Abstract
Recent studies involving DNAs bound strongly by bleomycins have documented that such DNAs are degraded by the antitumor antibiotic with characteristics different from those observed when studying the cleavage of randomly chosen DNAs in the presence of excess Fe•BLM. In the present study, surface plasmon resonance has been used to characterize the dynamics of BLM B2 binding to a strongly bound hairpin DNA, to define the effects of Fe3+, salt and temperature on BLM–DNA interaction. One strong primary DNA binding site, and at least one much weaker site was documented. In contrast, more than one strong cleavage site was found, an observation also made for two other hairpin DNAs. Evidence is presented for BLM equilibration between the stronger and weaker binding sites in a way that renders BLM unavailable to other, less strongly bound DNAs. Thus enhanced binding to a given site does not necessarily result in increased DNA degradation at that site, i.e. for strongly bound DNAs, the facility of DNA cleavage must involve parameters in addition to the intrinsic rate of C-4′ H atom abstraction from DNA sugars.
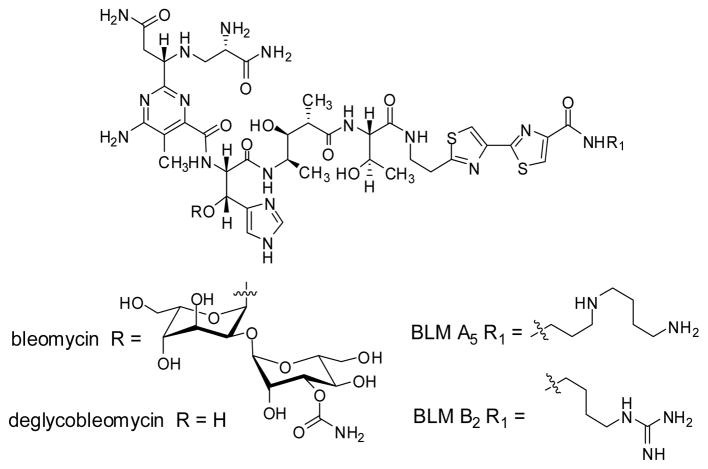
The bleomycins (BLMs, Figure 1) are a family of antitumor antibiotics, members of which are used clinically for the treatment of squamous cell carcinomas and other malignancies.1 While well established clinically as part of multidrug regimens,2 new strategies for bleomycin administration hold the promise of additional clinical applications as a single agent.3 The antitumor activity of bleomycin is believed to derive from its ability to degrade DNA,4 and possibly also RNA.4d,5 DNA cleavage has been especially well characterized, and the mechanism of degradation and derived products have been described in some detail.6
Figure 1.
Structures of bleomycin A5, bleomycin B2 and deglycobleomycin A5.
Until recently, virtually all studies of BLM-mediated DNA degradation were carried out using a large excess of a metalloBLM. However, since the clinical dose of bleomycin is atypically low (~5 μmol) it seemed logical to inquire whether the large excess of DNA relative to bleomycin in a clinical setting might result in the preferential binding of the drug to DNA sequences whose cleavage might differ from what has been observed in the presence of excess BLM. In fact, the isolation of (hairpin) DNAs strongly bound to immobilized BLM from a large library of DNAs having randomized sequences afforded DNA sequences not at all typical of those identified from studies of DNA cleavage in the presence of excess metalloBLMs.7 BLM-mediated cleavage of the strongly bound DNAs gave unusual cleavage patterns, variable efficiency of cleavage, and enhanced amounts of a previously observed alkali labile product.7b,c
A serious limitation of these, and all earlier studies has been the lack of a quantitative method for assessing nucleic acid binding by BLM. Fluorescence quenching of the bithiazole moiety of BLM accompanies binding to B-form DNA,8 and such quenching has been shown to have both ionic and non-ionic components.8b The measurement of DNA binding by fluorescence quenching has not been validated quantitatively by an independent method, and does not seem to reliably predict binding affinities for RNAs, or non-B-form DNAs.9 More recently, we have employed a competition assay, measuring the ability of a selected hairpin DNA to inhibit the (binding and) cleavage of a second profluorescent hairpin DNA present in equimolar concentration.7a,b While promising, the interpretation of this assay involves a few key assumptions, the validity of which have not been established. Even if the relative equilibrium binding constants inferred from such studies prove accurate, it would not exclude the possibility that BLM binding to two DNAs with comparable affinities might involve significantly different on- and off-rates. Since the rate-limiting step for B-DNA cleavage has been shown to involve abstraction of H• from the C-4′ H of DNA sugars,6h variations in the dynamics of BLM binding to different DNAs could afford altered efficiencies of cleavage.
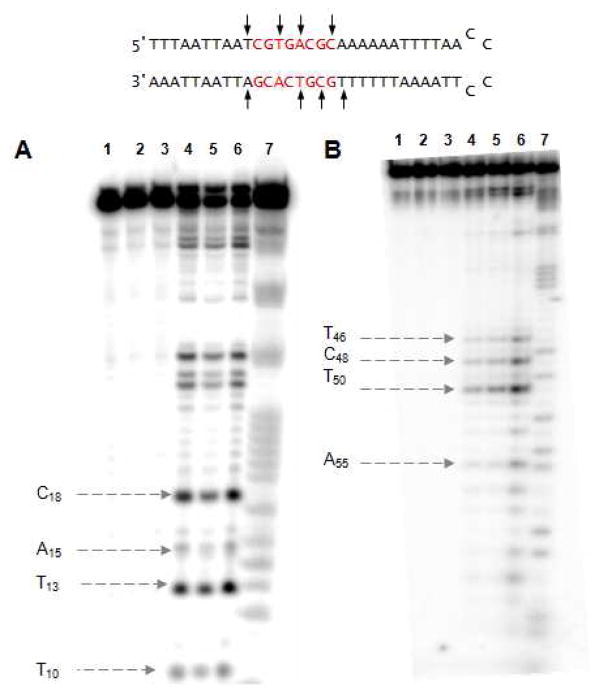
In order to assess the dynamics of BLM binding to hairpin DNAs selected for strong BLM binding,7 we employed surface plasmon resonance (SPR) which has been used to assess the interaction of other ligands with DNA.10 The main focus of the current study involved hairpin DNA 2 (Figure 2), which was shown in an earlier study to bind BLM A5 more strongly than most of the other hairpin DNAs studied.7a,b The 5′-32P end labeled DNA underwent cleavage at several sites when treated with Fe(II)•BLM A5 and Fe(II)• deglycoBLM A5; the sites of cleavage for the two BLM congeners were quite similar,7b,c but Fe(II)•BLM A5 was more potent as a DNA cleaving agent.7c Shown in Figure 2 is a summary of the sites of cleavage of the same hairpin DNA, radiolabeled alternatively at the 3′- and 5′-ends, with Fe(II)•BLM A5. The 5′-32P end labeled DNA underwent cleavage at T10, T13, A15 and C18 as noted previously,7b,c and the bands at T13 and C18 were the strongest, consistent with our earlier report, although the weak band detected previously at G147c was not observed in the present experiment. Treatment of the 5′-32P end labeled DNA with Fe(II)•BLM B2 gave quite similar results as well, with an intensity of cleavage similar to that of Fe(II)•BLM A5 (Figure S1, Supporting Information). As shown in Figure S2, cleavage of 5′-32P end labeled DNA 2 with Fe(II)•BLM B2 was minimally affected by the addition of NaCl at concentrations up to 150 mM.
Figure 2.
A) Sequence-selective cleavage of [5′-32P]-end labeled 64-nt hairpin DNA 2 by BLM A5. Lane 1, radiolabeled 2 alone; lane 2, 20 μM Fe2+; lane 3, 20 μM BLM A5; lane 4, 5 μM Fe(II)•BLM A5; lane 5, 10 μM Fe(II)•BLM A5; lane 6, 20 μM Fe(II)•BLM A5; lane 7, G+A lane. B) Sequence-selective cleavage of [3′-32P]-end labeled 64-nt hairpin DNA 2 by BLM A5. Lane 1, radiolabeled 2 alone; lane 2, 10 μM Fe2+; lane 3, 10 μM BLM A5; lane 4, 1 μM Fe(II)•BLM A5; lane 5, 5 μM Fe(II)•BLM A5; lane 6, 10 μM Fe(II)•BLM A5; lane 7, G+A lane.
Also shown in Figure 2 (panel B) is the result of cleavage of the same 3′-32P end labeled DNA with Fe(II)•BLM A5. Four cleavage bands were noted, at T46, C48, T50 and A55, the last of which was somewhat weaker than the other three. Thus this hairpin DNA is cleaved at multiple sites, which seem not to vary dramatically from one BLM congener to another, or in response to changes in ionic strength.
The hairpin DNA was attached to a BIAcore sensor chip via a biotin moiety, the latter of which was connected to the hairpin DNA through a tether at the 5′-end.11 Initially, the KA values for the Fe(III) chelates of BLM B2, deglycoBLM A5 and BLM A5 were measured, in order to assess relative affinities for metalloBLMs incapable of DNA cleavage. For all the BLM samples one strong binding site was noted and one, or probably more, sites that were significantly weaker. When measured at 25 °C and 10 mM NaCl, the KA values under these conditions were found to be approximately 3.2, 3.0 and 1.5 × 106 M−1, respectively. Thus the relative affinities of these metalloBLMs for hairpin DNA 2 (BLM B2 ≈ deglycoBLM A5 > BLM A5) was not in the same order as the relative potencies of cleavage of the same DNA by the Fe(II) derivatives (BLM B2 ≈ BLM A5 > deglycoBLM A5), although the actual sites of cleavage were quite similar for all three BLM congeners. The lack of correlation is illustrated in Figure S3, which shows the significantly greater affinity of deglycoBLM A5 at the strong and weaker binding sites, in comparison with the much weaker reported cleavage of DNA 2 by deglycoBLM A5.7c These experiments establish that the relative efficiency of cleavage of a strongly bound DNA by a given metalloBLM is not controlled simply by BLM–DNA affinity. The same conclusion can be reached from the finding that there was a single strong binding site for Fe(III)•BLM B2 but multiple sites of cleavage, two of which involved strong cleavage.
BLM B2 (Figure 1) has been shown to cleave chromosomal DNA quite efficiently in cultured mammalian cells relative to the other BLM congeners tested.12 Accordingly, the above results encouraged us to characterize the interaction of this congener with DNA 2 more carefully. Shown in Figure 3 (left panel) is an SPR sensorgram of the binding of Fe(III)•BLM B2 to immobilized DNA 2 at 25 °C in the presence of 25 mM NaCl, where the concentrations of metalloBLM employed were 50–500 nM. The KA value determined for this interaction was 3.9 × 106 M−1. The on-rate ka was determined as 2.9 × 105 M−1s−1, while the off-rate kd was found to be 0.074 s−1. The sensorgram for the same DNA measured at 25 °C in the presence of 10 mM NaCl is shown in the right panel of Figure 3.
Figure 3.
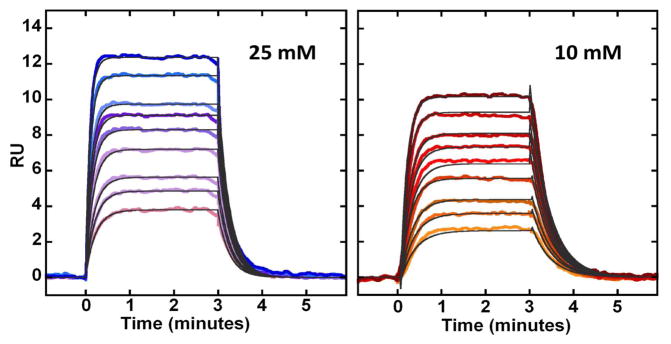
SPR sensorgrams for the interaction of Fe(III)•BLM B2 with DNA 2 at 25 mM (left panel) and 10 mM (right panel) NaCl concentrations and 25 °C. The individual sensorgrams (colored) represent responses at BLM B2 concentrations of 50, 75, 100, 150, 200, 250, 300, 400 and 500 nM (bottom to top). Global kinetic fit (black solid lines) with a 1:1 model was performed using Biacore T200 Evaluation Software to obtain kinetic association and dissociation rate constants.
A summary of the data determined at two salt concentrations and two temperatures is shown in Table 1. As is clear from these data, quite similar results for the on-rates were obtained at 15 and 25 °C, while the off-rates increased 2.5–3 fold at the higher temperature. Accordingly, KA was about three times larger at 15 °C due to the change in off-rate. Small, but consistent changes in all measured parameters resulted from the change in salt concentration. The structural factors in the hairpin DNA or Fe(III)•BLM B2 that cause these changes in affinity and dynamics of interaction are not presently clear, but should be amenable to analysis by systematic alterations of the structures of hairpin DNAs and BLM congeners. In this regard, one unanticipated result not yet investigated in detail was the dramatically lower affinity of metal free BLM B2 for DNA 2 (KA 2.2 × 104 M−1 at 10 mM NaCl and 25 °C), a result in good qualitative agreement with an earlier study that employed fluorescence quenching of the bithiazole moiety of BLM as an endpoint for measuring DNA binding in the presence of Fe(II) or Cu(II).8b
Table 1.
Binding of Fe(III)•BLM B2 to DNA 2a
| ka (× 105 M−1s−1) | kd (× 10−2 s−1) | KA (ka/kd) (× 106 M−1) | KD (kd/ka) (× 10−9 M) | KA (steady-state) (K1; K2 × 106 M−1) | |
|---|---|---|---|---|---|
|
|
|||||
| 15 °C | |||||
| 10 mM NaCl | 1.8 ± 0.23 | 1.7 ± 0.22 | 10.5 ± 1.3 | 95 ± 12 | 9.4 ± 0.38; 0.20 ± 0.08 |
| 25 mM NaCl | 3.5 ± 0.41 | 2.3 ± 0.28 | 15.2 ± 1.8 | 66 ± 7.9 | 9.3 ± 0.36; 0.38 ± 0.14 |
| 25 °C | |||||
| 10 mM NaCl | 1.4 ± 0.16 | 4.2 ± 0.50 | 3.2 ± 0.38 | 312 ± 37 | 3.1 ± 0.20 |
| 25 mM NaCl | 2.9 ± 0.17 | 7.4 ± 0.44 | 3.9 ± 0.23 | 256 ± 15 | 3.4 ± 0.29 |
50, 75, 100, 150, 200 and 250 nM concentrations of Fe (III)•BLM B2 were used to determine the kinetic rate constants. Quite similar results were obtained when higher (300 – 500 nM) concentrations were employed. Based on reproducibility of results, the errors in the strong binding constants and kinetics constants are ±15% and close to 40% for the weaker binding constants. The weaker binding constant (K2) could not be determined accurately at higher temperature.
As is clear from Figure 2, the Fe(II)•BLMs studied each cleaved the hairpin DNA substrate at multiple sites, albeit with somewhat different efficiencies. Since the cleavages observed in Figure 2 were carried out under conditions of single hit kinetics, it is logical to conclude that it must be possible for metalloBLMs to bind to the hairpin DNAs in multiple binding modes, each of which affords a different DNA cleavage pattern. To determine whether it is possible to detect more than one binding mode by surface plasmon resonance, the data in the sensorgrams was subjected to steady state analysis, using additional concentrations of Fe(III)•BLM B2 up to 1 μM.
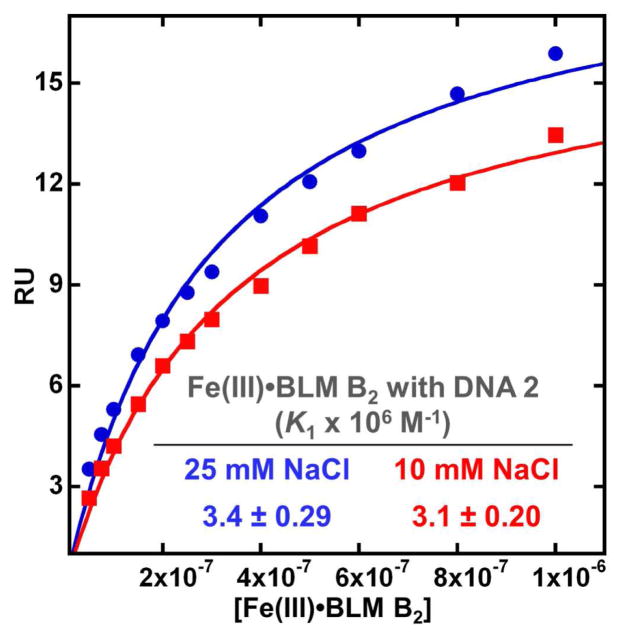
The SPR equilibrium binding plots for Fe(III)•BLM B2 and immobilized DNA 2 at the two salt concentrations (25 °C) are shown in Figure 4. The steady state analysis employed the same experimental data as above, but up to a higher (1 μM) concentration. The agreement of the strong binding constant with the kinetic binding constant was good (3.4 vs 3.9 × 106 at 25 mM NaCl and 3.1 vs 3.2 × 106 at 10 mM NaCl, cf Table 1).
Figure 4.
SPR equilibrium binding plots of Fe(III)•BLM B2 with DNA 2 at 10 mM and 25 mM NaCl concentrations and 25 °C. The steady-state response values were fitted as a function of free ligand concentration to a single-site interaction model. The binding affinities are listed in the inset and in Table 1.
Using the steady-state method it was also possible to estimate a value for the second binding mode that was found to have quite fast kinetics. The second binding was at least 10–20 times weaker than the primary binding and could be determined only with a large (~40%) error. The approximate values of the steady-state binding constants for Fe(III)•BLM B2 and of immobilized DNA 2 at 10 mM NaCl and 15 °C were 9.4 and 0.20 × 106 M−1 for the strong and weak binding sites, respectively. When measured at 25 mM NaCl and 15 °C, the comparable values were 9.3 and 0.38 × 106 M−1. Given the observation of multiple Fe•BLM cleavage sites on this DNA (Figure 2), there are probably multiple weaker binding sites on this DNA for Fe(III)•BLM B2, but they cannot be distinguished as it is difficult to achieve saturation and definitive maximum stoichiometry.
The existence of one strong, and one or more much weaker binding sites for Fe(III)•BLM B2 was not limited to DNA 2. As shown in Figure S4, previously reported7a,b DNAs 4 and 5 exhibited the same characteristics, with one strong binding site, and one or more weaker binding sites. These DNAs, particularly DNA 5, had numerous strong BLM cleavage sites in spite of the presence of only a single strong binding site.
In an earlier study,7b it was shown that a 16-nucleotide hair- pin DNA cleaved efficiently by one equivalent of Fe(II)•BLM failed to undergo significant cleavage in the additional presence one equivalent of any strongly bound 64-nt DNA. This implies that association of BLM with a strongly bound DNA effectively precludes binding to other species. In an effort to gain insights into the mechanism of DNA cleavage of hairpin DNA 2, we carried out competition experiments in which 32P-radiolabeled samples of DNA 2 or the 16-nt hairpin DNA of comparable specific activity were treated with Fe(II)•BLM in the presence of unlabeled competitor DNA. As shown in Figure S5, the Fe(II)•BLM B2-mediated cleavage of DNA 2 was little affected by 8 equivalents of the unlabeled 16-nt DNA. In contrast, the presence of a single equivalent of DNA 2 precluded cleavage of the radiolabeled 16-nt DNA. This suggests that even when not bound to the strong binding site of DNA 2, Fe•BLM remains in proximity to this DNA, resulting in significant cleavage at weakly bound sites in the same DNA in preference to efficient cleavage sites in another DNA.
In conclusion, we have characterized the binding and cleavage by Fe•BLM of a hairpin DNA selected for strong binding to BLM. The hairpin DNA undergoes cleavage on both arms by three different BLM congeners with similar sequence selectivity but different efficiencies. The relative efficiencies of cleavage do not correlate in a simple way with relative binding affinities, indicating that affinity is only one factor defining cleavage efficiency. SPR analysis of BLM binding to the hairpin DNA has permitted the dynamics of binding to be defined. The on-rate ka values did not change significantly from 25 to 15 °C, while the off-rate kd values decreased about three-fold at both salt concentrations studied. The kinetic and steady-state binding constants were in good agreement; steady-state analysis permitted the detection of at least one weaker binding mode for Fe(III)•BLM B2 to hairpin DNA 2. While the mode of BLM binding to DNA has been studied previously,13 the dynamics have not. The present analysis significantly advances our understanding of the quantitative parameters and dynamics of DNA binding by BLM and the relationship between DNA binding and cleavage by Fe•BLM.
Supplementary Material
Acknowledgments
This work was supported at ASU by NIH Research Grant CA140471, awarded by the National Cancer Institute, and at Georgia State University by NIH Research Grant AI064200, from the National Institute of Allergy and Infectious Diseases.
Footnotes
Cleavage of radiolabeled hairpin DNA 2 by BLM B2 under several different conditions, additional SPR experiments and experimental procedures for the DNA radiolabeling and cleavage. This information is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.(a) Carter SK, Crooke ST, Umezawa H, editors. Bleomycin: Current Status and New Developments; Academic Press; Orlando, FL: 1978. [Google Scholar]; (b) Sikic BI, Rozencweig M, Carter SK, editors. Bleomycin Chemotherapy. Academic Press; Orlando, FL: 1985. [Google Scholar]; (c) Cragg GM, Kingston DGI, Newman DJ, editors. Anticancer Agents from Natural Products; CRC Press; Boca Raton, FL: 2012. [Google Scholar]
- 2.(a) Einhorn LH, Donohue J. Ann Intern Med. 1977;87:293. doi: 10.7326/0003-4819-87-3-293. [DOI] [PubMed] [Google Scholar]; (b) Carlson RW, Sikic BI, Turbow MM, Ballon SC. J Clin Oncol. 1983;1:645. doi: 10.1200/JCO.1983.1.10.645. [DOI] [PubMed] [Google Scholar]; (c) Levi JA, Raghavan D, Harvey V, Thompson D, Sandeman T, Gill G, Stuart-Harris R, Snyder R, Byrne M, Kerestes Z. J Clin Oncol. 1993;11:1300. doi: 10.1200/JCO.1993.11.7.1300. [DOI] [PubMed] [Google Scholar]
- 3.(a) Poddevin B, Orlowski S, Belehradek J, Jr, Mir LM. Biochem Pharmacol. 1991;42:S67. doi: 10.1016/0006-2952(91)90394-k. [DOI] [PubMed] [Google Scholar]; (b) Munn SE, Higgins E, Marshall M, Clement M. Brit J Dermatol. 1996;135:969. doi: 10.1046/j.1365-2133.1996.d01-1104.x. [DOI] [PubMed] [Google Scholar]; (c) Nanda GS, Sun FX, Hofmann GA, Hoffman RM, Dev SB. Anticancer Res. 1998;18:1361. [PubMed] [Google Scholar]; (d) Horiuchi A, Nikaido T, Mitsushita J, Toki T, Konishi I, Fujii S. Int J Cancer. 2000;88:640. doi: 10.1002/1097-0215(20001115)88:4<640::aid-ijc19>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]; (e) Pollock B, Sheehan-Dare R. Lasers Surgery Med. 2002;30:135. doi: 10.1002/lsm.10024. [DOI] [PubMed] [Google Scholar]
- 4.(a) Stubbe J, Kozarich JW. Chem Rev. 1987;87:1107. [Google Scholar]; (b) Kane SA, Hecht SM. Prog Nucleic Acid Res Mol Biol. 1994;49:313. doi: 10.1016/s0079-6603(08)60054-9. [DOI] [PubMed] [Google Scholar]; (c) Claussen CA, Long EC. Chem Rev. 1999;99:2797. doi: 10.1021/cr980449z. [DOI] [PubMed] [Google Scholar]; (d) Hecht SM. J Nat Prod. 2000;63:158. doi: 10.1021/np990549f. [DOI] [PubMed] [Google Scholar]; (e) Chen J, Stubbe J. Nature Rev. 2005;5:102. [Google Scholar]
- 5.(a) Carter BJ, de Vroom E, Long EC, van der Marel GA, van Boom JH, Hecht SM. Proc Natl Acad Sci U S A. 1990;87:9373. doi: 10.1073/pnas.87.23.9373. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Holmes CE, Carter BJ, Hecht SM. Biochemistry. 1993;32:4293. doi: 10.1021/bi00067a019. [DOI] [PubMed] [Google Scholar]; (c) Holmes CE, Duff RJ, van der Marel GA, van Boom J, Hecht SM. Bioorg Med Chem. 1997;5:1235. doi: 10.1016/s0968-0896(97)00038-2. [DOI] [PubMed] [Google Scholar]; (d) Abraham AT, Lin JJ, Newton DL, Rybak S, Hecht SM. Chem Biol. 2003;10:45. doi: 10.1016/s1074-5521(02)00306-x. [DOI] [PubMed] [Google Scholar]; (e) Tao ZF, Konishi K, Keith G, Hecht SM. J Am Chem Soc. 2006;128:14806. doi: 10.1021/ja066187x. [DOI] [PubMed] [Google Scholar]
- 6.(a) Giloni L, Takeshita M, Johnson F, Iden C, Grollman AP. J Biol Chem. 1981;256:8608. [PubMed] [Google Scholar]; (b) Burger RM, Peisach J, Horwitz SB. J Biol Chem. 1982;257:8612. [PubMed] [Google Scholar]; (c) Sugiyama H, Xu C, Murugesan N, Hecht SM. J Am Chem Soc. 1985;107:4104. [Google Scholar]; (d) Sugiyama H, Kilkuskie RE, Hecht SM, van der Marel GA, van Boom JH. J Am Chem Soc. 1985;107:7765. [Google Scholar]; (e) Wu JC, Kozarich JW, Stubbe J. Biochemistry. 1985;24:7562. doi: 10.1021/bi00347a009. [DOI] [PubMed] [Google Scholar]; (f) Rabow L, Stubbe J, Kozarich JW, Gerlt JA. J Am Chem Soc. 1986;108:7130. [Google Scholar]; (g) Sugiyama H, Xu C, Murugesan N, Hecht SM, van der Marel GA, van Boom JH. Biochemistry. 1988;27:58. doi: 10.1021/bi00401a011. [DOI] [PubMed] [Google Scholar]; (h) Kozarich JW, Worth L, Jr, Frank BL, Christner DF, Vanderwall DE, Stubbe J. Science. 1989;245:1396. doi: 10.1126/science.2476851. [DOI] [PubMed] [Google Scholar]; (i) Rabow LE, Stubbe J, Kozarich JW. J Am Chem Soc. 1990;112:3196. [Google Scholar]; (j) Rabow LE, Stubbe J, Kozarich JW, McGall GH. J Am Chem Soc. 1990;112:3203. [Google Scholar]
- 7.(a) Akiyama Y, Ma Q, Edgar E, Laikhter A, Hecht SM. J Am Chem Soc. 2008;130:9650. doi: 10.1021/ja802905g. [DOI] [PubMed] [Google Scholar]; (b) Ma Q, Akiyama Y, Xu Z, Konishi K, Hecht SM. J Am Chem Soc. 2009;131:2013. doi: 10.1021/ja808629s. [DOI] [PubMed] [Google Scholar]; (c) Giroux RA, Hecht SM. J Am Chem Soc. 2010;132:16987. doi: 10.1021/ja107228c. [DOI] [PubMed] [Google Scholar]
- 8.(a) Chien M, Grollman AP, Horwitz SB. Biochemistry. 1977;16:3641. doi: 10.1021/bi00635a021. [DOI] [PubMed] [Google Scholar]; (b) Huang CH, Galvan L, Crooke ST. Biochemistry. 1980;19:1761. doi: 10.1021/bi00550a006. [DOI] [PubMed] [Google Scholar]
- 9.(a) Holmes CE, Hecht SM. J Biol Chem. 1993;268:25909. [PubMed] [Google Scholar]; (b) Guenther R, Forrest B, Newman W, Malkiewicz A, Agris PF. Acta Biochim Pol. 1998;45:13. [PubMed] [Google Scholar]
- 10.(a) Myszka DG. J Mol Recognit. 1999;12:279. doi: 10.1002/(SICI)1099-1352(199909/10)12:5<279::AID-JMR473>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]; (b) Nanjunda R, Munde M, Liu Y, Wilson WD. Methods for Studying Nucleic Acid/Drug Interactions. Chap 4. CRC Press; 2011. pp. 91–134. [Google Scholar]; (c) Liu Y, Chai Y, Kumar A, Tidwell RR, Boykin DW, Wilson WD. J Am Chem Soc. 2012;134:5290. doi: 10.1021/ja211628j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) The hairpin DNA oligonucleotide was purchased from IDT and contained a six carbon alkyl linker between the 5′ phosphate group on the oligonucleotide and a biotin moiety. Attachment to an SPR streptavidin-coated SA sensor chip was done according to previously described methods.10Wang S, Munde M, Wang S, Wilson WD. Biochemistry. 2011;35:7674. doi: 10.1021/bi201010g.
- 12.Berry DE, Chang LH, Hecht SM. Biochemistry. 1985;24:3207. doi: 10.1021/bi00334a020. [DOI] [PubMed] [Google Scholar]
- 13.(a) Manderville RA, Ellena JF, Hecht SM. J Am Chem Soc. 1995;117:7891. [Google Scholar]; (b) Wu W, Vanderwall DE, Lui SM, Tang XJ, Turner CJ, Kozarich JW. J Am Chem Soc. 1996;118:1281. [Google Scholar]; (c) Cortes JC, Sugiyama H, Ikudome K, Saito I, Wang AH. Biochemistry. 1997;36:9995. doi: 10.1021/bi9708951. [DOI] [PubMed] [Google Scholar]; (d) Lui SM, Vanderwall DE, Wu W, Tang X-J, Turner CJ, Kozarich JW, Stubbe J. J Am Chem Soc. 1997;119:9603. doi: 10.1016/s1074-5521(97)90128-9. [DOI] [PubMed] [Google Scholar]; (e) Goodwin KD, Lewis MA, Long EC, Georgiadis MM. Proc Natl Acad Sci U S A. 2008;105:5052. doi: 10.1073/pnas.0708143105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.