Abstract
Cognitive reserve (CR) is a concept meant to account for the frequent discrepancy between an individual’s measured level of brain pathology and her expected cognitive performance. It is particularly important within the context of aging and dementia, but has wider applicability to all forms of brain damage. As such, it has intimate links to related compensatory and neuroprotective concepts, as well as to the related notion of brain reserve. In this article, we introduce the concept of cognitive reserve and explicate its potential cognitive neural implementation. We conclude that cognitive reserve is compatible and complementary with many related concepts, but that each much draw sharper conceptual boundaries in order to truly explain preserved cognitive function in the face of aging or brain damage.
Keywords: cognitive reserve, aging, Alzheimer’s disease, imaging, epidemiology
The reserve concept
CR (see Glossary) has been proposed to account for the frequent discrepancy between a person’s underlying level of brain pathology (or age-related changes) and the observed functional and/or cognitive deficits that are expected to result from that pathology [1, 2]. There is extensive epidemiological and experimental evidence for the existence of such reserve: life exposures, such as educational and occupational attainment, and engagement in leisure and social activities have each been associated with decreased risk of developing dementia [3–6], more successful aging [7], and reduced clinical changes in several other conditions, including traumatic brain injuries [8], Parkinson’s disease (PD) [9], multiple sclerosis (MS) [10], and HIV-related dementia [11] (Figure 1).
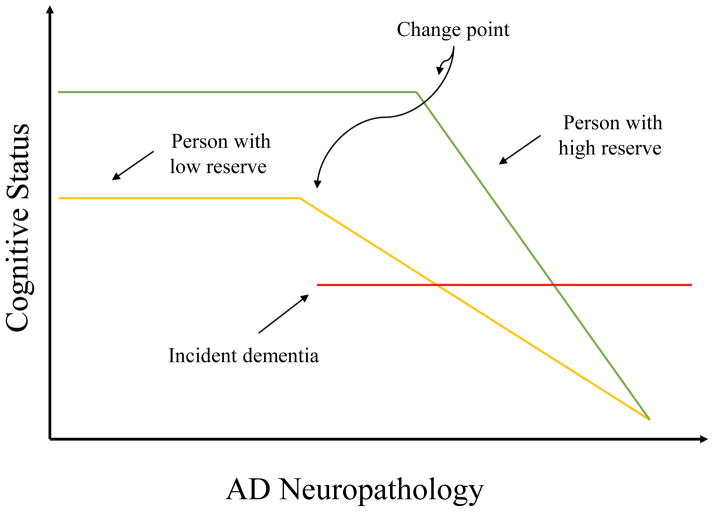
Figure 1.
Representation of how cognitive reserve may mediate between AD pathology and its clinical expression based on epidemiological and imaging studies. The x-axis represents AD pathology, slowly increasing over time. The y-axis represents cognitive function. We assume that AD pathology increases over time at the same rate in two individuals with high and low reserve. The amount of pathology needed before cognitive function is affected is greater with higher CR, leading to a later change point [91, 92]. It follows that more pathology will be needed for the person with higher CR to meet clinical diagnostic criteria for AD, thus delaying the onset of the disease. Also, at any level of cognitive performance, AD pathology will be more severe in the individual with higher CR [27, 93]. Once cognitive decline begins, it is more rapid in the person with higher CR [92, 94].
The status of CR as a concept has been debated vis-à-vis other related concepts, such as brain reserve (BR) [12] and more recently brain maintenance (BM) [13]. Several other related concepts have also been proposed [14]. In this review, we discuss recent work on these and related concepts, and attempt to delineate the subtle distinctions between them. We argue that, although the models differ in important respects, they are complementary as opposed to competing.
Models of reserve
Below we outline some of the dominant theories of preserved cognitive function in the face of advanced age, dementia, and/or brain damage. These theories focus either on compensatory mechanisms (emphasizing adaptations to diminished function or impaired brain structure), neuroprotective mechanisms (emphasizing factors which prevent diminished function and impaired structure), or some combination of both. The main models we discuss are BR, CR, BM, and neurocognitive scaffolding.
Brain reserve
The notion of BR posits that differential susceptibility of people to brain damage or pathology is a function of i) the extent of the brain damage and ii) a purely quantitative measure of brain reserve capacity (BRC) (such as the overall size of the brain, the number of neurons, the number of synapses, etc.) [12]. When pathology reduces BRC beyond a certain threshold, functional decline occurs. This can explain how pathology that is relatively equal between two people can yield differing functional manifestations (e.g., two people with equal levels of AD pathology, such as amyloid or tau deposition, can perform vastly differently on cognitive tests).
This conceptualization of BR can be considered a passive, threshold model, because once a certain ratio of pathological quantity to brain quantity is reached, functional impairment is inevitable. The general threshold model can be applied to virtually any pathology the brain may face. To take one example, a higher number of large pyramidal neurons in the cortex has been suggested as one potential quantitative measure that could predict differences in the rate of functional impairment given the same amount of AD pathology [15]. Most other related studies of BR typically use more generalized measures of BRC, such as total intracranial volume. However, although associations between head size and resistance to dementia have been found [16], often more sophisticated analyses that control for genetic factors, such as the apolipoprotein E (Apo-E) gene, have dampened this association [17]. Moreover, the link between head circumference and dementia is often significant only towards the extremely low ranges of the former [18].
More nuanced potential measures of BR are more feasible. For instance, imaging measures, such as diffusion tensor imaging (DTI), provide a new quantitative measure that can be used in the traditional threshold model (see, e.g., [19, 20]). Eventually, microstructural anatomical differences in measures such dendritic spine length, dendritic density, or synaptic proteins may be employed [21, 22]. Although in practice these measures are difficult to obtain in humans, except in autopsy studies, their homologues in animals can be examined using histology and their effects on animal behavior can be observed [23].
Cognitive reserve
In contrast to BR, CR can be considered to be an ‘active’ model, in that the threshold for functional decline is not fixed by quantitative brain measures, but can be altered based on experience. Thus, individuals with the same amount of BRC can have different levels of CR [1]. The CR model posits that cognitive processes are crucial for explaining the differences between someone who is functionally impaired and someone who is not, despite equal brain changes or pathology. These cognitive processes consist of differences in cognitive efficiency, capacity, or flexibility that are shaped by life experiences. Thus CR is ‘active’ in two senses: i) it relies on current neural activity to explain functional differences much more heavily than BR and ii) it suggests that current neural activity is shaped by disparate cognitive exposures/activities throughout the lifespan.
CR is often estimated using proxy variables for lifetime exposures and cognitive activity. Hence, years of education, measures of crystallized intelligence, such as vocabulary or knowledge, literacy level, number of intellectually stimulating leisure activities, degree of occupational complexity, and socioeconomic status are all commonly used to create an estimate of CR [1]. There are important methodological issues to consider in combining these CR proxies [24]. In addition, an alternative CR measure has been proposed that comprises the variance in cognitive performance not explained by socio-demographic measures and measures of brain pathology [25].
Recent evidence for the role of CR (as inferred via a number of proxies) in modulating the cognitive effects of pathology and normal aging is vast. Here, we provide a few representative recent studies as examples. In one study, the relationship between low plasma beta-amyloid (Aβ) and cognitive performance was modulated by CR: elders with higher education showed a weaker association [26]. Similarly, higher education was associated with diminished fludeoxyglucose positron emission tomography (FDG-PET) activation in subjects positive for cerebrospinal fluid Aβ, suggesting a compensatory role for CR [27]. Beyond AD, higher education has been associated with slower transition from mild cognitive impairment (MCI) to dementia in PD [9]. Similarly, higher CR (as measured using reading score on the WRAT-3) is protective against cognitive decline in women following chemotherapy treatment for breast cancer [28]. In the context of normal aging, higher CR (measured with educational and occupational history, as well as verbal IQ) was associated with lower functional MRI activation in right inferior frontal cortex during a working memory task (consistent with increased network efficiency – see below), as well as with decreased brain volumes in older adults with MCI and AD (consistent with more preservation of function in the presence of brain pathology) [29]. In the same vein, the strength of the blood oxygen level-dependent (BOLD) signal in task-related areas as-yet unaffected by AD pathology or gray matter atrophy was found to be positively correlated with CR proxies (again using occupational–educational history and verbal IQ, plus a measure of leisure activities) in MCI and AD subjects, but negatively in healthy subjects [30]. These findings suggest that CR operates in a compensatory manner even before pathology begins to diminish function in a particular area [30].
Because CR is a cognitive concept, it is important to identify its neural implementation. Stern [31] posited two neural mechanisms: neural reserve and neural compensation (Figure 2). Neural reserve addresses the cognitive networks that have developed over the lifespan as a function of innate capacity and lifetime exposures. Someone with higher neural reserve, may therefore have more efficient cognitive networks (i.e., networks that need to activate to a smaller degree than a less efficient network to perform the same task at a comparable level of performance), higher capacity networks (i.e., ones that can activate to a greater degree given increasing task difficulty), or greater flexibility in network selection. Thus, neural reserve encapsulates most of the differences observed between healthy individuals on cognitive tasks and posits that these differences may account for differential susceptibility to brain changes or pathology. Interestingly, recent theoretical models also equate higher intelligence to higher neural efficiency [32]. The neural reserve concept is compatible with Fabiani’s view of normal healthy aging as the continuation of processes that are already present earlier in life and which continually sculpt and transform the brain [33].
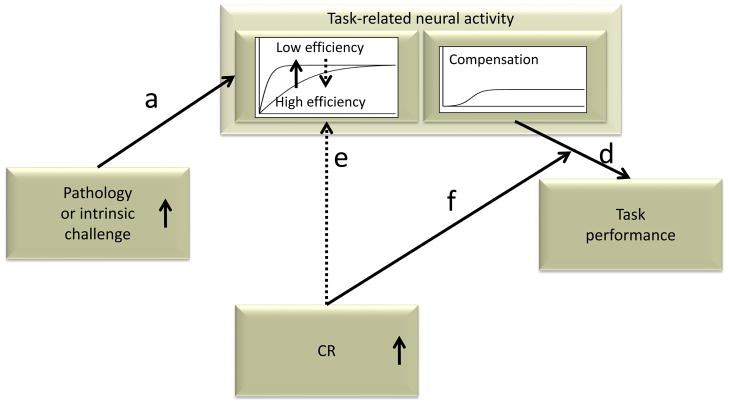
Figure 2.
Possible instantiation of CR via neural reserve and neural compensation. One study [82] using this model in the context of a working memory task observed decreasing network efficiency with volume loss within the network (path d). However, this decreasing efficiency was mitigated by higher measured CR (path f), consistent with the concept of neural reserve. Neural compensation was also observed via a second network expressed by older but not younger adults when efficiency was sufficiently impaired in the first network. More activation in the compensatory network was associated with diminished task performance (path e), which demonstrates that compensation need not always improve performance but may only maintain it, albeit at a lower level. Higher measured CR moderated the detrimental effect of the compensatory network on performance (path g), which suggests that individuals with higher CR may recruit additional networks not directly captured by task-related activation.
Neural compensation refers to situations where pathology (or age-related changes) afflicts primary task-related networks and necessitates the use of additional, compensatory networks to accomplish the same task. Individuals with higher levels of CR may be more capable of drawing on alternative neural networks in the face of brain changes in order to maintain function.
Although BR and CR began as very distinct concepts, much recent work that demonstrates the plasticity of the brain itself in response to experience suggests that the boundaries between the two models should be softened. In animal studies, voluntary aerobic exercise has been associated with dramatic structural changes, including increased neurogenesis in the dentate gyrus of the hippocampus [34] and upregulation of brain-derived neurotrophic factor (BDNF) [35]. Similarly, cognitive experiences have been associated with similar brain changes [22, 36]. Furthermore, ‘cognitive’ training can result in focal volume changes in areas relevant to task demand, which suggests that both long-term intellectual stimulation, as well as focused cognitive interventions can produce structural alterations [23,37] (Box 1).
Box 1. Implications for intervention.
Epidemiological data indicate that lifelong exposures are associated with more successful aging [44, 56, 60]. However, the transition from observation to intervention is not straightforward. It is unknown whether short-term engagement in any set of activities is sufficient to impart reserve; it is possible that reserve accrues only over long periods of time. Also, although intervention strategies can be guided by epidemiology, their optimal instantiation is not straightforward.
Aerobic exercise has well documented benefits and has resulted in improved performance on cognitive tests, especially in executive function [61]. A recent meta-analysis of 29 studies concluded that aerobic exercise is associated with improvements in various cognitive domains, such as processing speed, executive function, memory, and attention [62].
Cognitive or behavioral interventions have yielded more mixed results. A large-scale study found some improvements on trained domains, but no transfer of training across domains or improvement in activities of daily living. Studies that have immersed subjects in complex gameplay are also promising. Playing a complex role-playing game was associated with improved performance on a wide range of cognitive tasks [63] and playing the Space Fortress task with emphasis-change training was associated with improved working memory [64]. Although evidence of genuine transfer-of-training effects remains slim, and improvements in day-to-day cognitive functions are elusive [65], one study found that training on a working memory task generalized to improvements in measures of fluid intelligence [66]. Moreover, speed of processing training has been shown to lead to increased driving mobility for elder subjects [67].
Cognitive training has also been shown to induce structural changes. Training on several cognitive domains improved white matter (WM) microstructure in both younger and older healthy subjects [68]. Intensive memory training resulted in changes to gray matter volume and WM integrity in elders, and these increases correlated with improved memory performance [69, 70]. Changes in the structural integrity of WM have been induced with cognitive and visuomotor training [4,71], and video-game playing [72]. Other studies of training on working memory [73], mirror-reading [53], and Morse code [74] have also resulted in structural changes. Such changes may happen very quickly, even after just two hours of category learning [75] or spatial learning [76].
Although epidemiological data suggest that life exposures can enhance reserve, the exact ‘recipe’ for intervention remains unknown. The most meaningful endpoints for intervention in elders would be slowed rate of age-related cognitive decline or reduced risk of developing AD. Controlled studies that use such endpoints will undoubtedly be very expensive and will have to be conducted over long periods of time. Most likely, these studies should use multiple intervention strategies including exercise, cognitive stimulation, and social stimulation.
These structural changes are not confined to animal models. Many cross-sectional studies have also suggested that volumetric changes occur in humans following years of intellectual stimulation associated with higher education [38], specialized occupation [39], literacy [40], and so on. Caution is warranted against uncritically accepting this conclusion, given the vague causal directionality of the association between enriched experience and more intact brain structure [22]. Nonetheless, prospective studies support this idea. Increased hippocampal size in humans following a 6-month aerobic exercise intervention has been reported [41]. Moreover, three months of training on juggling skills was associated with increases in left intra-parietal sulcus and mid-temporal cortical grey matter [42]. Similarly, learning the layout of London was associated with gray matter increases in trainee taxi drivers, specifically in the hippocampus [43]. There are even suggestions that higher CR may be associated with reduced pathologies, such as reduced rate of hippocampal reduction in aging [44] and lower deposition of Aβ [45]. The latter observations suggest strongly that such exposures associated with CR may not only help the brain adapt to structural changes, but it may also help prevent those changes to begin with.
Brain maintenance
Similar to BR models is the notion of BM [13]. Rather than addressing differential susceptibility to impairment given equal levels of pathology, maintenance models instead ask which factors may protect against pathology or age-related changes, and posit that there are certain genetic factors and favorable life experiences that imbue people with a capacity to resist undergoing these changes. It is an open question whether BM models could be extended to predict that certain individuals are better than others at resisting not only the advent of pathological processes, but also resisting the harmful biological effects of the normal aging process itself. Disentangling the effects of CR and BM on levels of functional impairment is difficult due to practical challenges in identifying and measuring brain changes associated with aging or disease processes. In addition, this approach does not explain variability of functioning in the face of identified brain pathologies such as stroke, AD, MS or white matter hyperintensities, nor does it account for recent empirical observations that much age-related cognitive decline is unaccounted for by common neuropathologies [46]. Moreover, there is direct evidence that CR proxies can operate in a compensatory way and not in a neuroprotective way as BM predicts. For instance, Brayne et al. [47] found that years of education were not associated with neurodegeneration or vascular pathology themselves, but they did moderate the effects of such pathologies on clinical expression. Nonetheless, more direct studies are needed to disentangle the effects of BM from those of reserve theories.
The BM account has intimate links with research into neuroplasticity in animal models, but these two research strands are starkly different in that, whereas one looks explicitly at which factors are associated with the absence of any structural changes, the other looks at which factors facilitate either i) beneficial structural changes (e.g., [42, 48]), or b) compensatory functional responses, given harmful structural changes (e.g., [49]). The neural reserve and neural compensation implementations of CR are highly related to more recent notions of compensation emerging from the neuroplasticity literature conducted largely with animal studies, but also from observations in human subjects (e.g. [50]; Box 2).
Box 2. Using imaging to explore the neural basis of cognitive reserve.
Functional imaging has strengths and weaknesses for evaluating the neural basis of CR. It is not ideal for identifying networks that underlie alternate problem-solution strategies because imaging analyses are more attuned to common rather than different patterns of activation within a group of people. However, functional imaging can be used to probe individual differences in task-related activation as a function of task performance and CR proxies. Consideration of the degree of pathology aids in exploring how some individuals cope with pathology better than others [77].
Neural reserve would predict that individuals with and without a given pathology use the same brain networks, albeit with differing efficiency or capacity. It is important to consider that task-related activation can increase with task difficulty. Because aging or brain changes increase subjective task difficulty and reduce efficiency of brain networks, it is common to observe greater task-related activation in more affected than less affected individuals [29]. However, at higher levels of task demand, there can be greater activation in more intact individuals, because of greater network capacity [58]. Controlling for subjective task demand can produce equivalent levels of task-related activation in young and old individuals [78]. The compensation-related utilization of neural network hypothesis (CRUNCH) [50] encompasses these findings, with the additional observation that activation in more affected individuals might actually decline in response to increased task difficulty, perhaps reflecting overwhelming of their networks’ capacity. Because young and old use the same networks in this scheme, Stern [2] would not invoke the concept of compensation here.
Neural compensation would be invoked when the affected individuals engage networks not typically used by unaffected individuals. Generic models for compensation in aging include PASA [79] and HAROLD [80]. These models assume that compensatory activation is associated with better performance, with some support [81]. The actual implementation of compensation will depend closely on the networks that underlie the functions in question, and possible supporting networks. Studies have also demonstrated compensatory activation that is not associated with better performance [54, 82]. In this case, the use of an alternate network may maintain as opposed to improve performance. There is also the possibility of a ‘generic’ CR network that supports multiple functions [83].
These observations of functional activation patterns consistent with neural reserve and neural compensation can be tied to the CR model when it can be shown that in the face of a comparable amount of brain pathology individuals expected to have higher CR can maintain greater network efficiency or capacity, can compensate in an advantageous way, or can avoid resorting to a less advantageous alternate networks.
Bridging cognitive reserve and brain reserve
Another approach taken in a review by Lövdén et al. [51] creates a bridge between CR and BR. It suggests that people use knowledge-based strategies (which can be considered to be within their current range of cognitive flexibility) to perform tasks, but when faced with a prolonged mismatch between functional supply and challenge (of either the intrinsic or extrinsic nature), the brain itself must exhibit plastic and compensatory alterations that may result in a mechanism akin to neural compensation. When the brain is confronted by a challenge it relies on compensatory network activations to maintain performance; and although these compensatory networks could be less efficient at performing a given task than the primary networks used, without the additional activation performance would be severely impaired. Eventually, these challenges prompt changes in the brain itself, which Lövdén et al. define as true plasticity. When this happens, it seems plausible that more ‘process-based’ rather than knowledge-based mechanisms (i.e., compensatory mechanisms based on implicit improvements in abilities such as working memory rather than ones based on using explicit strategies such as mnemonics) begin to underlie performance differences. Indeed, Shing et al. [52] demonstrated that some cognitive domains (such as episodic memory) can be broken down into strategic and process-based components (Box 3).
Box 3. Cognitive manifestations of CR.
Despite the overwhelming emphasis on the neural underpinnings of reserve, Lövdén et al.’s [51] conceptual work on cognitive flexibility as a counterpart to neural plasticity should remind us that there is a purely behavioral dimension to CR that is based solely on cognitive networks – that is, acquired knowledge or ‘representations’ that do not reflect structural differences beyond synaptic connectivity.
These behavioral manifestations of CR may be reflected, for instance, in an increased ability to select and employ the best strategy for performing a task. Barulli et al. [57] report that higher CR (operationalized using verbal IQ and years of education) in healthy adults is associated with better strategy selection abilities in a computational estimation task: older adults with higher verbal IQs employed the best strategy often than those with lower verbal IQ, an effect not observed in a young group. This finding suggests that it is not overall cognitive ability which determines strategy selection, but CR based on a lifetime of acquired knowledge.
Other more specific cognitive domains – particularly memory, where mnemonic strategies would be very effective [84, 85] – may also be useful for evaluating the cognitive implementation of reserve. Woods et al. [86] report that in a sample of HIV-infected participants mnemonic strategy use was associated with higher verbal IQ and moderated the effects of the disease on visual working memory. There is also a growing body of evidence to suggest that higher CR is associated with better use of compensatory strategies. For example, Czernochowski et al. [87] found that higher-socioeconomic status (SES) older adults had access to compensatory mnemonic strategies that lower-SES elders did not. Boyle et al. [88] reported that poor decision-making was associated with a fourfold increased risk of mortality in a sample of 675 older adults, even after controlling for other cognitive domains. Whether improved strategy selection or decision-making abilities are an instantiation of CR or a basis for it is an open question.
Although very preliminary, these results suggest one largely unexplored path to studying the cognitive mechanisms of CR and related concepts. Identifying the particular cognitive strategies or compensatory mechanisms may help to identify the underlying neural mechanisms, as well as point towards effective interventions.
The scaffolding theory of aging and cognition (STAC)
Similar to Lövdén et al. [51], Park and Reuter-Lorenz [14] reason that compensatory mechanisms reflect a general feature of the brain that they call scaffolding: the ability to adapt to structural alterations (and task-induced functional limitations) by engaging in functional reorganization – that is, developing and/or relying on compensatory networks when the primary networks are no longer efficient at performing some task. This can happen either because more specialized networks are recruited, which accomplish the task better (an instance of scaffolding that happens during learning) or because the primary networks are being subjected to damage of any kind that renders them suboptimal (an instance of scaffolding that happens with age-related deterioration). Thus, age-related brain changes would be met with functional adaptations in affected areas and those downstream in order to minimize the cognitive impact of the brain changes. Such a compensatory mechanistic interpretation is consistent with much empirical data (e.g., [53]). This observation is comparable to that in neural reserve and compensation, which encompass similar mechanisms, including ‘compensatory’ networks that are associated with poorer performance [54].
Proponents of STAC suggest that it differs from CR in that it applies not only to aging, but also to the brain’s response to other pathologies or afflictions throughout the lifespan, as well as to normal task-related challenges, such as learning a new skill. [14] However, this overlooks the fact that CR is built up over a lifetime of experiences [55] and has been associated with reduced susceptibility to many forms of brain damage across the lifespan [56]. Moreover, CR has also been used to predict discrepancies in performance in the case of normal aging (e.g., [57]), as well as differences in performance and functional MRI activation resulting from differences in task difficulty (e.g., [58]). Moreover, in a longitudinally-followed sample of young people, low childhood IQ was found to be predictive of increased risk for psychiatric disorders, such as depression, schizophrenia, and anxiety in young adulthood [59]. This suggests that CR may be important throughout the lifespan for resisting not only cognitive decline, but psychiatric afflictions, as well. Thus, CR is a model with wide applicability across the lifespan and across a range of brain challenges, but despite its generality it can also account for qualitative shifts that occur in compensatory behavior (e.g., shifts in functional activation upon diagnosis, dramatic shifts in the rate of cognitive decline, etc.) in a way that a more generalized theory such as scaffolding cannot.
Concluding remarks
In this review, we attempted to clarify some of the conceptual relationships between CR and closely related models. As imaging methods become more advanced and macrostructural changes reveal their microstructural correlates, BR and CR may grow more interconnected. Much more fine-grained brain measures are necessary than the standard proxies for BR, such as brain size, and CR can point towards which of these more subtle measures are relevant. BM theories, meanwhile, are complementary to CR, but it is not clear to what extent they can account for age-related variability in cognitive performance, given their inability to explain compensatory mechanisms. Even with normal aging, some degree of structural change seems to be inevitable; therefore, these changes present a challenge with which the healthy ager needs to cope. Finally, scaffolding and related theories are also compatible with CR and, we have suggested, propose many similar overarching principles and mechanisms. The challenge for all theories now is to specify the underlying neural and cognitive mechanisms that mediate the relationship between brain challenge and cognitive performance (Box 4).
Box 4. Outstanding questions.
What are the neural mechanisms by which CR operates? To what extent are they captured by the neural reserve and neural compensation hypotheses?
Could there be a general ‘CR network’ – that is, a network developed over a lifetime of cognitive stimulation that is active and playing a compensatory role across very disparate tasks?
How do CR and BR interact? When and how is cognitive experience converted to structural change?
Can life experiences foster BM? Would this apply only to normal aging or to disease pathologies, such as plaques and tangles?
Can experiences be provided that impart CR?
To what extent is CR based on acquired knowledge (analogous to crystallized intelligence) versus cognitive processes (analogous to fluid intelligence)?
What role do genetic factors play in CR and BR? Do genetic factors merely set the initial limits for cognitive flexibility or could they also set limits for neuroplastic structural changes?
Might CR be easier to build at younger ages, consistent with the observation in animal studies of ‘critical periods’ [89, 90], during which certain neuroplastic changes are more likely?
How much normal age-related cognitive variance is accounted for by BM theories and CR theories, respectively? Is this question possible to answer without a clearer conceptual distinction between what constitutes normal aging and what constitutes pathology?
Highlights.
We examine cognitive reserve and related concepts (brain reserve/maintenance, cognitive flexibility, scaffolding)
These concepts are complementary rather than competing
Cognitive and brain reserve influence one another and are interconnected
Cognitive reserve is compatible with neuroprotective theories such as brain maintenance
To be truly useful, all models must identify underlying neural and cognitive mechanisms
Acknowledgments
Supported by NIH/NIA RO1 AG26158.
Glossary
- Brain reserve (BR)
differences in brain size and other quantitative aspects of the brain that explain differential susceptibility to functional impairment in the presence of pathology or other neurological insult
- Cognitive reserve (CR)
differences in cognitive processes as a function of lifetime intellectual activities and other environmental factors that explain differential susceptibility to functional impairment in the presence of pathology or other neurological insult
- Neural reserve
one proposed neural basis of cognitive reserve involving cognitive networks used by unimpaired individuals. Individual differences in network efficiency/capacity or the use of alternative strategies may provide reserve against the impact of brain changes
- Efficiency
the degree to which a task-related brain network must become activated in order to accomplish a given task
- Capacity
the degree to which a task-related brain network can maximally be activated to keep performing a task even in the face of increasing demands
- Neural compensation
one proposed neural basis cognitive reserve involving the utilization of alternative networks not typically used by healthy individuals in order to maintain or improve cognitive performance
- Brain maintenance (BM)
differences in individuals’ susceptibility to pathology, particularly in the context of aging; whereas reserve theories emphasize compensatory mechanisms, maintenance theories emphasize neuroprotective mechanisms
- Scaffolding theory of aging and cognition (STAC)
scaffolding is the recruitment of additional neural circuits or networks when the primary networks have become inefficient or damaged due to age, pathology, or even some normal task-related challenge. This process is in theory a general and life-long property of the brain
- Compensation-related utilization of neural circuits hypothesis (CRUNCH)
the theory that, as a task becomes more difficult, a network will be recruited to an increasing degree. At some point increased difficulty overwhelms the network, which ceases to function effectively
- Cognitive flexibility
the capacity to achieve best performance on a particular task, given the range of ability currently supported by the brain’s underlying neuroanatomical structure; transient fluctuations in function that do not result in long-term structural or anatomical changes fall under this umbrella.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- 2.Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stern Y, et al. Influence of education and occupation on the incidence of Alzheimer’s disease. J Am Med Assoc. 1994;271:1004–1010. [PubMed] [Google Scholar]
- 4.Scarmeas N, et al. Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology. 2001;57:2236–2242. doi: 10.1212/wnl.57.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson RS. Elderly women with larger social networks are less likely to develop dementia. Evid Based Ment Health. 2009;12:22. doi: 10.1136/ebmh.12.1.22. [DOI] [PubMed] [Google Scholar]
- 6.Valenzuela MJ, Sachdev P. Brain reserve and dementia: a systematic review. Psychol Med. 2005;25:1–14. doi: 10.1017/S0033291705006264. [DOI] [PubMed] [Google Scholar]
- 7.Suchy Y, et al. Instrumental activities of daily living among community-dwelling older adults: discrepancies between self-report and performance are mediated by cognitive reserve. J Clin Exp Neuropsychol. 2011;33:92–100. doi: 10.1080/13803395.2010.493148. [DOI] [PubMed] [Google Scholar]
- 8.Fay TB, et al. Cognitive reserve as a moderator of postconcussive symptoms in children with complicated and uncomplicated mild traumatic brain injury. J Int Neuropsychol Soc. 2010;16:94–105. doi: 10.1017/S1355617709991007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poletti M, et al. Mild cognitive impairment and cognitive reserve in Parkinson’s disease. Parkinsonism Relat Disord. 2011;17:579–586. doi: 10.1016/j.parkreldis.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Sumowski JF, et al. Cognitive reserve protects against cognitive dysfunction in multiple sclerosis. J Clin Exp Neuropsychol. 2009;31:913–926. doi: 10.1080/13803390902740643. [DOI] [PubMed] [Google Scholar]
- 11.Foley JM, et al. Cognitive reserve as a protective factor in older HIV-positive patients at risk for cognitive decline. Appl Neuropsychol. 2012;19:16–25. doi: 10.1080/09084282.2011.595601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satz P, et al. Brain and cognitive reserve: mediator(s) and construct validity, a critique. J Clin Exp Neuropsychol. 2011;33:121–130. doi: 10.1080/13803395.2010.493151. [DOI] [PubMed] [Google Scholar]
- 13.Nyberg L, et al. Memory aging and brain maintenance. Trends Cogn Sci. 2012;16:292–305. doi: 10.1016/j.tics.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sachdev PS, Valenzuela M. Brain and cognitive reserve. Am J Geriatr Psychiatry. 2009;17:175–178. doi: 10.1097/JGP.0b013e318196a661. [DOI] [PubMed] [Google Scholar]
- 16.Mortimer JA, et al. Head circumference, education and risk of dementia: findings from the Nun Study. J Clin Exp Neuropsychol. 2003;25:671–679. doi: 10.1076/jcen.25.5.671.14584. [DOI] [PubMed] [Google Scholar]
- 17.Graves AB, et al. Head circumference and incident Alzheimer’s disease: modification by apolipoprotein E. Neurology. 2001;57:1453–1460. doi: 10.1212/wnl.57.8.1453. [DOI] [PubMed] [Google Scholar]
- 18.Schofield PW, et al. An association between head circumference and Alzheimer’s disease in a population based study of aging. Neurology. 1997;49:30–37. doi: 10.1212/wnl.49.1.30. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, et al. Selective changes in white matter integrity in MCI and older adults with cognitive complaints. Biochim Biophys Acta-Mol Basis Dis. 2012;1822:423–430. doi: 10.1016/j.bbadis.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chao LL, et al. Associations between white matter hyperintensities and β amyloid on integrity of projection, association, and limbic fiber tracts measured with diffusion tensor MRI. PLoS One. 2013;8:e65175. doi: 10.1371/journal.pone.0065175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 22.Lövdén M, et al. Structural brain plasticity in adult learning and development. Neurosci Biobehav Rev. 2013 doi: 10.1016/j.neubiorev.2013.02.014. http://dx.doi.org/10.1016/j.neubiorev.2013.02.014. [DOI] [PubMed]
- 23.Lerch JP, et al. Maze training in mice induces MRI-detectable brain shape changes specific to the type of learning. Neuroimage. 2011;54:2086–2095. doi: 10.1016/j.neuroimage.2010.09.086. [DOI] [PubMed] [Google Scholar]
- 24.Jones RN, et al. Conceptual and measurement challenges in research on cognitive reserve. J Int Neuropsychol Soc. 2011;17:593–601. doi: 10.1017/S1355617710001748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reed BR, et al. Measuring cognitive reserve based on the decomposition of episodic memory variance. Brain. 2010;133:2196–2209. doi: 10.1093/brain/awq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yaffe K, et al. Association of plasma β-amyloid level and cognitive reserve with subsequent cognitive decline. JAMA. 2011;305:261–266. doi: 10.1001/jama.2010.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ewers M, et al. Cognitive reserve associated with FDG-PET in preclinical Alzheimer disease. Neurology. 2013;80:1194–1201. doi: 10.1212/WNL.0b013e31828970c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahles TA, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol. 2010;28:4434–4440. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solé-Padullés C, et al. Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2009;30:1114–1124. doi: 10.1016/j.neurobiolaging.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Bosch B, et al. Cognitive reserve modulates task-induced activations and deactivations in healthy elders, amnestic mild cognitive impairment and mild Alzheimer’s disease. Cortex. 2010;46:451–461. doi: 10.1016/j.cortex.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:S69–S74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- 32.Neubauer AC, Fink A. Intelligence and neural efficiency. Neurosci Biobehav Rev. 2009;33:1004–1023. doi: 10.1016/j.neubiorev.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Fabiani M. It was the best of times, it was the worst of times: a psychophysiologist’s view of cognitive aging. Psychophysiology. 2012;49:283–304. doi: 10.1111/j.1469-8986.2011.01331.x. [DOI] [PubMed] [Google Scholar]
- 34.van Praag H, et al. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aguiar AS, Jr, et al. Short bouts of mild-intensity physical exercise improve spatial learning and memory in aging rats: involvement of hippocampal plasticity via AKT, CREB and BDNF signaling. Mech Ageing Dev. 2011;132:560–567. doi: 10.1016/j.mad.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 37.Nithianantharajah J, Hannan AJ. The neurobiology of brain and cognitive reserve: mental and physical activity as modulators of brain disorders. Prog Neurobiol. 2009;89:369–382. doi: 10.1016/j.pneurobio.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Coffey CE, et al. Relation of education to brain size in normal aging: implications for the reserve hypothesis. Neurology. 1999;53:189–196. doi: 10.1212/wnl.53.1.189. [DOI] [PubMed] [Google Scholar]
- 39.Gaser C, Schlaug G. Brain structures differ between musicians and non-musicians. J Neurosci. 2003;23:9240–9245. doi: 10.1523/JNEUROSCI.23-27-09240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carreiras M, et al. An anatomical signature for literacy. Nature. 2009;461:983–986. doi: 10.1038/nature08461. [DOI] [PubMed] [Google Scholar]
- 41.Erickson KI, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Draganski B, May A. Training-induced structural changes in the adult human brain. Behav Brain Res. 2008;192:137–142. doi: 10.1016/j.bbr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 43.Woollett K, Maguire EA. Acquiring ‘the Knowledge’ of London’s layout drives structural brain changes. Curr Biol. 2011;21:2109–2114. doi: 10.1016/j.cub.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valenzuela MJ, et al. Lifespan mental activity predicts diminished rate of hippocampal atrophy. PLoS One. 2008;3:e2598. doi: 10.1371/journal.pone.0002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jagust WJ, Mormino EC. Lifespan brain activity, beta-amyloid, and Alzheimer’s disease. Trends Cogn Sci. 2011;15:520–526. doi: 10.1016/j.tics.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boyle PA, et al. Much of late life cognitive decline is not due to common neurodegenerative pathologies. Ann Neurol. 2013 doi: 10.1002/ana.23964. http://dx.doi.org/10.1002/ana.23964. [DOI] [PMC free article] [PubMed]
- 47.Brayne C, et al. Education, the brain and dementia: neuroprotection or compensation? EClipSE Collaborative Members. Brain. 2010;133:2210–2216. doi: 10.1093/brain/awq185. [DOI] [PubMed] [Google Scholar]
- 48.Wenger E, et al. Cortical thickness changes following spatial navigation training in adulthood and aging. Neuroimage. 2012;59:3389–3397. doi: 10.1016/j.neuroimage.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 49.Mandolesi L, et al. Environmental enrichment provides a cognitive reserve to be spent in the case of brain lesion. J Alzheimer Dis. 2008;15:11–28. doi: 10.3233/jad-2008-15102. [DOI] [PubMed] [Google Scholar]
- 50.Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Sci. 2008;17:177–182. [Google Scholar]
- 51.Lövdén M, et al. A theoretical framework for the study of adult cognitive plasticity. Psychol Bull. 2010;136:659–676. doi: 10.1037/a0020080. [DOI] [PubMed] [Google Scholar]
- 52.Shing YL, et al. Associative and strategic components of episodic memory: a lifespan dissociation. J Exp Psychol Gen. 2008;137:495–513. doi: 10.1037/0096-3445.137.3.495. [DOI] [PubMed] [Google Scholar]
- 53.Ilg R, et al. Gray matter increase induced by practice correlates with task-specific activation: a combined functional and morphometric magnetic resonance imaging study. J Neurosci. 2008;28:4210–4215. doi: 10.1523/JNEUROSCI.5722-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steffener J, et al. The impact of structure on age-related changes in working memory functional activity. Brain Imaging Behav. 2008;3:142–153. doi: 10.1007/s11682-008-9056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valenzuela MJ, Sachdev P. Assessment of complex mental activity across the lifespan: development of the Lifetime of Experiences Questionnaire (LEQ) Psychol Med. 2007;37:1015–1026. doi: 10.1017/S003329170600938X. [DOI] [PubMed] [Google Scholar]
- 56.Katzman R. Education and the prevalence of dementia and Alzheimer’s disease. Neurology. 1993;43:13–20. doi: 10.1212/wnl.43.1_part_1.13. [DOI] [PubMed] [Google Scholar]
- 57.Barulli DJ, et al. The influence of cognitive reserve on strategy selection in normal aging. J Int Neuropsychol Soc. 2013;19:1–4. doi: 10.1017/S1355617713000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stern Y, et al. Task difficulty modulates young-old differences in network expression. Brain Res. 2012;1435:130–145. doi: 10.1016/j.brainres.2011.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koenen KC, et al. Childhood IQ and adult mental disorders: a test of the cognitive reserve hypothesis. Am J Psychiatry. 2009;166:50–57. doi: 10.1176/appi.ajp.2008.08030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11:1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Erickson K, Kramer AF. Aerobic exercise effects on cognitive and neural plasticity in older adults. Br J Sports Med. 2009;43:22–24. doi: 10.1136/bjsm.2008.052498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith PJ, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72:239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Basak C, et al. Can training in a real-time strategy video game attenuate cognitive decline in older adults? Psychol Aging. 2008;23:765–777. doi: 10.1037/a0013494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stern Y, et al. Space Fortress game training and executive control in older adults: a pilot intervention. Neuropsychol Dev Cogn. 2011;18:653–677. doi: 10.1080/13825585.2011.613450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Owen AM, et al. Putting brain training to the test. Nature. 2010;465:775–778. doi: 10.1038/nature09042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jaeggi SM, et al. Improving fluid intelligence with training on working memory. Proc Natl Acad Sci U S A. 2008;105:6829–6833. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Edwards JD, et al. The longitudinal impact of cognitive speed of processing training on driving mobility. The Gerontologist. 2009;49:485–494. doi: 10.1093/geront/gnp042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lovden M, et al. Experience-dependent plasticity of white-matter microstructure extends into old age. Neuropsychologia. 2010;48:3878–3883. doi: 10.1016/j.neuropsychologia.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 69.Engvig A, et al. Memory training impacts short-term changes in aging white matter: a longitudinal diffusion tensor imaging study. Hum Brain Mapp. 2012;33:2390–2406. doi: 10.1002/hbm.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Engvig A, et al. Effects of memory training on cortical thickness in the elderly. Neuroimage. 2010;52:1667–1676. doi: 10.1016/j.neuroimage.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 71.Scholz J, et al. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Colom R, et al. Structural changes after videogame practice related to a brain network associated with intelligence. Intelligence. 2012;40:479–489. [Google Scholar]
- 73.Takeuchi H, et al. Training of working memory impacts structural connectivity. J Neurosci. 2010;30:3297–3303. doi: 10.1523/JNEUROSCI.4611-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schmidt-Wilcke T, et al. Distinct patterns of functional and structural neuroplasticity associated with learning Morse code. Neuroimage. 2010;51:1234–1241. doi: 10.1016/j.neuroimage.2010.03.042. [DOI] [PubMed] [Google Scholar]
- 75.Kwok V, et al. Learning new color names produces rapid increase in gray matter in the intact adult human cortex. Proc Natl Acad Sci U S A. 2011;108:6686–6688. doi: 10.1073/pnas.1103217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sagi Y, et al. Learning in the fast lane: new insights into neuroplasticity. Neuron. 2012;73:1195–1203. doi: 10.1016/j.neuron.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 77.Steffener J, Stern Y. Exploring the neural basis of cognitive reserve in aging. Biochim Biophys Acta. 2012;1822:467–473. doi: 10.1016/j.bbadis.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schneider-Garces NJ, et al. Span, CRUNCH, and Beyond: Working Memory Capacity and the Aging Brain. J Cogn Neurosci. 2009;22:655–669. doi: 10.1162/jocn.2009.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Davis SW, et al. Que PASA? The posterior-anterior shift in aging. Cereb Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Berlingeri M, et al. Reassessing the HAROLD model: is the hemispheric asymmetry reduction in older adults a special case of compensatory-related utilisation of neural circuits? Exp Brain Res. 2013;224:393–410. doi: 10.1007/s00221-012-3319-x. [DOI] [PubMed] [Google Scholar]
- 81.Rossi S, et al. Age-related functional changes of prefrontal cortex in long-term memory: a repetitive transcranial magnetic stimulation study. J Neurosci. 2004;24:7939–7944. doi: 10.1523/JNEUROSCI.0703-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Steffener J, et al. Supporting performance in the face of age-related neural changes: testing mechanistic roles of cognitive reserve. Brain Imaging Behav. 2011;22:655–669. doi: 10.1007/s11682-011-9125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stern Y, et al. A common neural network for cognitive reserve in verbal and object working memory in young but not old. Cereb Cortex. 2008;18:959–967. doi: 10.1093/cercor/bhm134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garrett DD, et al. Everyday memory compensation: the impact of cognitive reserve, subjective memory, and stress. Psychol Aging. 2010;25:74–83. doi: 10.1037/a0017726. [DOI] [PubMed] [Google Scholar]
- 85.Uttner I, et al. Reduced benefit from mnemonic strategies in early-stage Alzheimer’s disease: a brief testing-the-limits paradigm for clinical practice. J Neurol. 2010;257:1718–1726. doi: 10.1007/s00415-010-5610-8. [DOI] [PubMed] [Google Scholar]
- 86.Woods SP, et al. Spontaneous strategy use protects against visual working memory deficits in older adults infected with HIV. Arch Clin Neuropsychol. 2010;25:724–733. doi: 10.1093/arclin/acq069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Czernochowski D, et al. Use it or lose it? SES mitigates age-related decline in a recency/recognition task. Neurobiol Aging. 2008;29:945–958. doi: 10.1016/j.neurobiolaging.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boyle PA, et al. Poor decision making is associated with an increased risk of mortality among community-dwelling older persons without dementia. Neuroepidemiology. 2013;40:247–252. doi: 10.1159/000342781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wagner A, et al. Brain plasticity: to what extent do aged animals retain the capacity to coordinate gene activity in response to acute challenges. Exp Gerontol. 2000;35:1211–1227. doi: 10.1016/s0531-5565(00)00154-6. [DOI] [PubMed] [Google Scholar]
- 90.Bloss EB, et al. Evidence for reduced experience-dependent dendritic spine plasticity in the aging prefrontal cortex. J Neurosci. 2011;31:7831–7839. doi: 10.1523/JNEUROSCI.0839-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Amieva H, et al. The 9 year cognitive decline before dementia of the Alzheimer type: a prospective population-based study. Brain. 2005;128:1093–1101. doi: 10.1093/brain/awh451. [DOI] [PubMed] [Google Scholar]
- 92.Hall CB, et al. Education delays accelerated decline on a memory test in persons who develop dementia. Neurology. 2007;69:1657–1664. doi: 10.1212/01.wnl.0000278163.82636.30. [DOI] [PubMed] [Google Scholar]
- 93.Stern Y, et al. Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer’s disease. Ann Neurol. 1992;32:371–375. doi: 10.1002/ana.410320311. [DOI] [PubMed] [Google Scholar]
- 94.Stern Y, et al. Rate of memory decline in AD is related to education and occupation: Cognitive reserve? Neurology. 1999;53:1942–1957. doi: 10.1212/wnl.53.9.1942. [DOI] [PubMed] [Google Scholar]