Abstract
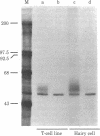
The origin and exact stage of differentiation of the neoplastic cells that comprise hairy cell leukemia have remained uncertain. As Ig heavy and light chain genes must both undergo a DNA rearrangement during B-cell development but rarely do so within other hematopoietic lineages, we examined these genes in this leukemia. The neoplastic cells of all eight cases demonstrated rearranged heavy and light chain genes and, in two cases examined, contained the corresponding mRNA for heavy and light chain Ig. Consistent with this B-cell genotype, all cases displayed cell surface HLA-DR and B-cell-associated antigens. Unexpectedly, all cases demonstrated cell surface Tac antigen, which previously had been restricted predominantly to select T-cell malignancies and activated T cells. Prior studies suggested that the anti-Tac monoclonal antibody recognized a peptide associated with the binding of interleukin 2 (T-cell growth factor) in such T cells. Immunoprecipitation with anti-Tac and NaDodSO4/polyacrylamide gel electrophoresis revealed an antigen on leukemic hairy cells with a Mr of 53,000-57,000, identical in size to the receptor on activated T cells. This apparent biphenotypic status might reflect a transformation-associated expression of the Tac antigen in this leukemia. Alternatively, hairy cell leukemia may be a malignancy of a unique stage of normal B-cell differentiation in which the Tac antigen is expressed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson C. S., Kersey J. H., LeBien T. W. A monoclonal antibody (BA-1) reactive with cells of human B lymphocyte lineage. J Immunol. 1981 Jan;126(1):83–88. [PubMed] [Google Scholar]
- Bouroncle B. A. Leukemic reticuloendotheliosis (hairy cell leukemia). Blood. 1979 Mar;53(3):412–436. [PubMed] [Google Scholar]
- Braylan R. C., Burke J. S. Hairy cell leukemia. Annu Rev Med. 1979;30:17–24. doi: 10.1146/annurev.me.30.020179.000313. [DOI] [PubMed] [Google Scholar]
- Breard J., Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with human peripheral blood monocytes. J Immunol. 1980 Apr;124(4):1943–1948. [PubMed] [Google Scholar]
- Burns G. F., Cawley J. C., Worman C. P., Karpas A., Barker C. R., Goldstone A. H., Hayhoe F. G. Multiple heavy chain isotypes on the surface of the cells of hairy cell leukemia. Blood. 1978 Dec;52(6):1132–1147. [PubMed] [Google Scholar]
- Catovsky D. Hairy-cell leukaemia and prolymphocytic leukaemia. Clin Haematol. 1977 Feb;6(1):245–268. [PubMed] [Google Scholar]
- Cawley J. C., Burns G. F., Nash T. A., Higgy K. E., Child J. A., Roberts B. E. Hairy-cell leukemia with T-cell features. Blood. 1978 Jan;51(1):61–69. [PubMed] [Google Scholar]
- Cohen H. J., George E. R., Kremer W. B. Hairy cell leukemia: cellular characteristics including surface immunoglobulin dynamics and biosynthesis. Blood. 1979 Apr;53(4):764–765. [PubMed] [Google Scholar]
- Davis M. M., Calame K., Early P. W., Livant D. L., Joho R., Weissman I. L., Hood L. An immunoglobulin heavy-chain gene is formed by at least two recombinational events. Nature. 1980 Feb 21;283(5749):733–739. doi: 10.1038/283733a0. [DOI] [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Fox R. I., Fong S., Sabharwal N., Carstens S. A., Kung P. C., Vaughan J. H. Synovial fluid lymphocytes differ from peripheral blood lymphocytes in patients with rheumatoid arthritis. J Immunol. 1982 Jan;128(1):351–354. [PubMed] [Google Scholar]
- Golde D. W., Stevens R. H., Quan S. G., Saxon A. Immunoglobulin synthesis in hairy cell leukaemia. Br J Haematol. 1977 Mar;35(3):359–365. doi: 10.1111/j.1365-2141.1977.tb00595.x. [DOI] [PubMed] [Google Scholar]
- Golomb H. M., Catovsky D., Golde D. W. Hairy cell leukemia: a clinical review based on 71 cases. Ann Intern Med. 1978 Nov;89(5 Pt 1):677–683. doi: 10.7326/0003-4819-89-5-677. [DOI] [PubMed] [Google Scholar]
- Guglielmi P., Preud'homme J. L., Flandrin G. Phenotypic changes of phytohaemagglutinin-stimulated hairy cells. Nature. 1980 Jul 10;286(5769):166–168. doi: 10.1038/286166a0. [DOI] [PubMed] [Google Scholar]
- Hardy R. R., Hayakawa K., Haaijman J., Herzenberg L. A. B-cell subpopulations identified by two-colour fluorescence analysis. Nature. 1982 Jun 17;297(5867):589–591. doi: 10.1038/297589a0. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Mann D. L., Hemler M. E., Schroer J. A., Shelhamer J. H., Eisenbarth G. S., Strominger J. L., Thomas C. A., Mostowski H. S., Fauci A. S. Characterization of a monoclonal antibody that defines an immunoregulatory T cell subset for immunoglobulin synthesis in humans. Proc Natl Acad Sci U S A. 1980 May;77(5):2914–2918. doi: 10.1073/pnas.77.5.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
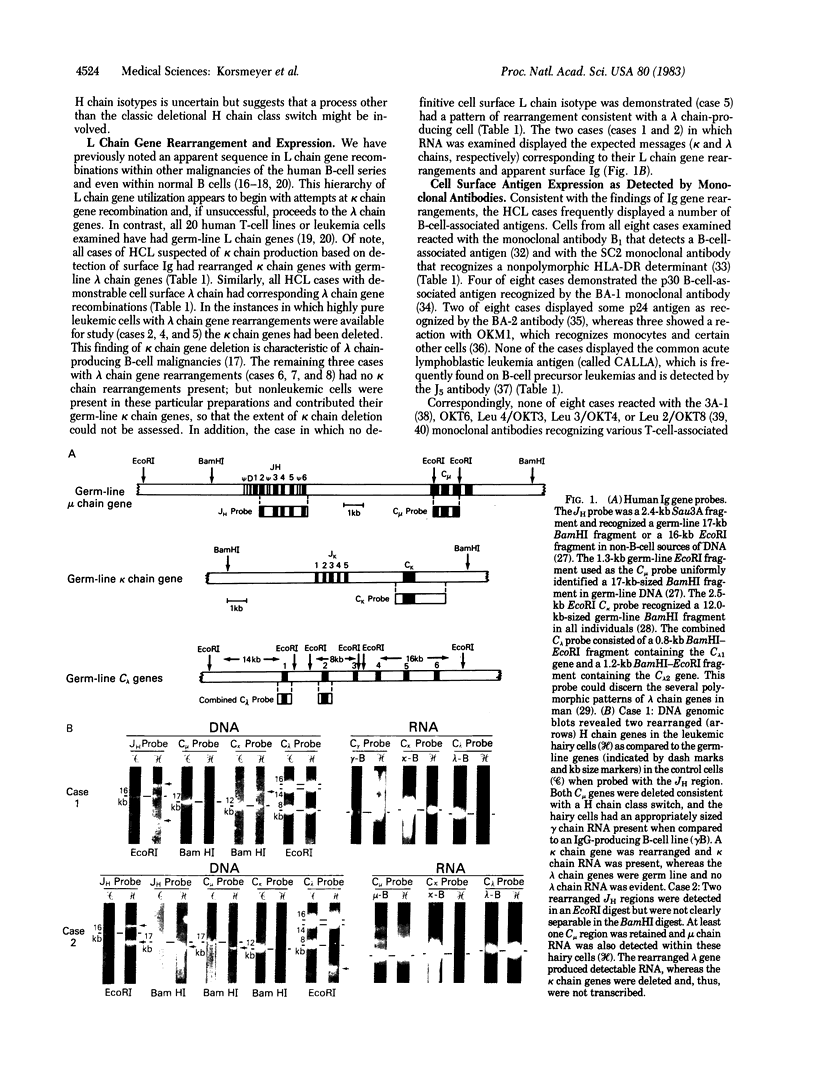
- Hieter P. A., Hollis G. F., Korsmeyer S. J., Waldmann T. A., Leder P. Clustered arrangement of immunoglobulin lambda constant region genes in man. Nature. 1981 Dec 10;294(5841):536–540. doi: 10.1038/294536a0. [DOI] [PubMed] [Google Scholar]
- Hieter P. A., Korsmeyer S. J., Waldmann T. A., Leder P. Human immunoglobulin kappa light-chain genes are deleted or rearranged in lambda-producing B cells. Nature. 1981 Apr 2;290(5805):368–372. doi: 10.1038/290368a0. [DOI] [PubMed] [Google Scholar]
- Hieter P. A., Max E. E., Seidman J. G., Maizel J. V., Jr, Leder P. Cloned human and mouse kappa immunoglobulin constant and J region genes conserve homology in functional segments. Cell. 1980 Nov;22(1 Pt 1):197–207. doi: 10.1016/0092-8674(80)90168-3. [DOI] [PubMed] [Google Scholar]
- Howard M., Kessler S., Chused T., Paul W. E. Long-term culture of normal mouse B lymphocytes. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5788–5792. doi: 10.1073/pnas.78.9.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. A comparative study of the peroxidase-antiperoxidase method and an avidin-biotin complex method for studying polypeptide hormones with radioimmunoassay antibodies. Am J Clin Pathol. 1981 May;75(5):734–738. doi: 10.1093/ajcp/75.5.734. [DOI] [PubMed] [Google Scholar]
- Jaffe E. S., Shevach E. M., Frank M. M., Green I. Leukemic reticuloendotheliosis: presence of a receptor for cytophilic antibody. Am J Med. 1974 Jul;57(1):108–114. doi: 10.1016/0002-9343(74)90775-x. [DOI] [PubMed] [Google Scholar]
- Jansen J., LeBien T. W., Kersey J. H. The phenotype of the neoplastic cells of hairy cell leukemia studied with monoclonal antibodies. Blood. 1982 Mar;59(3):609–614. [PubMed] [Google Scholar]
- Jansen J., Schuit H. R., Meijer C. J., van Nieuwkoop J. A., Hijmans W. Cell markers in hairy cell leukemia studied in cells from 51 patients. Blood. 1982 Jan;59(1):52–60. [PubMed] [Google Scholar]
- Kalyanaraman V. S., Sarngadharan M. G., Robert-Guroff M., Miyoshi I., Golde D., Gallo R. C. A new subtype of human T-cell leukemia virus (HTLV-II) associated with a T-cell variant of hairy cell leukemia. Science. 1982 Nov 5;218(4572):571–573. doi: 10.1126/science.6981847. [DOI] [PubMed] [Google Scholar]
- Kersey J. H., LeBien T. W., Abramson C. S., Newman R., Sutherland R., Greaves M. P-24: a human leukemia-associated and lymphohemopoietic progenitor cell surface structure identified with monoclonal antibody. J Exp Med. 1981 Mar 1;153(3):726–731. doi: 10.1084/jem.153.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsmeyer S. J., Arnold A., Bakhshi A., Ravetch J. V., Siebenlist U., Hieter P. A., Sharrow S. O., LeBien T. W., Kersey J. H., Poplack D. G. Immunoglobulin gene rearrangement and cell surface antigen expression in acute lymphocytic leukemias of T cell and B cell precursor origins. J Clin Invest. 1983 Feb;71(2):301–313. doi: 10.1172/JCI110770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsmeyer S. J., Hieter P. A., Ravetch J. V., Poplack D. G., Waldmann T. A., Leder P. Developmental hierarchy of immunoglobulin gene rearrangements in human leukemic pre-B-cells. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7096–7100. doi: 10.1073/pnas.78.11.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsmeyer S. J., Hieter P. A., Sharrow S. O., Goldman C. K., Leder P., Waldmann T. A. Normal human B cells display ordered light chain gene rearrangements and deletions. J Exp Med. 1982 Oct 1;156(4):975–985. doi: 10.1084/jem.156.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Evans R. L., Lipinski M., Cunningham-Rundles C., Good R. A., Herzenberg L. A. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981 Feb 1;153(2):310–323. doi: 10.1084/jem.153.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Uchiyama T., Smith K. A., Waldmann T. A., Greene W. C. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor; partial characterization of the receptor. Nature. 1982 Nov 18;300(5889):267–269. doi: 10.1038/300267a0. [DOI] [PubMed] [Google Scholar]
- Liebson H. J., Marrack P., Kappler J. B cell helper factors. II. Synergy among three helper factors in the response of T cell- and macrophage-depleted B cells. J Immunol. 1982 Oct;129(4):1398–1402. [PubMed] [Google Scholar]
- Nadler L. M., Ritz J., Hardy R., Pesando J. M., Schlossman S. F., Stashenko P. A unique cell surface antigen identifying lymphoid malignancies of B cell origin. J Clin Invest. 1981 Jan;67(1):134–140. doi: 10.1172/JCI110005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posnett D. N., Chiorazzi N., Kunkel H. G. Monoclonal antibodies with specificity for hairy cell leukemia cells. J Clin Invest. 1982 Aug;70(2):254–261. doi: 10.1172/JCI110612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch J. V., Siebenlist U., Korsmeyer S., Waldmann T., Leder P. Structure of the human immunoglobulin mu locus: characterization of embryonic and rearranged J and D genes. Cell. 1981 Dec;27(3 Pt 2):583–591. doi: 10.1016/0092-8674(81)90400-1. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz J., Pesando J. M., Notis-McConarty J., Lazarus H., Schlossman S. F. A monoclonal antibody to human acute lymphoblastic leukaemia antigen. Nature. 1980 Feb 7;283(5747):583–585. doi: 10.1038/283583a0. [DOI] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Saxon A., Stevens R. H., Golde D. W. T-lymphocyte variant of hairy-cell leukemia. Ann Intern Med. 1978 Mar;88(3):323–326. doi: 10.7326/0003-4819-88-3-323. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Max E. E., Leder P. A kappa-immunoglobulin gene is formed by site-specific recombination without further somatic mutation. Nature. 1979 Aug 2;280(5721):370–375. doi: 10.1038/280370a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Uchiyama T., Nelson D. L., Fleisher T. A., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. II. Expression of Tac antigen on activated cytotoxic killer T cells, suppressor cells, and on one of two types of helper T cells. J Immunol. 1981 Apr;126(4):1398–1403. [PubMed] [Google Scholar]
- Zatz M. M., Mathieson B. J., Kanellopoulos-Langevin C., Sharrow S. O. Separation and characterization of two component tumor lines within the AKR lymphoma, AKTB-1, by fluorescence-activated cell sorting and flow microfluorometry analysis. I. the coexistence of sIg+ and sIg- sublines. J Immunol. 1981 Feb;126(2):608–613. [PubMed] [Google Scholar]