Country) [13,19,24]. A recent study in the USA found more longitudinal (3.5/10,000 births) than transverse reduction defects (1.9/10,000 births) for the periods 1972–1974 and 1979–2000 [3].
The differences in BP between countries are most likely to be the result of variations in coding methods, ascertainment, notification and inclusion criteria and, until a consistent and compatible system (such as that of EUROCAT) is universally adopted, there will be no way of determining whether BP variation has an environmental or other cause.
Localisation and laterality of limb defects
We found upper limbs were more commonly affected, which agrees with the literature [9,13,23,33]. Left-sided limb defects prevailed in our study, as in other studies [9,14,24], but others have reported more right-sided defects [16,34] or both sides being equally affected [35]. Longitudinal and multiple reduction defects were more frequently localised on the right side [34].
Gender distribution
Our male:female ratio for CLD was 1.2:1 in both total births and in live births, compared with a male:female ratio of 1.1:1 for live births in the northern Netherlands (1981–2010) [36]. This male excess has been reported before, but its aetiology remains unexplained [17,19,37].
Trends
In the northern Netherlands, the BP of non-syndromic syndactyly has shown a significant decrease since 1992. EUROCAT-NNL has a reliable and well-established network that notifies the registry of new cases shortly after birth, ensuring that the parents of almost all children with congenital anomalies in the region are contacted for registration, thus we do not feel many cases will have been missed. In addition, the diagnosis of syndactyly as part of a genetic syndrome is more commonly detected now than in the early 1990s. Excluding cases with genetic syndromes from our time-trend analysis may have influenced our finding of a drop in cases with non-syndromic syndactyly over time. Another reason for the decline might be a change in parental attitude towards giving informed consent for registering syndactyly, which might now be seen as a rather minor anomaly. The introduction of the informed consent procedure in 1992 may also have influenced the number of registrations compared to the period before 1992. In a post-hoc analysis, no other significant trends were found for the period 1981–1991. Finally, not all CLD cases may have been reported yet. By including data from recent years in our study, the decline may be due to the fact that some of the cases still need to be registered. Therefore, we cannot fully rule out that the decline observed is a registration artefact.
Associated birth defects and recognised conditions
In comparison with polydactyly, syndactyly and “other CLD”, we saw reduction defects more often in combination with congenital anomalies affecting the central nervous system, digestive system (the only significant association in current study, Table 5), genital or urinary system. Associations of reduction defects with these kinds of anomalies have been reported previously [17,19,24]. However, in our study, reduction defects were not the CLD type that occurred most frequently in combination with cardiovascular anomalies, as seen in other studies [17,19,24]. We found polydactyly (equally preaxial and postaxial) occurred most often in combination with cardiovascular anomalies, especially with ventricular septum defects. Another study reported polydactyly occurring mostly with central nervous system anomalies [38]. They also found significant associations of polydactyly with recognised syndromes: trisomy 13, Meckel, and Down syndrome [38]. We only found trisomy 13 occurring often in our cases with postaxial polydactyly of the upper limb.
In our NNL study, amniotic bands were the second most frequently recognised condition after trisomy 13, and they also occurred more often in combination with transversal limb defects and syndactyly, as reported in the literature [34]. We also found syndactyly occurring often with trisomy 18 (mostly syndactyly of lower limbs), Apert, and Poland syndrome, also reported previously [39].
Longitudinal preaxial reduction defects were found to be the most common reduction defects occurring in cases with other congenital anomalies [17,34]. Preaxial defects, for instance, can occur in the VATER association and in many genetic conditions, while transverse defects are usually isolated single defects in a family and may be caused by disruptive events in early pregnancy [17,34]. We also found radius aplasia/dysplasia most frequently in cases with VATER/VACTERL, trisomy 18, and Holt-Oram syndrome.
To our knowledge, there are no genetic predispositions that are specific to the Dutch population that could have influenced the birth prevalences of CLDs in this study. In addition, the tables on the official EUROCAT website did not reveal any particular recognized condition specific for the Netherlands compared to the other six registries (Dublin, Ireland; Odense, Denmark; Paris, France; Hainaut, Belgium; Emilia-Romagna, and Tuscany, Italy) [32]. These observations, and the fact that our birth prevalences of CLD are comparable to those in other EUROCAT registries, imply that our birth prevalences are relatively good estimates of world-wide prevalences. However, studies on larger populations with CLD and with the same inclusion criteria would allow for better generalizability.
Study strengths and limitations
The EUROCAT-NNL database contains specific and detailed information about cases with birth defects. All the cases were verified and corrected for any miscoding of the CLD. Updated information, if available, was retrieved from the notifying hospital to clarify doubtful coding or diagnosis.
Our data will be of clinical relevance to clinicians treating children with CLD and to their parents, not only the parents of live-born children who were unaware of their child’s defect prior to delivery, but also the parents who know they are expecting a child with a CLD. The latter group is becoming more and more significant with all the possibilities for information now offered by prenatal diagnostics.
A limitation of our study may be the relatively small number of children with MCA included in the analysis for associations. The response rate for the parental consent for the registration of their child is fairly high (80%). Nevertheless, we cannot exclude the fact that underestimation of the true BP of CLD may have occurred. This type of underestimation, due to the voluntary participation of parents or notifying sources, is also likely to be present in other birth defects registries (e.g., Tuscany and Emilia-Romagna, Italy; Paris, France; United Kingdom; Austria; Switzerland; Spain) [28]. A further limitation may be the classification system used. Although there are several classifications of CLD [1,40,41] available, they all pose difficulties. The International Federation of Societies for Surgery of the Hand has adopted the morphological classification of Swanson et al. [40]. However, this classification for CLD has not been accepted by all surgeons [14], and difficulties based on this classification have been reported [20]. Furthermore, Swanson’s classification is a more morphological classification, and does not conform to the aim of our study, which was to provide a descriptive overview of CLD. Stoll et al. [42] proposed a purely descriptive classification of CLD, which complied with the purposes of this article. However, Stoll et al.’s classification did not allow for CLD that were included in the “other CLD” category (e.g. arthrogryposis). We therefore used a classification based on ICD-9 and ICD-10 [29,30].
Conclusions
We established a prevalence of 21.1 children with CLD per 10,000 births in NNL after a thorough coding and correction procedure, which makes this figure reasonably reliable. The decreasing trend observed in non-syndromic syndactyly in 1992–2010 may be real or the result of a registration artefact. CLD occurred frequently in combination with cardiovascular and urinary tract anomalies, and were significantly associated with digestive-tract anomalies.
Abbreviations
BPA: British Paediatric Association; BP: Birth prevalences; CLD: Congenital limb defects; EUROCAT: European Surveillance of Congenital Anomalies; ICD: International Classification of Diseases; MCA: Multiple congenital anomalies; SPSS: Statistical Package for the Social Sciences.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
EV, CvdS, HRM, and HdW participated in the design of the study. EV and HdW prepared the database. EV, CvdS, HdW, AvE, and JB participated in the assessment of diagnoses and correction of the incorrect codes describing the birth defects. EV, HRM, PUD, and HdW participated in the analysis of the data. EV has written the paper. CvdS, AvE, JB, PUD, HRM, and HdW contributed with critical revision of manuscript for important intellectual content. All authors have approved this manuscript for submission.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Ecaterina Vasluian, Email: e.golea.vasluian@umcg.nl.
Corry K van der Sluis, Email: c.k.van.der.sluis@umcg.nl.
Anthonie J van Essen, Email: a.j.van.essen@umcg.nl.
Jorieke E H Bergman, Email: j.e.h.van.kammen@umcg.nl.
Pieter U Dijkstra, Email: p.u.dijkstra@umcg.nl.
Heleen A Reinders-Messelink, Email: h.a.reinders@umcg.nl.
Hermien E K de Walle, Email: h.e.k.de.walle@umcg.nl.
Acknowledgements
We thank Marian Bakker and Nicole Siemensma-Muhlenberg (EUROCAT-NNL, Groningen, The Netherlands) for their contribution to the description of the EUROCAT-NNL registry, collection of data, preparation and correction of the database, and for methodological advice. We thank Jackie Senior and Kate Mc Intyre (editors, Department of Genetics, UMCG, Groningen, the Netherlands) for their professional editing of the manuscript.
References
- Day HJB. The ISO/ISPO classification of congenital limb deficiency. Prosthet Orthot Int. 1991;15(2):67–69. doi: 10.3109/03093649109164635. [DOI] [PubMed] [Google Scholar]
- EUROCAT Guide 3 (2nd Ed): For the Description and Classification of Congenital Limb Defects. http://www.eurocat-network.eu/content/EUROCAT-Guide-3.pdf.
- Gold NB, Westgate MN, Holmes LB. Anatomic and etiological classification of congenital limb deficiencies. Am J Med Genet A. 2011;155A(6):1225–1235. doi: 10.1002/ajmg.a.33999. [DOI] [PubMed] [Google Scholar]
- Tayel SM, Fawzia MM, Al Naqeeb NA, Gouda S, Al Awadi SA, Naguib KK. A morpho-etiological description of congenital limb anomalies. Ann Saudi Med. 2005;25(3):219–227. doi: 10.5144/0256-4947.2005.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froster UG, Baird PA. Maternal factors, medications, and drug exposure in congenital limb reduction defects. Environ Health Perspect. 1993;101(Suppl 3):269–274. doi: 10.1289/ehp.93101s3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes LB. Teratogen-induced limb defects. Am J Med Genet. 2002;112(3):297–303. doi: 10.1002/ajmg.10781. [DOI] [PubMed] [Google Scholar]
- European Surveillance of Congenital Anomalies. http://www.eurocat-network.eu.
- International Clearinghouse for Birth Defects Surveillance and Research. http://www.icbdsr.org.
- Aro T, Heinonen OP, Saxen L. Incidence and secular trends of congenital limb defects in Finland. Int J Epidemiol. 1982;11(3):239–244. doi: 10.1093/ije/11.3.239. [DOI] [PubMed] [Google Scholar]
- Rogala EJ, Wynne-Davies R, Littlejohn A, Gormley J. Congenital limb anomalies: frequency and aetiological factors. Data from the Edinburgh Register of the Newborn (1964–68) J Med Genet. 1974;11(3):221–233. doi: 10.1136/jmg.11.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo-Sanchez E, Cuevas L, Amar E, Bakker MK, Bianca S, Bianchi F, Canfield MA, Castilla EE, Clementi M, Cocchi G, Feldkamp ML, Landau D, Leoncini E, Li Z, Lowry RB, Mastroiacovo P, Mutchinick OM, Rissmann A, Ritvanen A, Scarano G, Siffel C, Szabova E, Martinez-Frias ML. Amelia: a multi-center descriptive epidemiologic study in a large dataset from the International Clearinghouse for Birth Defects Surveillance and Research, and overview of the literature. Am J Med Genet C Semin Med Genet. 2011;157C(4):288–304. doi: 10.1002/ajmg.c.30319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo-Sanchez E, Cuevas L, Amar E, Bianca S, Bianchi F, Botto LD, Canfield MA, Castilla EE, Clementi M, Cocchi G, Landau D, Leoncini E, Li Z, Lowry RB, Mastroiacovo P, Mutchinick OM, Rissmann A, Ritvanen A, Scarano G, Siffel C, Szabova E, Martinez-Frias ML. Phocomelia: a worldwide descriptive epidemiologic study in a large series of cases from the International Clearinghouse for Birth Defects Surveillance and Research, and overview of the literature. Am J Med Genet C Semin Med Genet. 2011;157C(4):305–320. doi: 10.1002/ajmg.c.30320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzolari E, Manservigi D, Garani GP, Cocchi G, Magnani C, Milan M. Limb reduction defects in Emilia Romagna, Italy: epidemiological and genetic study in 173,109 consecutive births. J Med Genet. 1990;27(6):353–357. doi: 10.1136/jmg.27.6.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom AG, Laurell T, Arner M. Epidemiology of congenital upper limb anomalies in 562 children born in 1997 to 2007: a total population study from Stockholm. Sweden. J Hand Surg. 2010;35(11):1742–1754. doi: 10.1016/j.jhsa.2010.07.007. [DOI] [PubMed] [Google Scholar]
- McGuirk CK, Westgate MN, Holmes LB. Limb deficiencies in newborn infants. Pediatrics. 2001;108(4):E64. doi: 10.1542/peds.108.4.e64. [DOI] [PubMed] [Google Scholar]
- Rijnders LJ, Boonstra AM, Groothoff JW, Cornel MC, Eisma WH. Lower limb deficient children in The Netherlands: epidemiological aspects. Prosthet Orthot Int. 2000;24(1):13–18. doi: 10.1080/03093640008726517. [DOI] [PubMed] [Google Scholar]
- Rosano A, Botto LD, Olney RS, Khoury MJ, Ritvanen A, Goujard J, Stoll C, Cocchi G, Merlob P, Mutchinick O, Cornel MC, Castilla EE, Martinez-Frias ML, Zampino G, Erickson JD, Mastroiacovo P. Limb defects associated with major congenital anomalies: clinical and epidemiological study from the International Clearinghouse for Birth Defects Monitoring Systems. Am J Med Genet. 2000;93(2):110–116. doi: 10.1002/1096-8628(20000717)93:2<110::AID-AJMG6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Smith ES, Dafoe CS, Miller JR, Banister P. An epidemiological study of congenital reduction deformities of the limbs. Br J Prev Soc Med. 1977;31(1):39–41. doi: 10.1136/jech.31.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll C, Calzolari E, Cornel M, Garcia-Minaur S, Garne E, Nevin N, Ten Kate L. A study on limb reduction defects in six European regions. Ann Genet. 1996;39(2):99–104. [PubMed] [Google Scholar]
- Koskimies E, Lindfors N, Gissler M, Peltonen J, Nietosvaara Y. Congenital upper limb deficiencies and associated malformations in Finland: a population-based study. J Hand Surg. 2011;36(6):1058–1065. doi: 10.1016/j.jhsa.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Froster-Iskenius UG, Baird PA. Amelia: incidence and associated defects in a large population. Teratology. 1990;41(1):23–31. doi: 10.1002/tera.1420410104. [DOI] [PubMed] [Google Scholar]
- Froster-Iskenius UG, Baird PA. Limb reduction defects in over one million consecutive livebirths. Teratology. 1989;39(2):127–135. doi: 10.1002/tera.1420390205. [DOI] [PubMed] [Google Scholar]
- Ephraim PL, Dillingham TR, Sector M, Pezzin LE, Mackenzie EJ. Epidemiology of limb loss and congenital limb deficiency: a review of the literature. Arch Phys Med Rehabil. 2003;84(5):747–761. doi: 10.1016/s0003-9993(02)04932-8. [DOI] [PubMed] [Google Scholar]
- Stoll C, Alembik Y, Dott B, Roth MP. Risk factors in limb reduction defects. Paediatr Perinat Epidemiol. 1992;6(3):323–338. doi: 10.1111/j.1365-3016.1992.tb00773.x. [DOI] [PubMed] [Google Scholar]
- Monango AD, Buitendijk SE. Congenital malformations in The Netherlands 1997–2007, report based on Prenatal Registration Netherlands. Netherlands Organisation for Applied Scientific Research. 2009;112:46. KvL/P&Z 2009. [Google Scholar]
- EUROCAT Guide 1.3: Instructions for the Registration and Surveillance of Congenital Anomalies. http://www.eurocat-network.eu/content/EUROCAT-Guide-1.3.pdf.
- Prevalence of congenital malformations in the Northern Netherlands 1981–2007. http://www.rug.nl/umcg/faculteit/disciplinegroepen/medischegenetica/eurocat/pdf/Prevalence_of_Congenital_Malformations_in_the_Northern_Netherlands_1981_2007.pdf.
- Greenlees R, Neville A, Addor MC, Amar E, Arriola L, Bakker M, Barisic I, Boyd PA, Calzolari E, Doray B, Draper E, Vollset SE, Garne E, Gatt M, Haeusler M, Kallen K, Khoshnood B, Latos-Bielenska A, Martinez-Frias ML, Materna-Kiryluk A, Dias CM, McDonnell B, Mullaney C, Nelen V, O’Mahony M, Pierini A, Queisser-Luft A, Randrianaivo-Ranjatoelina H, Rankin J, Rissmann A, Ritvanen A, Salvador J, Sipek A, Tucker D, Verellen-Dumoulin C, Wellesley D, Wertelecki W. Paper 6: EUROCAT member registries: organization and activities. Birth Defects Res A Clin Mol Teratol. 2011;91(Suppl 1):S51–S100. doi: 10.1002/bdra.20775. [DOI] [PubMed] [Google Scholar]
- EUROCAT ICD9 (with BPA extension) http://www.eurocat-network.eu/content/EUROCAT-ICD9-with-BPA-Extension.pdf.
- EUROCAT ICD10. http://www.eurocat-network.eu/content/EUROCAT-Q-Chapter-2008.pdf.
- Manouvrier-Hanu S, Holder-Espinasse M, Lyonnet S. Genetics of limb anomalies in humans. Trends Genet. 1999;15(10):409–417. doi: 10.1016/S0168-9525(99)01823-5. [DOI] [PubMed] [Google Scholar]
- EUROCAT Prevalence Tables. http://www.eurocat-network.eu/ACCESSPREVALENCEDATA/PrevalenceTables.
- Schulman J, Edmonds LD, McClearn AB, Jensvold N, Shaw GM. Surveillance for and comparison of birth defect prevalences in two geographic areas–United States, 1983–88. MMWR CDC Surveill Summ. 1993;42(1):1–7. [PubMed] [Google Scholar]
- Evans JA, Vitez M, Czeizel A. Congenital abnormalities associated with limb deficiency defects: a population study based on cases from the Hungarian Congenital Malformation Registry (1975–1984) Am J Med Genet. 1994;49(1):52–66. doi: 10.1002/ajmg.1320490111. [DOI] [PubMed] [Google Scholar]
- Stoll C, Rosano A, Botto LD, Erickson D, Khoury MJ, Olney RS, Castilla EE, Cocchi G, Cornel MC, Goujard J, Bermejo E, Merlob P, Mutchinick O, Ritvanen A, Zampino G, Mastroiacovo P. On the symmetry of limb deficiencies among children with multiple congenital anomalies. Ann Genet. 2001;44(1):19–24. doi: 10.1016/S0003-3995(01)01036-X. [DOI] [PubMed] [Google Scholar]
- Tables: Population dynamics; birth, death and migration per region. Central Bureau of Statistics. http://statline.cbs.nl/StatWeb/publication/default.aspx?DM=SLEN&PA=37422ENG&D1=0%2c4-5&D2=31-60&LA=EN&STB=T%2cG1&VW=T.
- Giele H, Giele C, Bower C, Allison M. The incidence and epidemiology of congenital upper limb anomalies: a total population study. J Hand Surg Am. 2001;26(4):628–634. doi: 10.1053/jhsu.2001.26121. [DOI] [PubMed] [Google Scholar]
- Castilla EE, Lugarinho R, da Graca Dutra M, Salgado LJ. Associated anomalies in individuals with polydactyly. Am J Med Genet. 1998;80(5):459–465. doi: 10.1002/(SICI)1096-8628(19981228)80:5<459::AID-AJMG5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Jordan D, Hindocha S, Dhital M, Saleh M, Khan W. The epidemiology, genetics and future management of syndactyly. Open Orthop J. 2012;6:14–27. doi: 10.2174/1874325001206010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson AB, Swanson GD, Tada K. A classification for congenital limb malformation. J Hand Surg Am. 1983;8(5 Pt 2):693–702. doi: 10.1016/s0363-5023(83)80249-4. [DOI] [PubMed] [Google Scholar]
- Kay HW, Day HJ, Henkel HL, Kruger LM, Lamb DW, Marquardt E, Mitchell R, Swanson AB, Willert HG. The proposed international terminology for the classification of congenital limb deficiencies. Dev Med Child Neurol Suppl. 1975;34(34):1–12. [PubMed] [Google Scholar]
- Stoll C, Duboule D, Holmes LB, Spranger J. Classification of limb defects. Am J Med Genet. 1998;77(5):439–441. doi: 10.1002/(SICI)1096-8628(19980605)77:5<439::AID-AJMG16>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]