Abstract
Suicide and bipolar disorder (BD) are challenging, complex, and intertwined areas of study in contemporary psychiatry. Indeed, BD is associated with the highest lifetime risk for suicide attempt and completion of all the psychiatric conditions. Given that several clinical risk factors for both suicide and BD have been well noted in the literature, exploring the neurobiological aspects of suicide in BD may provide insights into both preventive measures and future novel treatments. This review synthesizes findings regarding the neurobiological aspects of suicide and, when applicable, their link to BD. Neurochemical findings, genes/epigenetics, and potential molecular targets for current or future treatments are discussed. The role of endophenotypes and related proximal and distal risk factors underlying suicidal behavior are also explored. Lastly, we discuss the manner in which preclinical work on aggression and impulsivity may provide additional insights for the future development of novel treatments.
Keywords: Suicide, Suicidal behavior, Bipolar disorder, Glutamate, Neurobiology, Depression, Treatment
Introduction
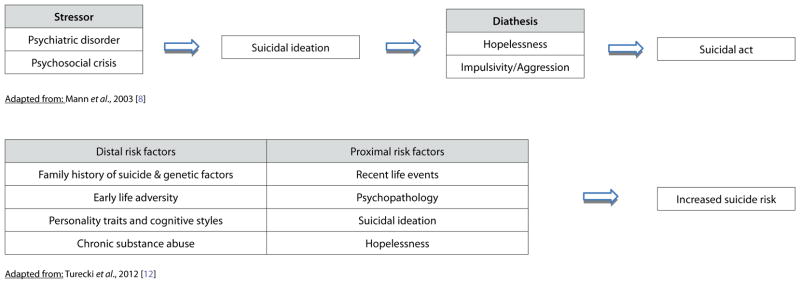
Of all the psychiatric conditions, bipolar disorder (BD) is associated with the highest lifetime risk for suicide attempt and completion [1]. Indeed, the lifetime risk of completing suicide in patients with BD ranges from 8–20%, nearly 10–20 times that of the U.S. general population [2]. Strikingly, between 25% and 56% of patients with BD attempt suicide; many of those who survive experience resulting morbidity and, on average, have shorter life expectancies [3,4]. The World Health Organization’s Global Burden of Disease study ranked BD seventh among all medical disorders with regard to years of life lost to death or disability [5]. Although several clinical risk factors for suicide have been noted in the literature [6,7], most research in this area has focused on the stress-diathesis model of suicidal behavior [8–11] and on evaluating potential proximal and distal risk factors [12] (see Tables 1 and 2). Nevertheless, suicidal behavior remains extremely difficult to predict even when comprehensive clinical and neurocognitive information is available [13]. Thus, understanding the neurobiological aspects that underlie suicidal behavior may provide important insights into more effective preventive measures and/or future novel treatments.
Table 1.
Clinical variables significantly associated with suicidal behavior in patients with bipolar disorder.
| Risk factors | Adjusted odds ratio |
|---|---|
| Female gender | 2.08 |
| History of alcohol abuse | 2.05 |
| History of substance abuse | 1.99 |
| Current benzodiazepine use | 1.59 |
| Higher overall symptom severity | 1.45 |
| Treatment non-adherence | 1.40 |
| Greater depressive symptom severity baseline | 1.09 |
| Longer disease duration | 1.02 |
| Young age at first treatment for mood episode | 0.98 |
Adapted from: Bellivier et al., 2011 [7]
Study design: Two-year, prospective, observational study that enrolled 3,684 adult patients with bipolar disorder and initiated or changed oral treatment for an acute manic/mixed episode
Table 2.
Suicide models.
|
The concept of suicide as a neurobiological phenomenon emerged in the mid-1960s in response to a seminal study that noted increased mean urinary levels of 17-hydoxycorticosteroid (a metabolic product of cortisol) in depressed patients who went on to commit suicide [14]. Since then, many studies have explored the neurobiological underpinnings of suicide using a variety of approaches, including post-mortem brain tissue analysis, changes in cerebrospinal fluid (CSF), and alterations in peripheral markers in suicide completers compared to matched control groups. Each of these methods is associated with significant limitations and confounders. In postmortem studies, such confounders include prior medication exposure status, proxy-based psychiatric diagnoses, integrity of the samples, variability in postmortem interval, and co-morbid substance abuse. Similarly, the limitations of using in vivo samples include the temporal relationship between the time of the suicide attempt and the point of collection, and the inability to establish the presence or lack of suicidal ideation in attempters. Despite limitations, post-mortem methods are still valuable for in-depth molecular and cellular analyses not otherwise possible in living subjects [15].
Table 3 highlights findings from several excellent reviews that detail neurobiological alterations associated with suicide/suicidal behavior [8,9,12,16–20]. It is beyond the scope of this review to explore all of these topics; instead, this article selectively discusses those findings and clinical trials that clinicians may find of greatest value. It should be noted that, across the literature, many of these findings are inconsistent, and not all are specific to BD. However, it has been argued that the vast majority of suicide attempters and victims suffered from an underlying mood disorder at the time of the attempt [21,22]. As a result, these findings may very well be relevant or have overlapping involvement in BD, and positive findings may ultimately lead to new targets and enhance subsequent drug discovery efforts [23].
Table 3.
Summary of putative targets explored in the neurobiology of suicide.
| Neurotransmitters | Examples of findings in suicide victims/Attempters compared to controls | Citation |
|---|---|---|
| Serotonin | Decreased 5-HIAA levels in CSF; decreased serotonin uptake and transporter levels in platelets; blunting of fenfluramine-induced prolactin response; increased 5-HT1A and 5-HT2A receptor binding and expression in PFC; increased TPH immunoreactive neurons in DRN, 5-HTT promoter | [24,25,28,31,32,67,120–129] |
| Norepinephrine/Adrenaline | Lower urinary and plasma MHPG (major metabolite of NE); MHPG levels negatively correlated with lethality of attempts; fewer noradrenergic neurons in LC; increase in α2-adrenergic receptor densities and mRNA expression in hypothalamus and frontal cortex; increased β-adrenergic binding | [33,35,130,131] [34,132–135] |
| Dopamine | Lower CSF HVA; lower urinary HVA, DOPAC, and dopamine | [136,137] |
| Glutamate and glial involvement | Density of AMPA receptors may be increased in caudate nucleus; downregulation of SLC1A3 and GLUL and other differential gene expression; elevated microglial density; alteration in NMDA receptor complex in frontal cortex | [138,139–143] |
| GABA | Increased density of GABA neurons in hippocampus/neocortical areas; upregulation of GABAAα5 in frontal cortex; increase in relative density of GAD-ir neuropil in hippocampal formation; differential gene expression | [139,141,142,144,145] |
| Opioids | Higher density of μ-opioid receptors in frontal and temporal cortex | [146] |
| Acetylcholine | Increased [3H]-pirenzepine binding of M1 and M4 receptor binding in ACC | [147] |
|
| ||
| Cell signaling | ||
|
| ||
| G-proteins | Lower basal and GTPγS-simulated AC activity; decreased GTPγS-stimulated PI hydrolysis and decreased GSα protein expression in BA 10; decreased levels of Gi2α and GOα and increased levels of GSα-S in PFC | [148–150] |
| Phospholipase C (PLC) | Decreased protein expression of isoenzymes in PFC | [151] |
| Protein Kinase C (PKC) | Decreased PKC activity in PFC | [152–154] |
| Protein Kinase A (PKA) | Decreased protein and mRNA expression of PKA subunits of PFC | [155,156] |
| CREB | Decreased protein and mRNA expression in PFC and hippocampus | [73,74] |
| BDNF and Trk-B receptor | Decreased mRNA expression of BDNF and Trk-B isoforms noted in postmortem brain | [75,157] |
| Stress systems | ||
| HPA Axis | Decreased CRF receptors and altered CRF receptor type ratios; higher POMC mRNA density; increased levels of CRF immunoreactivity; DST-non-suppressors more likely to commit and complete suicide; increased adrenal weight; altered GR mRNA expression | [42,43,46,158–165] |
| Polyamines | SSAT downregulation in frontal cortex; upregulation of ARG2, AMD1, OAZ1, and OAZ2 (roles in polyamine biosynthesis) | [166,167] |
| Cytokines | Increased mRNA expression of IL-4 in PFC of females and increased IL-13 in males; increased IL-6 levels in CSF; elevated quinolinic acid in CSF of suicide attempters | [116,168,169] |
| Testosterone | Testosterone levels positively correlate with number of manic episodes and number of suicide attempts | [61] |
|
| ||
| Others | ||
|
| ||
| Lipid metabolism | Lower levels of serum cholesterol and triglycerides; increased suicidal behaviors in Smith-Lemli-Opitz syndrome; lower serum concentrations of the n-6, arachidonic acid (AA) in subjects with suicide attempts | [55,57,58,170,171] |
| Epigenetics | Increased cytosine methylation of NR3C1 glucocorticoid receptor promoter; increased DNMT 3b mRNA expression; increased histone methylation; increased BDNF promoter methylation in Wernicke area | [76,164,172,173] |
| Cognitive alterations | Impaired decision making (Iowa Gambling Task); increased attentional bias toward suicide-related stimuli; impairments in neuropsychological tasks (e.g. attention/memory) | [174–178] |
Abbreviations:
CSF – cerebrospinal fluid; 5-HIAA - 5-Hydroxyindoleacetic acid; TPH – tryptophan hydroxylase; DRN – dorsal raphe nucleus; 5-HTT – serotonin transporter; NE – norepinephrine; MHPG - 3-Methoxy- 4-hydroxyphenylglycol; LC – locus coeruleus; mRNA – messenger ribonucleic acid; HVA – homovanillic acid; DOPAC – dihydroxyphenylacetic acid; AMPA - α-amino-3-hydroxy-5-methyl-4- isoxazolepropionic acid; GABA - gamma-aminobutyric acid; GAD – glutamic acid decarboxylase; ir – immunoreactive; M1 and M4 – muscarinic acetylcholine receptors; ACC – anterior cingulate cortex; CREB - cAMP response element-binding protein; GTP – guanosine triphosphate; AC – adenylyl cyclase; PI – Phosphatidylinositol; BA – Brodmann area; PFC – prefrontal cortex; BDNF - brain-derived neurotrophic factor; Trk-b – tyrosine related kinase; HPA – hypothalamic-pituitary-adrenal; DST - dexamethasone suppression test; CRF - corticotropin-releasing factor; GR- glucocorticoid receptor; POMC - pro-opiomelanocortin; ARG2 - arginase II; AMD1 - S-adenosylmethionine decarboxylase; OAZ1 and OAZ2 - antizymes 1 and 2; SSAT - Spermidine/spermine N1-acetyltransferase; IL – interleukin; SLC1A3 - solute carrier family 1 (glial high affinity glutamate transporter) member; GLUL – glutamate-ammonia ligase; NR3C1 - neuron-specific glucocorticoid receptor; DNMT - DNA methyltransferase
Neurochemical findings in target systems
Decades of research have documented abnormalities in the hypothalamic pituitary adrenal (HPA) axis as well as the serotonergic, dopaminergic, and noradrenergic systems with regard to the neurobiology of suicide. These systems are integral to stress response, and their altered functioning may be influenced by genetic, epigenetic, and/or adverse life events [18].
Serotonergic system
As regards the serotonergic system, depressed patients with suicidal behavior were found to have significantly lower CSF levels of the serotonin metabolite 5-hydroxyindoleacetic acid (5-HIAA) than depressed patients without suicidal behavior or controls [24–26]. Low CSF 5-HIAA levels were also shown to predict future suicide attempts and completions [27,28]. Interestingly, lower levels of the same metabolite were shown to correspond with the lethality of the suicide attempt [29]. Similarly, low serotonergic function was observed in suicide attempters with major depressive disorder (MDD), as indicated by a blunted prolactin response to challenge dosing with a serotonin reuptake inhibitor, fenfluramine [30]. Among the many serotonin (5-HT) receptor subtypes, extensive postmortem studies implicate 5-HT2A receptors [19]. Early studies found increased 5-HT2A binding in suicide victims compared to controls [31], and subsequent studies similarly showed patterns of increased protein expression of 5-HT2A in the prefrontal cortex (PFC) and hippocampus in suicide victims [32]. It thus appears that post-synaptic serotonin receptor upregulation reflected in increased gene expression may be a compensatory response to reduced serotonin neuronal activity [32].
Noradrenergic system
Fewer noradrenergic neurons were found in the loci cerulei of suicide victims with MDD [33], and increased β-adrenergic receptor binding was found in the PFC of suicide victims [34]. In addition, lower urinary and plasma levels of 3-methoxy-4-hydroxyphenylglycol (MHGP), a major metabolite of norepinephrine, were found in patients displaying suicidal behaviors compared with controls [35]. Another study found that the lethality of suicide attempts in BD patients negatively correlated with MHGP levels [36].
Dopaminergic system
Few post-mortem studies examining the dopaminergic system have shown conclusive trends; however, one study demonstrated higher cortical levels of homovanillic acid (HVA) (dopamine’s primary metabolite) in suicide and homicide victims compared to those who died of physical disease, but not accident victims [37]. High CSF levels of HVA have also been shown to correlate with human aggression [38].
HPA system
With respect to the HPA axis, considerable evidence suggests that early-life adverse events can produce enduring changes in the regulation of stress-response systems in humans [39,40]. In addition, the HPA axis has bidirectional relationships with the neurotransmitter systems discussed above [41]. As a result, many investigators have examined components of the HPA axis in suicide victims. For example, one postmortem study identified a significant decrease in the number of corticotropin releasing factor (CRF) receptor binding sites in suicide completers compared to controls [42]. Similarly, increased CRF immunoreactivity was found in the frontal cortex of suicide victims [43]. A recent study also demonstrated that BD patients with a past history of attempted suicide had bedtime salivary cortisol levels 7.4% higher than unaffected healthy controls [44]. BD alone was not associated with a cortisol increase, suggesting a role for stress dysregulation in mediating the high suicide risk in BD.
Interestingly, the dexamethasone suppression test (DST), a measure used to assess HPA axis functioning, showed that non-suppressors were more likely to commit and complete suicide than DST suppressors over long-term follow-up (e.g. 15 years) [45–47]. A recent meta-analysis of seven studies concluded that DST non-suppression increased suicide risk by a factor of 4.6 [48]. The DST has also been explored as a potential biological marker for depression [49,50] and, indeed, DST non-suppression has been associated with severity and poorer prognosis in major depressive episodes, suggesting that it may be a potential state-dependent marker for suicide risk [8]. DST non-suppression has also been found more often in the depressed phase of BD compared to patients with MDD [51]. For additional neurobiological findings regarding HPA dysfunction and suicidal behavior, the interested reader is referred to an excellent review by Turecki and colleagues [12].
Other hormonal systems: cholesterol and testosterone
The link between lipids and suicidal behavior was investigated after large randomized clinical trials and other meta-analyses revealed an increase in violence-related activities— including suicide [52,53]—in patients taking cholesterol-lowering medications. Specifically, clinical studies suggested a relationship between reduced total cholesterol levels and suicidal behavior [54–56]. One recent study found lower serum cholesterol and triglyceride levels in men with BD who attempted suicide compared with BD men who had not [57]. Additional support for this potential biological marker comes from a study noting that the biological relatives of Smith-Lemli-Opitz syndrome carriers—an autosomal recessive condition characterized by abnormally low cholesterol levels resulting from mutations in the genes involved in cholesterol biosynthesis—had an increased number of suicide attempts and completions compared to controls [58]. Low cholesterol levels have been associated with decreased serotonergic activity [59] and lower cholesterol in the brain, which may lead to reduced synaptic plasticity and brain dysfunction [60]. As a result, Smith-Lemli-Opitz patients and carriers may be important sub-groups for further study with regard to suicide neurobiology.
A recent exploratory study in male and female BD patients experiencing a depressive or mixed episode who had at least one past suicide attempt found that testosterone levels were positively correlated with both the number of previous manic episodes and with suicide attempts [61]. Notably, cholesterol serves as a precursor to testosterone and other hormones such as cortisol and estrogen. While the relationship between serum testosterone levels and suicidal behavior is not consistent [62], testosterone and other androgens are believed to be involved in the pathophysiology of mood disorders and suicidal behavior [63,64].
Genetics and epigenetics
Between twins, heritability estimates for suicide range from 21–50%, and 30–55% for suicidal behavior (attempts, suicidal ideation, planning) [65]. Both linkage and association studies can be used to identify relevant genes and pathways associated with suicide. While it is beyond the scope of this review to offer a comprehensive overview of this large and rapidly growing field, it should be noted that several new technologies are powering this research, including emerging microarray technologies and functional genomic studies that can profile the expression of thousands of genes and perform genome-wide arrays for thousands of single nucleotide polymorphisms (SNPs) [66].
Genetics
An earlier systemic review of association studies evaluating suicidal behavior and genes coding for serotonin receptors and for the serotonin transporter gene (5-HTT) noted a significant, robust association with the 5-HTT promoter locus, but not for the 5-HT2A 102 T/C variant. The review, which included a meta-analysis of nearly 12 studies (n=1599), found a significant association between the 5-HTTLPR low-expressing S allele and suicidal behavior [67]. Other candidate genes have included tryptophan hydroxylase (TPH 1 and TPH 2), catechol-O-methyltransferase (COMT), and nitric oxide synthase types I and III (NOS I and NOS III) [68–70]. A recent analysis of genome-wide association studies of BD-I and BD-II, as well as MDD, found that the strongest association for suicide attempts in BD was observed in a region without identified genes (rs1466846). While five other loci showed suggestive associations, overall results suggested that the inherited risk for suicide in mood disorders is unlikely to be secondary to individual common variants of large effect [71].
Candidate genes for association studies in suicide have generally been drawn from prior neurobiologic studies that identified many of the systems described above as putative targets; in addition, areas related to neurotrophins and cell signaling systems are now also being investigated [72]. A recent review by Pandey and colleagues discussed the role of key transcription factors and target genes involved in suicide [19]. Most studies discussed used postmortem brain samples, and results broadly implicate serotonin subtype receptor abnormalities, along with variations in G proteins, the effector phospholipase C (PLC), and protein kinases A and C (PKA and PKC) (see Table 3) [19]. PKC activates the transcription factor cyclic adenosine monophosphate response element-binding protein (CREB) that, in turn, regulates the expression of target plasticity genes such as brain derived neurotrophic factor (BDNF). Interestingly, studies have shown decreased CREB and BDNF (along with tyrosine related kinase (TrkB) receptors) in the postmortem brains of suicide victims [73–75].
Epigenetics
The study of epigenetics, a relatively new branch of molecular biology, may provide insight into the way the environment influences gene expression by examining phenotypic changes not coded by DNA. Common epigenetic mechanisms include DNA methylation, histone acetylation/deacetylation, and phosphorylation. One recent study reported epigenetic alterations in suicide victims; DNA methyltransferase (DNMT) 3b (an enzyme that de novo methylates CpG islands) had increased expression in the frontopolar cortex of suicide completers compared to controls [76]. Similarly, another study implicated altered TrkB expression in epigenetic processes related to suicide [75]. However, caution must be employed in assuming that epigenetic changes are involved in particular disease/outcome states, as this field is still evolving; more knowledge is required to understand how these alterations are mediated [77].
Endophenotypes and potential medication targets
Suicide endophenotypes: impulsivity and aggression
The concept of candidate suicide endophenotypes is also being used to explore the neurobiological basis of suicide [78–81]. Prominent researchers have defined an endophenotype as a “measurable component along the pathway between disease and distal genotype” [82]. Endophenotypes include neurophysiological, biochemical, and neuropsychological constructs, where heritability and stability (state independence) represent key components of an ideal marker [78]. In particular, impulsivity and aggression have been well studied candidate endophenotypes associated with suicidal behavior [79]. Other endophenotypic approaches include studying impaired decision-making, altered skin conductance, and response to functional neuroimaging paradigms [80]. Overall, these constructs could be used in conjunction with other endophenotypes already explored in BD, including abnormal regulation of circadian rhythms, response to certain medication challenges, cognitive deficits, and neuroimaging findings (e.g. early-onset white matter abnormalities) [83,84] to better predict suicidal behavior.
With respect to BD patients, a recent regression analysis showed that lifetime aggressive traits were associated with past suicide attempts [85]. Interestingly, previous studies have suggested that lithium may have strong anti-aggressive properties in humans [86,87] and anti-impulsivity properties in preclinical models [88–90]. Notably, of all current treatments for BD, long-term lithium therapy is the only intervention associated with decreased rates of suicidal behavior and mortality in well-conducted meta-analytic reviews [91–93]. Putative molecular targets for lithium include cyclic adenosine monophosphate (cAMP)-mediated signal transduction, CREB activation, increased BDNF expression, the phosphatidylinositol cascade, PKC inhibition, glycogen synthase kinase 3 (GSK-3) inhibition, and B-cell lymphoma 2 (Bcl-2) expression [94]. Complementary work has also been undertaken to develop relevant animal models of suicide (e.g. shock-induced aggression) in order to test potentially protective pharmaceutical compounds [78,95].
Nevertheless, a recent, randomized, double-blind trial evaluating lithium compared to valproate detected no difference between treatments with regard to time to suicide attempt or suicide event [96]. However, it should be noted that given the challenge of recruiting very ill BD patients for research, the authors were not able to meet their target enrollment; as a result, the study was significantly underpowered to detect suicide deaths or suicide attempts. Similarly, the study may not have been enriched for impulsive suicidal individuals. These findings should not discourage practitioners from using lithium as a first-line agent in BD; indeed, a recent editorial highlights the ethical, logistic, and feasibility hurdles involved in conducting such “hard outcome” clinical studies [97]. Table 4 summarizes other valuable randomized controlled studies that evaluated suicide or suicidal behavior as the primary outcome in their clinical trials. In addition to lithium, clozapine—although primarily used to treat schizophrenia—has also been shown to reduce suicidal behavior [98,99]. With regard to clozapine’s putative mechanism of action, one animal study found that clozapine inhibits system A-mediated glycine transport in cortical synaptosomes [100]. System A transporters may play a role in regulating synaptic glycine levels and provide an indirect mechanism for clozapine to potentiate N-methyl-D-aspartate (NMDA) receptor mediated neurotransmission [100].
Table 4.
Summary of randomized medication trials evaluating suicidal ideation/Behavior as a primary outcome.
| Study | Design/Sample | Primary measures | Results | Citations |
|---|---|---|---|---|
| Grunebaum et al. (2012) | DB, RCT n=74, paroxetine (max 50 mg/d) vs. bupropion (max 450 mg/d), MDD pts with hx of suicide attempt or SI, 16 wks | Suicidal attempt classification by weekly consensus; suicidal events by Columbia Suicide History Form; SSI | Depressed patients with greater baseline SI treated with paroxetine compared to bupropion appeared to experience greater acute improvement in suicidal ideation, after adjusting for global depression | [179] |
| Oquendo et al. (2011) | DB, RCT, n=98, BD with past suicide attempts, lithium vs. valproate, 2.5 yrs | Time to suicide completion; time to suicide attempt; time to suicide event; SSI | Intent-to-treat showed no differences between treatment groups in time to suicide attempt or to suicide event | [96] |
| Khan et al. (2011) | DB, RCT, parallel group; MDD; citalopram (20 mg/d) + placebo vs. citalopram + lithium (300 mg/d), n=80, 4 wks | At screening and trial end: suicidal thoughts/behaviors; S-STS; MADRS; C-SSRS | No significant differences in primary outcome measures at 4 wks; post-hoc analysis showed patients assigned to citalopram + therapeutic lithium had significantly higher S-STS remission rates. | [180] |
| Rucci et al. (2011) | Two-site, RCT, MDD, allocated to IPT or SSRI, n=291, 4 months | SI; Suicidality items from HDRS and QIDS | Time to suicidal ideation was significantly longer in patients allocated to SSRI compared to those allocated to IPT, even after controlling for treatment augmentation, benzodiazepine use, and co-morbidity with anxiety disorders | [181] |
| Lauterbach et al. (2008) | DB, RCT, pts with recent suicide attempts (<3 months), treatment with lithium or placebo, n =167, 12 months | Suicide attempt; SSI | Survival analysis showed no significant difference of suicidal acts between lithium and placebo. Post-hoc analysis revealed that all completed suicides had occurred in the placebo group, accounting for a significant difference in incidence rate | [182] |
| Reeves et al. (2008) | DB, RCT, placebo-controlled, n=24, MDD on antidepressant receiving risperidone (0.25 mg – 2 mg/d) vs. placebo, 8 wks | Severity of suicidality; SSI | Risperidone significantly reduced SI in MDD patients and the overall effect of risperidone appeared to be superior to placebo. The onset of effect was within 2 weeks of treatment and sustained along the course of the treatment. | [183] |
| Lauterbach et al. (2005) | DB, RCT, placebocontrolled multi-center trial evaluating proposed suicide preventive effects of lithium in patients with suicidal behavior | Number of suicide attempts or completed suicides; SIS; Medical Damage Scale; Risk-Rescue Scale; SSI | SUPLI-Study terminated because number of enrolled individuals after 5 years was still below necessary estimated sample size | [184] |
| Meltzer et al. (2003) | Multicenter, RCT, international, clozapine vs. olanzapine, n = 980 schizophrenia or schizoaffective disorder, 2 yrs | Suicide attempts/completion; hospitalizations to prevent suicide; rating of “much worsening of suicidality” from baseline; CGI-SS | Clozapine therapy was superior to olanzapine therapy in preventing suicide attempts in patients with schizophrenia and schizoaffective disorder at high risk for suicide | [99] |
| Verkes et al. (1998) | DB, RCT, paroxetine (40 mg/day) vs. placebo in 91 patients who had recently attempted suicide for at least a second time, 1 yr | Suicide attempt; self-rating scales for depressive symptoms, anger; Axis II diagnoses | With adjustment for the number of previous suicide attempts, paroxetine showed significant efficacy in the prevention of recurrent suicidal behavior | [185] |
Abbreviations:
Hx – history; Tx – treatment; MADRS - Montgomery-Asberg Depression Rating Scale; HDRS – Hamilton Rating Scale for Depression; QIDS – Quick Inventory of Depressive Symptomatology; DB – double blind; RCT – randomized controlled trial; MDD – major depressive disorder; BD – bipolar disorder; SI – suicidal ideation; SSRI – selective serotonin reuptake inihibitor; IPT – interpersonal therapy; SSI - scale for suicide ideation; S-STS - Sheehan-Suicidality Tracking Scale; C-SSRS - Columbia Suicide Severity Rating Scale; SIS – Suicide Intent Scale; SUPLI - Suicide Prevention by Lithium - the Lithium Intervention Study; CGI-SS- Clinical Global Impression of Suicide Severity
Finally, studying other syndromes with features that contribute to suicide risk (e.g. increased impulsivity) may generate other neurobiologic targets. For example, children affected with Lesch-Nyhan syndrome, a rare inherited condition characterized by overproduction and accumulation of uric acid, will often have an irresistible urge to hurt themselves (e.g. biting, head banging). Interestingly, allopurinol, a xanthine oxidase inhibitor used to treat hyperuricemia has been shown to be effective in treating mania [101–103]. Impulsivity in mania is pervasive, known to affect attention and behavioral inhibition, and can often lead to suicide attempts [104]. Interestingly, an experimental D1 dopamine receptor antagonist is currently being evaluated to relieve self-injurious behavior in patients with Lesch-Nyhan syndrome (NCT01065558). Table 5 highlights potential medication targets for decreasing suicidal behavior.
Table 5.
Clinical compounds implicating molecular targets for suicidal behavior.
| Drug compound | Target | Clinical findings | Citations |
|---|---|---|---|
| Lithium | cAMP mediated signal transduction; CREB activation; BDNF; PI cascade; PKC inhibition; GSK-3 inhibition; Bcl-2 expression | Mood stabilizing and anti-depressant effects; thought to also target impulsivity and aggression (oral formulation) | [94,186] |
| Ketamine | mTOR; eEF2; BDNF; Arc; GSK-3 inhibition | Rapid antidepressant and antisuicidal effects (IV) | [111,112] |
| TRH | HPT axis | Rapid antidepressant effect (IV) and antisuicidal effects (IT) | [114,187] |
| Scopolamine | Muscarinic receptors; NMDA receptor expression; mTOR | Rapid antidepressant effects (IV); sub-analysis with suicide item score decreased in clinical rating scales | [188,189] |
| Allopurinol | Purinergic system | Shown to have efficacy in bipolar mania; may reduce impulsivity associated with suicidal behavior | [190,191] |
Abbreviations:
cAMP: cyclic adenosine monophosphate; CREB: cAMP response element binding protein; BDNF: brain-derived neurotrophic factor; PI: phosphatidylinositide; PKC: protein kinase C; GSK-3: glycogen synthase kinase 3; Bcl-2: B-cell lymphoma 2; eEF2: eukaryotic elongation factor; TRH: thyrotropin releasing hormone; Arc: activity-regulated cytoskeletal associated protein; HPT: hypothalamic pituitary axis; NMDA: N-methyl-D-aspartate; IV: intravenous; IT: intrathecal; mTOR: mammalian target of rapamycin.
Novel drug developments
Ketamine
Remarkably, a recent experimental therapeutic has also demonstrated promising anti-suicidal effects. An open-label study found that a low, intravenous dose of the NMDA antagonist ketamine (0.5 mg/kg)—previously shown to have rapid antidepressant effects in randomized controlled trials of MDD [105] and bipolar depression [106]—rapidly (within 40 minutes) and significantly decreased suicidal ideation scores. This effect remained significant for four hours post-infusion [107]. Another study found that ketamine was associated with reductions in the suicidality item score in the Montgomery-Asberg Depression Rating Scale (MADRS) scale and with other implicit suicidal associations within 24 hours after a single infusion [108]. Another recent trial that replicated ketamine’s antidepressant effect in bipolar depression also found that this agent rapidly improved suicidal ideation [109]. In addition, a recent naturalistic study with low-dose ketamine (IV bolus 0.2mg/kg over one to two minues) in depressed emergency room patients with acute suicidal ideation found significant improvements in suicidal ideation that lasted up to 10 days post-infusion [110]. A large, randomized, double-blind trial evaluating ketamine for suicidal ideation is currently underway (NCT01507181).
Mechanisms underlying ketamine’s rapid antidepressant effects have implicated costimulation (along with NMDA receptors) of α-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA) ionotropic receptors and several downstream signaling cascades including the serine/threonine protein kinase mammalian target of rapamycin (mTOR) pathway and GSK-3. Ketamine’s mediated blockade of NMDA receptors has also been shown to deactivate eukaryotic elongation factor (eEF2), leading to reduced eEF2 phosphorylation and de-suppression of rapid dendritic protein translation (including BDNF) [111]. Ketamine similarly activates cytoskeleton-associated protein (Arc), which is involved in actin polymerization and stable expansion of dendritic spines. These dynamic processes overall contribute to the synaptic plasticity mechanisms that mediate ketamine’s rapid antidepressant effects [112]. As such, these targets—including GSK-3 inhibition— may also be responsible for ketamine’s rapid antisuicidal effects. Future studies will aim to further elucidate this area of investigation.
Thyrotropin-releasing hormone
The antisuicidal effects of intranasal thyrotropin-releasing hormone (TRH) are now being evaluated. TRH is a hypothalamic tripeptide-releasing hormone that stimulates the pituitary release of thyroid stimulating hormone (TSH), thus regulating the production of thyroid hormones. TRH receptors are found throughout the brain, with highest densities occurring in the amygdala and hippocampus and lower levels in the cortex, diencephalon, and basal ganglia [113]. Since the 1970s, TRH has been relevant to the pathophysiology of mood disorders; early studies evaluating the rapid antidepressant effects of oral and intravenous TRH had mixed results (reviewed in [114]). Challenges with this agent include minimal blood-brain barrier penetration and rapid degradation of TRH in the periphery given its short half-life [115]. Nevertheless, one pilot study (n=8) that used a lumbar intrathecal (IT) injection (half-life 54.5 +/− 5.4min in CSF) of TRH (500μg of protirelin) found that it had both rapid antidepressant and antisuicidal effects [115]. The authors cautioned against generalizing the results due to the small sample size and refractory patient population. In addition, no subsequent studies replicated this finding. Nevertheless, TRH may lead to promising avenues in suicide research, particularly with respect to therapeutic options that could emerge from efforts to optimize delivery routes.
Conclusions
Suicide is a significant, longstanding public health issue. Individuals with BD are at significantly higher risk of morbidity and mortality from suicidal behavior than the general population. As noted above, a great deal of research has focused on dysfunction of the HPA axis and alterations of major neurotransmitter systems. Glutamatergic involvement may play an important role, given recent antisuicidal findings with the NMDA antagonist ketamine [116]. Additional research has also emerged at the gene/epigenetic level, and from the study of neurotrophic factors and lipid metabolites. Future studies will investigate proximal and distal risk factors with various endophenotypes underlying suicidal behavior. Unfortunately, designing meaningful clinical trials to study proximal risk factors such as suicidal ideation, while necessary, is fraught with ethical and logistical challenges. With respect to new compounds, preclinical work in the areas of aggression and impulsivity may provide additional insights for drug development. Novel therapeutics such as ketamine and the repurposing of TRH may also meaningfully contribute to future preventive measures.
Kim and colleagues suggested a model to predict suicide risk that uses a classification system similar to the Child-Pugh classification system used in chronic liver disease [117]. In this model, points would be assigned to risk factors such as DST-non suppression, lower CSF-5-HIAA levels, abnormal neuropsychological test results, genetic factors, cholesterol levels, and validated psychosocial risk factors. As the cost of neuroimaging decreases, these technologies could further be applied in clinical populations. While such models have limitations (e.g. low specificity [50]), hypothetically the total scores of these accumulated risk factors would correlate with suicide risk.
Finally, future treatments should not solely focus on suicide molecular “targets”, but continue to address comorbid conditions associated with suicide. Results from the National Comorbidity Survey Replication study revealed that approximately 80% of those who attempt suicide in the United States have a prior mental disorder; in particular, the comorbid presence of disorders characterized by severe anxiety/agitation (e.g. PTSD) and poor impulse control (e.g. substance abuse disorders) may predict which individuals with suicidal ideation go on to plan or attempt suicide. Future studies will need to examine the bi-directional interaction between such comorbidities and molecular targets of suicide to point to therapeutic anti-suicide targets and to better estimate overall suicide risk in individuals [118]. To further address risk, combining non-pharmacological approaches (e.g. cognitive behavioral therapy, dialectical behavior therapy) with evidence based medication regimens may also reduce suicide risk in BD [119]. Because suicide is a complex phenomenon and requires a multidimensional approach, the time to conduct rigorous studies applying neurobiological frameworks to reduce suicide and suicidal behavior is now.
Acknowledgments
The authors gratefully acknowledge the support of the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH). Ioline Henter provided invaluable editorial assistance.
Role of Funding Source: This review was supported by the Intramural Research Program of the National Institute of Mental Health (Bethesda, Maryland). The NIMH had no further role in the writing of this review; or in the decision to submit the paper for publication.
Footnotes
Financial Disclosures: The authors gratefully acknowledge the support of the Intramural Research Program of the National Institute of Mental Health (NIMH), and the NARSAD Independent Investigator Award and Brain & Behavior Foundation Bipolar Research Award (Dr. Zarate). Dr. Zarate is listed as a co-inventor on a patent application for the use of ketamine and its metabolites in major depression. He has assigned his rights in the patent to the U.S. government but will share a percentage of any royalties that may be received by the government. All remaining authors have no conflict of interest to disclose, financial or otherwise.
References
- 1.Abreu LN, Lafer B, Baca-Garcia E, Oquendo MA. Suicidal ideation and suicide attempts in bipolar disorder type I: an update for the clinician. Rev Bras Psiquiatr. 2009;31:271–280. doi: 10.1590/s1516-44462009005000003. [DOI] [PubMed] [Google Scholar]
- 2.Goodwin FK, Fireman B, Simon GE, Hunkeler EM, Lee J, Revicki D. Suicide risk in bipolar disorder during treatment with lithium and divalproex. JAMA. 2003;290:1467–1473. doi: 10.1001/jama.290.11.1467. [DOI] [PubMed] [Google Scholar]
- 3.Jamison KR. Suicide and bipolar disorder. J Clin Psychiatry. 2000;61(Suppl 9):47–51. [PubMed] [Google Scholar]
- 4.Harris EC, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry. 1997;170:205–228. doi: 10.1192/bjp.170.3.205. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. The World Health Report 2001 – Mental health: new understanding, new hope. World Health Organization; Geneva: 2001. [Google Scholar]
- 6.Undurraga J, Baldessarini RJ, Valenti M, Pacchiarotti I, Vieta E. Suicidal risk factors in bipolar I and II disorder patients. J Clin Psychiatry. 2012;73:778–782. doi: 10.4088/JCP.11m07041. [DOI] [PubMed] [Google Scholar]
- 7.Bellivier F, Yon L, Luquiens A, Azorin JM, Bertsch J, Gerard S, et al. Suicidal attempts in bipolar disorder: results from an observational study (EMBLEM) Bipolar Disord. 2011;13:377–386. doi: 10.1111/j.1399-5618.2011.00926.x. [DOI] [PubMed] [Google Scholar]
- 8.Mann JJ. Neurobiology of suicidal behaviour. Nat Rev Neurosci. 2003;4:819–828. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- 9.Turecki G. Suicidal behavior: is there a genetic predisposition? Bipolar Disord. 2001;3:335–349. doi: 10.1034/j.1399-5618.2001.30608.x. [DOI] [PubMed] [Google Scholar]
- 10.van Heeringen K. Stress-diathesis model of suicidal behavior. In: Dwivedi Y, editor. The neurobiological basis of suicide. CRC Press; Boca Raton, FL, USA: 2012. pp. 113–124. [Google Scholar]
- 11.Mann JJ, Waternaux C, Haas GL, Malone KM. Toward a clinical model of suicidal behavior in psychiatric patients. Am J Psychiatry. 1999;156:181–189. doi: 10.1176/ajp.156.2.181. [DOI] [PubMed] [Google Scholar]
- 12.Turecki G, Ernst C, Jollant F, Labonte B, Mechawar N. The neurodevelopmental origins of suicidal behavior. Trends Neurosci. 2012;35:14–23. doi: 10.1016/j.tins.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Gilbert AM, Garno JL, Braga RJ, Shaya Y, Goldberg TE, Malhotra AK, et al. Clinical and cognitive correlates of suicide attempts in bipolar disorder: is suicide predictable? J Clin Psychiatry. 2011;72:1027–1033. doi: 10.4088/JCP.10m06410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bunney WE, Jr, Fawcett JA. Possibility of a biochemical test for suicidal potential: an analysis of endocrine findings prior to three suicides. Arch Gen Psychiatry. 1965;13:232–239. doi: 10.1001/archpsyc.1965.01730030038006. [DOI] [PubMed] [Google Scholar]
- 15.Pandey GN, Dwivedi Y. What can post-mortem studies tell us about the pathoetiology of suicide? Future Neurol. 2010;5:701–720. doi: 10.2217/fnl.10.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanley M, Stanley B, Traskman-Bendz L, Mann JJ, Meyendorff E. Neurochemical findings in suicide completers and suicide attempters. Suicide Life-Threat. 1986;16:286–300. doi: 10.1111/j.1943-278x.1986.tb00356.x. [DOI] [PubMed] [Google Scholar]
- 17.Stanley M, Stanley B. Biochemical studies in suicide victims: current findings and future implications. Suicide Life-Threat. 1989;19:30–42. doi: 10.1111/j.1943-278x.1989.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 18.Mann JJ, Currier DM. Stress, genetics and epigenetic effects on the neurobiology of suicidal behavior and depression. Eur Psychiatry. 2010;25:268–271. doi: 10.1016/j.eurpsy.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pandey GN. Neurobiology of adult and teenage suicide. Asian J Psychiatr. 2011;4:2–13. doi: 10.1016/j.ajp.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandey GN, Dwivedi Y. Peripheral biomarkers for suicide. In: Dwivedi Y, editor. The neurobiological basis of suicide. CRC Press; Boca Raton, FL, USA: 2012. pp. 407–424. [Google Scholar]
- 21.Beautrais AL, Joyce PR, Mulder RT, Fergusson DM, Deavoll BJ, Nightingale SK. Prevalence and comorbidity of mental disorders in persons making serious suicide attempts: a case-control study. Am J Psychiatry. 1996;153:1009–1014. doi: 10.1176/ajp.153.8.1009. [DOI] [PubMed] [Google Scholar]
- 22.Shaffer D, Gould MS, Fisher P, Trautman P, Moreau D, Kleinman M, et al. Psychiatric diagnosis in child and adolescent suicide. Arch Gen Psychiatry. 1996;53:339–348. doi: 10.1001/archpsyc.1996.01830040075012. [DOI] [PubMed] [Google Scholar]
- 23.McCullumsmith RE, Meador-Woodruff JH. Novel approaches to the study of postmortem brain in psychiatric illness: old limitations and new challenges. Biol Psychiatry. 2011;69:127–133. doi: 10.1016/j.biopsych.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 24.Asberg M, Traskman L, Thoren P. 5-HIAA in the cerebrospinal fluid. A biochemical suicide predictor? Arch Gen Psychiatry. 1976;33:1193–1197. doi: 10.1001/archpsyc.1976.01770100055005. [DOI] [PubMed] [Google Scholar]
- 25.Agren H. Symptom patterns in unipolar and bipolar depression correlating with monoamine metabolites in the cerebrospinal fluid: I. General patterns. Psychiatry Res. 1980;3:211–223. doi: 10.1016/0165-1781(80)90038-4. [DOI] [PubMed] [Google Scholar]
- 26.Agren H, Niklasson F. Suicidal potential in depression: focus on CSF monoamine and purine metabolites. Psychopharmacol Bull. 1986;22:656–660. [PubMed] [Google Scholar]
- 27.Cooper SJ, Kelly CB, King DJ. 5-Hydroxyindoleacetic acid in cerebrospinal fluid and prediction of suicidal behaviour in schizophrenia. Lancet. 1992;340:940–941. doi: 10.1016/0140-6736(92)92819-2. [DOI] [PubMed] [Google Scholar]
- 28.Nordstrom P, Samuelsson M, Asberg M, Traskman-Bendz L, Aberg-Wistedt A, Nordin C, et al. CSF 5-HIAA predicts suicide risk after attempted suicide. Suicide Life-Threat. 1994;24:1–9. [PubMed] [Google Scholar]
- 29.Mann JJ, Malone KM. Cerebrospinal fluid amines and higher-lethality suicide attempts in depressed inpatients. Biol Psychiatry. 1997;41:162–171. doi: 10.1016/s0006-3223(96)00217-x. [DOI] [PubMed] [Google Scholar]
- 30.Mann JJ, McBride PA, Malone KM, DeMeo M, Keilp J. Blunted serotonergic responsivity in depressed inpatients. Neuropsychopharmacology. 1995;13:53–64. doi: 10.1016/0893-133X(95)00016-7. [DOI] [PubMed] [Google Scholar]
- 31.Stanley M, Mann JJ. Increased serotonin-2 binding sites in frontal cortex of suicide victims. Lancet. 1983;1:214–216. doi: 10.1016/s0140-6736(83)92590-4. [DOI] [PubMed] [Google Scholar]
- 32.Pandey GN, Dwivedi Y, Rizavi HS, Ren X, Pandey SC, Pesold C, et al. Higher expression of serotonin 5-HT(2A) receptors in the postmortem brains of teenage suicide victims. Am J Psychiatry. 2002;159:419–429. doi: 10.1176/appi.ajp.159.3.419. [DOI] [PubMed] [Google Scholar]
- 33.Arango V, Underwood MD, Mann JJ. Fewer pigmented locus coeruleus neurons in suicide victims: preliminary results. Biol Psychiatry. 1996;39:112–120. doi: 10.1016/0006-3223(95)00107-7. [DOI] [PubMed] [Google Scholar]
- 34.Mann JJ, Stanley M, McBride PA, McEwen BS. Increased serotonin2 and beta-adrenergic receptor binding in the frontal cortices of suicide victims. Arch Gen Psychiatry. 1986;43:954–959. doi: 10.1001/archpsyc.1986.01800100048007. [DOI] [PubMed] [Google Scholar]
- 35.Secunda SK, Cross CK, Koslow S, Katz MM, Kocsis J, Maas JW, et al. Biochemistry and suicidal behavior in depressed patients. Biol Psychiatry. 1986;21:756–767. doi: 10.1016/0006-3223(86)90241-6. [DOI] [PubMed] [Google Scholar]
- 36.Sher L, Carballo JJ, Grunebaum MF, Burke AK, Zalsman G, Huang YY, et al. A prospective study of the association of cerebrospinal fluid monoamine metabolite levels with lethality of suicide attempts in patients with bipolar disorder. Bipolar Disord. 2006;8:543–550. doi: 10.1111/j.1399-5618.2006.00319.x. [DOI] [PubMed] [Google Scholar]
- 37.Ohmori T, Arora RC, Meltzer HY. Serotonergic measures in suicide brain: the concentration of 5-HIAA, HVA, and tryptophan in frontal cortex of suicide victims. Biol Psychiatry. 1992;32:57–71. doi: 10.1016/0006-3223(92)90142-m. [DOI] [PubMed] [Google Scholar]
- 38.Soderstrom H, Blennow K, Manhem A, Forsman A. CSF studies in violent offenders. I. 5-HIAA as a negative and HVA as a positive predictor of psychopathy. J Neural Transm. 2001;108:869–878. doi: 10.1007/s007020170036. [DOI] [PubMed] [Google Scholar]
- 39.Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol. 2010;52:671–690. doi: 10.1002/dev.20494. [DOI] [PubMed] [Google Scholar]
- 40.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 41.Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Rev. 1991;43:425–473. [PubMed] [Google Scholar]
- 42.Nemeroff CB, Owens MJ, Bissette G, Andorn AC, Stanley M. Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry. 1988;45:577–579. doi: 10.1001/archpsyc.1988.01800300075009. [DOI] [PubMed] [Google Scholar]
- 43.Merali Z, Kent P, Du L, Hrdina P, Palkovits M, Faludi G, et al. Corticotropin-releasing hormone, arginine vasopressin, gastrin-releasing peptide, and neuromedin B alterations in stress-relevant brain regions of suicides and control subjects. Biol Psychiatry. 2006;59:594–602. doi: 10.1016/j.biopsych.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 44.Kamali M, Saunders EF, Prossin AR, Brucksch CB, Harrington GJ, Langenecker SA, et al. Associations between suicide attempts and elevated bedtime salivary cortisol levels in bipolar disorder. J Affect Disord. 2012;136:350–358. doi: 10.1016/j.jad.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coryell W, Schlesser MA. Suicide and the dexamethasone suppression test in unipolar depression. Am J Psychiatry. 1981;138:1120–1121. doi: 10.1176/ajp.138.8.1120. [DOI] [PubMed] [Google Scholar]
- 46.Yerevanian BI, Feusner JD, Koek RJ, Mintz J. The dexamethasone suppression test as a predictor of suicidal behavior in unipolar depression. J Affect Disord. 2004;83:103–108. doi: 10.1016/j.jad.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 47.Coryell W, Schlesser M. The dexamethasone suppression test and suicide prediction. Am J Psychiatry. 2001;158:748–753. doi: 10.1176/appi.ajp.158.5.748. [DOI] [PubMed] [Google Scholar]
- 48.Coryell W, Schlesser M. Combined biological tests for suicide prediction. Psychiatry Res. 2007;150:187–191. doi: 10.1016/j.psychres.2006.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carroll BJ, Martin FI, Davies B. Resistance to suppression by dexamethasone of plasma 11-O.H.C.S. levels in severe depressive illness. Br Med J. 1968;3:285–287. doi: 10.1136/bmj.3.5613.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mann JJ, Currier D, Stanley B, Oquendo MA, Amsel LV, Ellis SP. Can biological tests assist prediction of suicide in mood disorders? Int J Neuropsychopharmacol. 2006;9:465–474. doi: 10.1017/S1461145705005687. [DOI] [PubMed] [Google Scholar]
- 51.Rush AJ, Giles DE, Schlesser MA, Orsulak PJ, Parker CR, Jr, Weissenburger JE, et al. The dexamethasone suppression test in patients with mood disorders. J Clin Psychiatry. 1996;57:470–484. doi: 10.4088/jcp.v57n1006. [DOI] [PubMed] [Google Scholar]
- 52.Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317:1237–1245. doi: 10.1056/NEJM198711123172001. [DOI] [PubMed] [Google Scholar]
- 53.Muldoon MF, Manuck SB, Matthews KA. Lowering cholesterol concentrations and mortality: a quantitative review of primary prevention trials. BMJ. 1990;301:309–314. doi: 10.1136/bmj.301.6747.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kunugi H, Takei N, Aoki H, Nanko S. Low serum cholesterol in suicide attempters. Biol Psychiatry. 1997;41:196–200. doi: 10.1016/S0006-3223(95)00672-9. [DOI] [PubMed] [Google Scholar]
- 55.Golier JA, Marzuk PM, Leon AC, Weiner C, Tardiff K. Low serum cholesterol level and attempted suicide. Am J Psychiatry. 1995;152:419–423. doi: 10.1176/ajp.152.3.419. [DOI] [PubMed] [Google Scholar]
- 56.Papassotiropoulos A, Hawellek B, Frahnert C, Rao GS, Rao ML. The risk of acute suicidality in psychiatric inpatients increases with low plasma cholesterol. Pharmacopsychiatry. 1999;32:1–4. doi: 10.1055/s-2007-979181. [DOI] [PubMed] [Google Scholar]
- 57.Vuksan-Cusa B, Marcinko D, Nad S, Jakovljevic M. Differences in cholesterol and metabolic syndrome between bipolar disorder men with and without suicide attempts. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:109–112. doi: 10.1016/j.pnpbp.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 58.Lalovic A, Merkens L, Russell L, Arsenault-Lapierre G, Nowaczyk MJ, Porter FD, et al. Cholesterol metabolism and suicidality in Smith-Lemli-Opitz syndrome carriers. Am J Psychiatry. 2004;161:2123–2126. doi: 10.1176/appi.ajp.161.11.2123. [DOI] [PubMed] [Google Scholar]
- 59.Ringo DL, Lindley SE, Faull KF, Faustman WO. Cholesterol and serotonin: seeking a possible link between blood cholesterol and CSF 5-HIAA. Biol Psychiatry. 1994;35:957–959. doi: 10.1016/0006-3223(94)91242-4. [DOI] [PubMed] [Google Scholar]
- 60.Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, et al. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- 61.Sher L, Grunebaum MF, Sullivan GM, Burke AK, Cooper TB, Mann JJ, et al. Testosterone levels in suicide attempters with bipolar disorder. J Psychiatr Res. 2012;46:1267–1271. doi: 10.1016/j.jpsychires.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perez-Rodriguez MM, Lopez-Castroman J, Martinez-Vigo M, Diaz-Sastre C, Ceverino A, Nunez-Beltran A, et al. Lack of association between testosterone and suicide attempts. Neuropsychobiology. 2011;63:125–130. doi: 10.1159/000318085. [DOI] [PubMed] [Google Scholar]
- 63.Rubinow DR, Schmidt PJ. Androgens, brain, and behavior. Am J Psychiatry. 1996;153:974–984. doi: 10.1176/ajp.153.8.974. [DOI] [PubMed] [Google Scholar]
- 64.Sher L. Testosterone and suicidal behavior. Expert Rev Neurother. 2012;12:257–259. doi: 10.1586/ern.12.6. [DOI] [PubMed] [Google Scholar]
- 65.Voracek M, Loibl LM. Genetics of suicide: a systematic review of twin studies. Wien Klin Wochenschr. 2007;119:463–475. doi: 10.1007/s00508-007-0823-2. [DOI] [PubMed] [Google Scholar]
- 66.Mirnics K, Levitt P, Lewis DA. Critical appraisal of DNA microarrays in psychiatric genomics. Biol Psychiatry. 2006;60:163–176. doi: 10.1016/j.biopsych.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 67.Anguelova M, Benkelfat C, Turecki G. A systematic review of association studies investigating genes coding for serotonin receptors and the serotonin transporter: II. Suicidal behavior. Mol Psychiatry. 2003;8:646–653. doi: 10.1038/sj.mp.4001336. [DOI] [PubMed] [Google Scholar]
- 68.Bondy B, Buettner A, Zill P. Genetics of suicide. Mol Psychiatry. 2006;11:336–351. doi: 10.1038/sj.mp.4001803. [DOI] [PubMed] [Google Scholar]
- 69.Brezo J, Klempan T, Turecki G. The genetics of suicide: a critical review of molecular studies. Psychiatr Clin North Am. 2008;31:179–203. doi: 10.1016/j.psc.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 70.Rujescu D, Giegling I, Mandelli L, Schneider B, Hartmann AM, Schnabel A, et al. NOS-I and -III gene variants are differentially associated with facets of suicidal behavior and aggression-related traits. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:42–48. doi: 10.1002/ajmg.b.30569. [DOI] [PubMed] [Google Scholar]
- 71.Perlis RH, Huang J, Purcell S, Fava M, Rush AJ, Sullivan PF, et al. Genome-wide association study of suicide attempts in mood disorder patients. Am J Psychiatry. 2010;167:1499–1507. doi: 10.1176/appi.ajp.2010.10040541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Currier D, Mann JJ. Stress, genes and the biology of suicidal behavior. Psychiatr Clin North Am. 2008;31:247–269. doi: 10.1016/j.psc.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dwivedi Y, Rao JS, Rizavi HS, Kotowski J, Conley RR, Roberts RC, et al. Abnormal expression and functional characteristics of cyclic adenosine monophosphate response element binding protein in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:273–282. doi: 10.1001/archpsyc.60.3.273. [DOI] [PubMed] [Google Scholar]
- 74.Pandey GN, Dwivedi Y, Ren X, Rizavi HS, Roberts RC, Conley RR. Cyclic AMP response element-binding protein in post-mortem brain of teenage suicide victims: specific decrease in the prefrontal cortex but not the hippocampus. Int J Neuropsychopharmacol. 2007;10:621–629. doi: 10.1017/S1461145706007231. [DOI] [PubMed] [Google Scholar]
- 75.Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- 76.Poulter MO, Du L, Weaver IC, Palkovits M, Faludi G, Merali Z, et al. GABAA receptor promoter hypermethylation in suicide brain: implications for the involvement of epigenetic processes. Biol Psychiatry. 2008;64:645–652. doi: 10.1016/j.biopsych.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 77.Autry AE, Monteggia LM. Epigenetics in suicide and depression. Biol Psychiatry. 2009;66:812–813. doi: 10.1016/j.biopsych.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gould TD, Gottesman II. Psychiatric endophenotypes and the development of valid animal models. Genes Brain Behav. 2006;5:113–119. doi: 10.1111/j.1601-183X.2005.00186.x. [DOI] [PubMed] [Google Scholar]
- 79.Kovacsics CE, Gottesman II, Gould TD. Lithium’s antisuicidal efficacy: elucidation of neurobiological targets using endophenotype strategies. Annu Rev Pharmacol Toxicol. 2009;49:175–198. doi: 10.1146/annurev.pharmtox.011008.145557. [DOI] [PubMed] [Google Scholar]
- 80.Courtet P, Gottesman II, Jollant F, Gould TD. The neuroscience of suicidal behaviors: what can we expect from endophenotype strategies? Transl Psychiatry. 2011;1:e7. doi: 10.1038/tp.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carballo JJ, Akamnonu CP, Oquendo MA. Neurobiology of suicidal behavior. An integration of biological and clinical findings. Arch Suicide Res. 2008;12:93–110. doi: 10.1080/13811110701857004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 83.Lenox RH, Gould TD, Manji HK. Endophenotypes in bipolar disorder. Am J Med Genet. 2002;114:391–406. doi: 10.1002/ajmg.10360. [DOI] [PubMed] [Google Scholar]
- 84.Hasler G, Drevets WC, Gould TD, Gottesman II, Manji HK. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2006;60:93–105. doi: 10.1016/j.biopsych.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 85.Grunebaum MF, Ramsay SR, Galfalvy HC, Ellis SP, Burke AK, Sher L, et al. Correlates of suicide attempt history in bipolar disorder: a stress-diathesis perspective. Bipolar Disord. 2006;8:551–557. doi: 10.1111/j.1399-5618.2006.00304.x. [DOI] [PubMed] [Google Scholar]
- 86.Sheard MH, Marini JL, Bridges CI, Wagner E. The effect of lithium on impulsive aggressive behavior in man. Am J Psychiatry. 1976;133:1409–1413. doi: 10.1176/ajp.133.12.1409. [DOI] [PubMed] [Google Scholar]
- 87.Muller-Oerlinghausen B, Lewitzka U. Lithium reduces pathological aggression and suicidality: a mini-review. Neuropsychobiology. 2010;62:43–49. doi: 10.1159/000314309. [DOI] [PubMed] [Google Scholar]
- 88.Ohmura Y, Tsutsui-Kimura I, Kumamoto H, Minami M, Izumi T, Yamaguchi T, et al. Lithium, but not valproic acid or carbamazepine, suppresses impulsive-like action in rats. Psychopharmacology (Berl) 2012;219:421–432. doi: 10.1007/s00213-011-2496-9. [DOI] [PubMed] [Google Scholar]
- 89.Xu CM, Wang J, Wu P, Zhu WL, Li QQ, Xue YX, et al. Glycogen synthase kinase 3beta in the nucleus accumbens core mediates cocaine-induced behavioral sensitization. J Neurochem. 2009;111:1357–1368. doi: 10.1111/j.1471-4159.2009.06414.x. [DOI] [PubMed] [Google Scholar]
- 90.Xu CM, Wang J, Wu P, Xue YX, Zhu WL, Li QQ, et al. Glycogen synthase kinase 3beta in the nucleus accumbens core is critical for methamphetamine-induced behavioral sensitization. J Neurochem. 2011;118:126–139. doi: 10.1111/j.1471-4159.2011.07281.x. [DOI] [PubMed] [Google Scholar]
- 91.Cipriani A, Pretty H, Hawton K, Geddes JR. Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: a systematic review of randomized trials. Am J Psychiatry. 2005;162:1805–1819. doi: 10.1176/appi.ajp.162.10.1805. [DOI] [PubMed] [Google Scholar]
- 92.Baldessarini RJ, Tondo L, Davis P, Pompili M, Goodwin FK, Hennen J. Decreased risk of suicides and attempts during long-term lithium treatment: a meta-analytic review. Bipolar Disord. 2006;8(5 Pt 2):625–39. doi: 10.1111/j.1399-5618.2006.00344.x. [DOI] [PubMed] [Google Scholar]
- 93.Tondo L, Baldessarini RJ. Long-term lithium treatment in the prevention of suicidal behavior in bipolar disorder patients. Epidemiol Psichiatr Soc. 2009;18:179–183. doi: 10.1017/s1121189x00000439. [DOI] [PubMed] [Google Scholar]
- 94.Quiroz JA, Machado-Vieira R, Zarate CA, Jr, Manji HK. Novel insights into lithium’s mechanism of action: neurotrophic and neuroprotective effects. Neuropsychobiology. 2010;62:50–60. doi: 10.1159/000314310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.O’Donnell KC, Gould TD. The behavioral actions of lithium in rodent models: leads to develop novel therapeutics. Neurosci Biobehav Rev. 2007;31:932–962. doi: 10.1016/j.neubiorev.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oquendo MA, Galfalvy HC, Currier D, Grunebaum MF, Sher L, Sullivan GM, et al. Treatment of suicide attempters with bipolar disorder: a randomized clinical trial comparing lithium and valproate in the prevention of suicidal behavior. Am J Psychiatry. 2011;168:1050–1056. doi: 10.1176/appi.ajp.2011.11010163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Perlis RH. Hard outcomes: clinical trials to reduce suicide. Am J Psychiatry. 2011;168:1009–1011. doi: 10.1176/appi.ajp.2011.11081250. [DOI] [PubMed] [Google Scholar]
- 98.Reid WH, Mason M, Hogan T. Suicide prevention effects associated with clozapine therapy in schizophrenia and schizoaffective disorder. Psychiatr Serv. 1998;49:1029–1033. doi: 10.1176/ps.49.8.1029. [DOI] [PubMed] [Google Scholar]
- 99.Meltzer HY, Alphs L, Green AI, Altamura AC, Anand R, Bertoldi A, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT) Arch Gen Psychiatry. 2003;60:82–91. doi: 10.1001/archpsyc.60.1.82. [DOI] [PubMed] [Google Scholar]
- 100.Javitt DC, Duncan L, Balla A, Sershen H. Inhibition of system A-mediated glycine transport in cortical synaptosomes by therapeutic concentrations of clozapine: implications for mechanisms of action. Mol Psychiatry. 2005;10:275–287. doi: 10.1038/sj.mp.4001552. [DOI] [PubMed] [Google Scholar]
- 101.Machado-Vieira R, Lara DR, Souza DO, Kapczinski F. Therapeutic efficacy of allopurinol in mania associated with hyperuricemia. J Clin Psychopharmacol. 2001;21:621–622. doi: 10.1097/00004714-200112000-00017. [DOI] [PubMed] [Google Scholar]
- 102.Machado-Vieira R, Soares JC, Lara DR, Luckenbaugh DA, Busnello JV, Marca G, et al. A double-blind, randomized, placebo-controlled 4-week study on the efficacy and safety of the purinergic agents allopurinol and dipyridamole adjunctive to lithium in acute bipolar mania. J Clin Psychiatry. 2008;69:1237–1245. doi: 10.4088/jcp.v69n0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fan A, Berg A, Bresee C, Glassman LH, Rapaport MH. Allopurinol augmentation in the outpatient treatment of bipolar mania: a pilot study. Bipolar Disord. 2012;14:206–210. doi: 10.1111/j.1399-5618.2012.01001.x. [DOI] [PubMed] [Google Scholar]
- 104.Swann AC. Impulsivity in mania. Curr Psychiatry Rep. 2009;11:481–487. doi: 10.1007/s11920-009-0073-2. [DOI] [PubMed] [Google Scholar]
- 105.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 106.Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010;71:1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009;66:522–526. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zarate CA, Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol. 2011;14:1127–1131. doi: 10.1017/S1461145711000629. [DOI] [PubMed] [Google Scholar]
- 111.Kavalali ET, Monteggia LM. Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am J Psychiatry. 2012;169:1150–1156. doi: 10.1176/appi.ajp.2012.12040531. [DOI] [PubMed] [Google Scholar]
- 112.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Manaker S, Eichen A, Winokur A, Rhodes CH, Rainbow TC. Autoradiographic localization of thyrotropin releasing hormone receptors in human brain. Neurology. 1986;36:641–646. doi: 10.1212/wnl.36.5.641. [DOI] [PubMed] [Google Scholar]
- 114.Callahan AM, Frye MA, Marangell LB, George MS, Ketter TA, L’Herrou T, et al. Comparative antidepressant effects of intravenous and intrathecal thyrotropin-releasing hormone: confounding effects of tolerance and implications for therapeutics. Biol Psychiatry. 1997;41:264–272. doi: 10.1016/s0006-3223(97)00372-7. [DOI] [PubMed] [Google Scholar]
- 115.Marangell LB, George MS, Callahan AM, Ketter TA, Pazzaglia PJ, L’Herrou TA, et al. Effects of intrathecal thyrotropin-releasing hormone (protirelin) in refractory depressed patients. Arch Gen Psychiatry. 1997;54:214–222. doi: 10.1001/archpsyc.1997.01830150034007. [DOI] [PubMed] [Google Scholar]
- 116.Erhardt S, Lim CK, Linderholm KR, Janelidze S, Lindqvist D, Samuelsson M, et al. Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacology. 2013;38:743–752. doi: 10.1038/npp.2012.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee BH, Kim YK. Potential peripheral biological predictors of suicidal behavior in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:842–847. doi: 10.1016/j.pnpbp.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 118.Nock MK, Hwang I, Sampson NA, Kessler RC. Mental disorders, comorbidity and suicidal behavior: results from the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15:868–876. doi: 10.1038/mp.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fountoulakis KN, Gonda X, Siamouli M, Rihmer Z. Psychotherapeutic intervention and suicide risk reduction in bipolar disorder: a review of the evidence. J Affect Disord. 2009;113:21–29. doi: 10.1016/j.jad.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 120.Meltzer HY, Arora RC, Baber R, Tricou BJ. Serotonin uptake in blood platelets of psychiatric patients. Arch Gen Psychiatry. 1981;38:1322–1326. doi: 10.1001/archpsyc.1981.01780370024002. [DOI] [PubMed] [Google Scholar]
- 121.Rausch JL, Janowsky DS, Risch SC, Huey LY. A kinetic analysis and replication of decreased platelet serotonin uptake in depressed patients. Psychiatry Res. 1986;19:105–112. doi: 10.1016/0165-1781(86)90003-x. [DOI] [PubMed] [Google Scholar]
- 122.Lichtenberg P, Shapira B, Gillon D, Kindler S, Cooper TB, Newman ME, et al. Hormone responses to fenfluramine and placebo challenge in endogenous depression. Psychiatry Res. 1992;43:137–146. doi: 10.1016/0165-1781(92)90128-p. [DOI] [PubMed] [Google Scholar]
- 123.Mann JJ, McBride PA, Brown RP, Linnoila M, Leon AC, DeMeo M, et al. Relationship between central and peripheral serotonin indexes in depressed and suicidal psychiatric inpatients. Arch Gen Psychiatry. 1992;49:442–446. doi: 10.1001/archpsyc.1992.01820060022003. [DOI] [PubMed] [Google Scholar]
- 124.Mann JJ, Malone KM, Sweeney JA, Brown RP, Linnoila M, Stanley B, et al. Attempted suicide characteristics and cerebrospinal fluid amine metabolites in depressed inpatients. Neuropsychopharmacology. 1996;15(6):576–586. doi: 10.1016/S0893-133X(96)00102-9. [DOI] [PubMed] [Google Scholar]
- 125.Arora RC, Meltzer HY. Serotonergic measures in the brains of suicide victims: 5-HT2 binding sites in the frontal cortex of suicide victims and control subjects. Am J Psychiatry. 1989;146:730–736. doi: 10.1176/ajp.146.6.730. [DOI] [PubMed] [Google Scholar]
- 126.Arango V, Underwood MD, Gubbi AV, Mann JJ. Localized alterations in pre-and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Res. 1995;688:121–133. doi: 10.1016/0006-8993(95)00523-s. [DOI] [PubMed] [Google Scholar]
- 127.Hrdina PD, Demeter E, Vu TB, Sotonyi P, Palkovits M. 5-HT uptake sites and 5-HT2 receptors in brain of antidepressant-free suicide victims/depressives: increase in 5-HT2 sites in cortex and amygdala. Brain Res. 1993;614:37–44. doi: 10.1016/0006-8993(93)91015-k. [DOI] [PubMed] [Google Scholar]
- 128.Underwood MD, Khaibulina AA, Ellis SP, Moran A, Rice PM, Mann JJ, et al. Morphometry of the dorsal raphe nucleus serotonergic neurons in suicide victims. Biol Psychiatry. 1999;46:473–483. doi: 10.1016/s0006-3223(99)00043-8. [DOI] [PubMed] [Google Scholar]
- 129.Boldrini M, Underwood MD, Mann JJ, Arango V. More tryptophan hydroxylase in the brainstem dorsal raphe nucleus in depressed suicides. Brain Res. 2005;1041:19–28. doi: 10.1016/j.brainres.2005.01.083. [DOI] [PubMed] [Google Scholar]
- 130.Brown GL, Goodwin FK, Ballenger JC, Goyer PF, Major LF. Aggression in humans correlates with cerebrospinal fluid amine metabolites. Psychiatry Res. 1979;1:131–139. doi: 10.1016/0165-1781(79)90053-2. [DOI] [PubMed] [Google Scholar]
- 131.Galfalvy H, Currier D, Oquendo MA, Sullivan G, Huang YY, John Mann J. Lower CSF MHPG predicts short-term risk for suicide attempt. Int J Neuropsychopharmacol. 2009;12:1327–1335. doi: 10.1017/S1461145709990228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Meana JJ, Garcia-Sevilla JA. Increased alpha 2-adrenoceptor density in the frontal cortex of depressed suicide victims. J Neural Transm. 1987;70:377–381. doi: 10.1007/BF01253612. [DOI] [PubMed] [Google Scholar]
- 133.Callado LF, Meana JJ, Grijalba B, Pazos A, Sastre M, Garcia-Sevilla JA. Selective increase of alpha2A-adrenoceptor agonist binding sites in brains of depressed suicide victims. J Neurochem. 1998;70:1114–1123. doi: 10.1046/j.1471-4159.1998.70031114.x. [DOI] [PubMed] [Google Scholar]
- 134.Escriba PV, Ozaita A, Garcia-Sevilla JA. Increased mRNA expression of alpha2A-adrenoceptors, serotonin receptors and mu-opioid receptors in the brains of suicide victims. Neuropsychopharmacology. 2004;29:1512–1521. doi: 10.1038/sj.npp.1300459. [DOI] [PubMed] [Google Scholar]
- 135.Arango V, Ernsberger P, Marzuk PM, Chen JS, Tierney H, Stanley M, et al. Autoradiographic demonstration of increased serotonin 5-HT2 and beta-adrenergic receptor binding sites in the brain of suicide victims. Arch Gen Psychiatry. 1990;47:1038–1047. doi: 10.1001/archpsyc.1990.01810230054009. [DOI] [PubMed] [Google Scholar]
- 136.Roy A, Agren H, Pickar D, Linnoila M, Doran AR, Cutler NR, et al. Reduced CSF concentrations of homovanillic acid and homovanillic acid to 5-hydroxyindoleacetic acid ratios in depressed patients: relationship to suicidal behavior and dexamethasone nonsuppression. Am J Psychiatry. 1986;143:1539–1545. doi: 10.1176/ajp.143.12.1539. [DOI] [PubMed] [Google Scholar]
- 137.Roy A, Karoum F, Pollack S. Marked reduction in indexes of dopamine metabolism among patients with depression who attempt suicide. Arch Gen Psychiatry. 1992;49:447–450. doi: 10.1001/archpsyc.1992.01820060027004. [DOI] [PubMed] [Google Scholar]
- 138.Noga JT, Hyde TM, Herman MM, Spurney CF, Bigelow LB, Weinberger DR, et al. Glutamate receptors in the postmortem striatum of schizophrenic, suicide, and control brains. Synapse. 1997;27:168–176. doi: 10.1002/(SICI)1098-2396(199711)27:3<168::AID-SYN2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 139.Kim S, Choi KH, Baykiz AF, Gershenfeld HK. Suicide candidate genes associated with bipolar disorder and schizophrenia: an exploratory gene expression profiling analysis of post-mortem prefrontal cortex. BMC Genomics. 2007;8:413. doi: 10.1186/1471-2164-8-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, et al. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res. 2008;42:151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 141.Sequeira A, Mamdani F, Ernst C, Vawter MP, Bunney WE, Lebel V, et al. Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PLoS One. 2009;4:e6585. doi: 10.1371/journal.pone.0006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fiori LM, Turecki G. Broadening our horizons: gene expression profiling to help better understand the neurobiology of suicide and depression. Neurobiol Dis. 2012;45:14–22. doi: 10.1016/j.nbd.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 143.Nowak G, Ordway GA, Paul IA. Alterations in the N-methyl-Daspartate (NMDA) receptor complex in the frontal cortex of suicide victims. Brain Res. 1995;675:157–164. doi: 10.1016/0006-8993(95)00057-w. [DOI] [PubMed] [Google Scholar]
- 144.Bielau H, Steiner J, Mawrin C, Trubner K, Brisch R, Meyer-Lotz G, et al. Dysregulation of GABAergic neurotransmission in mood disorders: a postmortem study. Ann NY Acad Sci. 2007;1096:157–169. doi: 10.1196/annals.1397.081. [DOI] [PubMed] [Google Scholar]
- 145.Gos T, Gunther K, Bielau H, Dobrowolny H, Mawrin C, Trubner K, et al. Suicide and depression in the quantitative analysis of glutamic acid decarboxylase-immunoreactive neuropil. J Affect Disord. 2009;113:45–55. doi: 10.1016/j.jad.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 146.Gross-Isseroff R, Dillon KA, Israeli M, Biegon A. Regionally selective increases in mu opioid receptor density in the brains of suicide victims. Brain Res. 1990;530:312–316. doi: 10.1016/0006-8993(90)91301-v. [DOI] [PubMed] [Google Scholar]
- 147.Zavitsanou K, Katsifis A, Mattner F, Huang XF. Investigation of m1/m4 muscarinic receptors in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major depression disorder. Neuropsychopharmacology. 2004;29:619–625. doi: 10.1038/sj.npp.1300367. [DOI] [PubMed] [Google Scholar]
- 148.Cowburn RF, Marcusson JO, Eriksson A, Wiehager B, O’Neill C. Adenylyl cyclase activity and G-protein subunit levels in postmortem frontal cortex of suicide victims. Brain Res. 1994;633:297–304. doi: 10.1016/0006-8993(94)91552-0. [DOI] [PubMed] [Google Scholar]
- 149.Pacheco MA, Stockmeier C, Meltzer HY, Overholser JC, Dilley GE, Jope RS. Alterations in phosphoinositide signaling and G-protein levels in depressed suicide brain. Brain Res. 1996;723:37–45. doi: 10.1016/0006-8993(96)00207-7. [DOI] [PubMed] [Google Scholar]
- 150.Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. mRNA and protein expression of selective alpha subunits of G proteins are abnormal in prefrontal cortex of suicide victims. Neuropsychopharmacology. 2002;27:499–517. doi: 10.1016/S0893-133X(02)00335-4. [DOI] [PubMed] [Google Scholar]
- 151.Pandey GN, Dwivedi Y, Pandey SC, Teas SS, Conley RR, Roberts RC, et al. Low phosphoinositide-specific phospholipase C activity and expression of phospholipase C beta1 protein in the prefrontal cortex of teenage suicide subjects. Am J Psychiatry. 1999;156:1895–1901. doi: 10.1176/ajp.156.12.1895. [DOI] [PubMed] [Google Scholar]
- 152.Pandey GN, Dwivedi Y, Pandey SC, Conley RR, Roberts RC, Tamminga CA. Protein kinase C in the postmortem brain of teenage suicide victims. Neurosci Lett. 1997;228:111–114. doi: 10.1016/s0304-3940(97)00378-9. [DOI] [PubMed] [Google Scholar]
- 153.Pandey GN, Dwivedi Y, Rizavi HS, Ren X, Conley RR. Decreased catalytic activity and expression of protein kinase C isozymes in teenage suicide victims: a postmortem brain study. Arch Gen Psychiatry. 2004;61:685–693. doi: 10.1001/archpsyc.61.7.685. [DOI] [PubMed] [Google Scholar]
- 154.Coull MA, Lowther S, Katona CL, Horton RW. Altered brain protein kinase C in depression: a post-mortem study. Eur Neuropsychopharmacol. 2000;10:283–288. doi: 10.1016/s0924-977x(00)00084-5. [DOI] [PubMed] [Google Scholar]
- 155.Pandey GN, Dwivedi Y, Ren X, Rizavi HS, Mondal AC, Shukla PK, et al. Brain region specific alterations in the protein and mRNA levels of protein kinase A subunits in the post-mortem brain of teenage suicide victims. Neuropsychopharmacology. 2005;30:1548–1556. doi: 10.1038/sj.npp.1300765. [DOI] [PubMed] [Google Scholar]
- 156.Dwivedi Y, Conley RR, Roberts RC, Tamminga CA, Pandey GN. [(3)H]cAMP binding sites and protein kinase a activity in the prefrontal cortex of suicide victims. Am J Psychiatry. 2002;159:66–73. doi: 10.1176/appi.ajp.159.1.66. [DOI] [PubMed] [Google Scholar]
- 157.Ernst C, Deleva V, Deng X, Sequeira A, Pomarenski A, Klempan T, et al. Alternative splicing, methylation state, and expression profile of tropomyosin-related kinase B in the frontal cortex of suicide completers. Arch Gen Psychiatry. 2009;66:22–32. doi: 10.1001/archpsyc.66.1.22. [DOI] [PubMed] [Google Scholar]
- 158.Lopez JF, Palkovits M, Arato M, Mansour A, Akil H, Watson SJ. Localization and quantification of pro-opiomelanocortin mRNA and glucocorticoid receptor mRNA in pituitaries of suicide victims. Neuroendocrinology. 1992;56:491–501. doi: 10.1159/000126266. [DOI] [PubMed] [Google Scholar]
- 159.Szigethy E, Conwell Y, Forbes NT, Cox C, Caine ED. Adrenal weight and morphology in victims of completed suicide. Biol Psychiatry. 1994;36:374–380. doi: 10.1016/0006-3223(94)91212-2. [DOI] [PubMed] [Google Scholar]
- 160.Hiroi N, Wong ML, Licinio J, Park C, Young M, Gold PW, et al. Expression of corticotropin releasing hormone receptors type I and type II mRNA in suicide victims and controls. Mol Psychiatry. 2001;6:540–546. doi: 10.1038/sj.mp.4000908. [DOI] [PubMed] [Google Scholar]
- 161.Austin MC, Janosky JE, Murphy HA. Increased corticotropin-releasing hormone immunoreactivity in monoamine-containing pontine nuclei of depressed suicide men. Mol Psychiatry. 2003;8:324–332. doi: 10.1038/sj.mp.4001250. [DOI] [PubMed] [Google Scholar]
- 162.Roy A. Hypothalamic-pituitary-adrenal axis function and suicidal behavior in depression. Biol Psychiatry. 1992;32:812–816. doi: 10.1016/0006-3223(92)90084-d. [DOI] [PubMed] [Google Scholar]
- 163.Jokinen J, Nordstrom P. HPA axis hyperactivity and attempted suicide in young adult mood disorder inpatients. J Affect Disord. 2009;116:117–120. doi: 10.1016/j.jad.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 164.McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sinclair D, Fullerton JM, Webster MJ, Shannon Weickert C. Glucocorticoid receptor 1B and 1C mRNA transcript alterations in schizophrenia and bipolar disorder, and their possible regulation by GR gene variants. PLoS One. 2012;7:e31720. doi: 10.1371/journal.pone.0031720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sequeira A, Gwadry FG, Ffrench-Mullen JM, Canetti L, Gingras Y, Casero RA, Jr, et al. Implication of SSAT by gene expression and genetic variation in suicide and major depression. Arch Gen Psychiatry. 2006;63:35–48. doi: 10.1001/archpsyc.63.1.35. [DOI] [PubMed] [Google Scholar]
- 167.Fiori LM, Gross JA, Turecki G. Effects of histone modifications on increased expression of polyamine biosynthetic genes in suicide. Int J Neuropsychopharmacol. 2012;15:1161–1166. doi: 10.1017/S1461145711001520. [DOI] [PubMed] [Google Scholar]
- 168.Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, et al. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry. 2009;66:287–292. doi: 10.1016/j.biopsych.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 169.Tonelli LH, Stiller J, Rujescu D, Giegling I, Schneider B, Maurer K, et al. Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta Psychiatr Scand. 2008;117:198–206. doi: 10.1111/j.1600-0447.2007.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ellison LF, Morrison HI. Low serum cholesterol concentration and risk of suicide. Epidemiology. 2001;12:168–172. doi: 10.1097/00001648-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 171.Evans SJ, Prossin AR, Harrington GJ, Kamali M, Ellingrod VL, Burant CF, et al. Fats and factors: lipid profiles associate with personality factors and suicidal history in bipolar subjects. PLoS One. 2012;7:e29297. doi: 10.1371/journal.pone.0029297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ernst C, Chen ES, Turecki G. Histone methylation and decreased expression of TrkB.T1 in orbital frontal cortex of suicide completers. Mol Psychiatry. 2009;14:830–832. doi: 10.1038/mp.2009.35. [DOI] [PubMed] [Google Scholar]
- 173.Keller S, Sarchiapone M, Zarrilli F, Videtic A, Ferraro A, Carli V, et al. Increased BDNF promoter methylation in the Wernicke area of suicide subjects. Arch Gen Psychiatry. 2010;67:258–267. doi: 10.1001/archgenpsychiatry.2010.9. [DOI] [PubMed] [Google Scholar]
- 174.Jollant F, Bellivier F, Leboyer M, Astruc B, Torres S, Verdier R, et al. Impaired decision making in suicide attempters. Am J Psychiatry. 2005;162:304–310. doi: 10.1176/appi.ajp.162.2.304. [DOI] [PubMed] [Google Scholar]
- 175.Cha CB, Najmi S, Park JM, Finn CT, Nock MK. Attentional bias toward suicide-related stimuli predicts suicidal behavior. J Abnorm Psychol. 2010;119:616–622. doi: 10.1037/a0019710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Keilp JG, Sackeim HA, Brodsky BS, Oquendo MA, Malone KM, Mann JJ. Neuropsychological dysfunction in depressed suicide attempters. Am J Psychiatry. 2001;158:735–741. doi: 10.1176/appi.ajp.158.5.735. [DOI] [PubMed] [Google Scholar]
- 177.Jollant F, Lawrence NL, Olie E, Guillaume S, Courtet P. The suicidal mind and brain: a review of neuropsychological and neuroimaging studies. World J Biol Psychiatry. 2011;12:319–339. doi: 10.3109/15622975.2011.556200. [DOI] [PubMed] [Google Scholar]
- 178.Keilp JG, Gorlyn M, Russell M, Oquendo MA, Burke AK, Harkavy-Friedman J, et al. Neuropsychological function and suicidal behavior: attention control, memory and executive dysfunction in suicide attempt. Psychol Med. 2012;10:1–13. doi: 10.1017/S0033291712001419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Grunebaum MF, Ellis SP, Duan N, Burke AK, Oquendo MA, John Mann J. Pilot randomized clinical trial of an SSRI vs bupropion: effects on suicidal behavior, ideation, and mood in major depression. Neuropsychopharmacology. 2012;37:697–706. doi: 10.1038/npp.2011.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Khan A, Khan SR, Hobus J, Faucett J, Mehra V, Giller EL, et al. Differential pattern of response in mood symptoms and suicide risk measures in severely ill depressed patients assigned to citalopram with placebo or citalopram combined with lithium: role of lithium levels. J Psychiatr Res. 2011;45:1489–1496. doi: 10.1016/j.jpsychires.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 181.Rucci P, Frank E, Scocco P, Calugi S, Miniati M, Fagiolini A, et al. Treatment-emergent suicidal ideation during 4 months of acute management of unipolar major depression with SSRI pharmacotherapy or interpersonal psychotherapy in a randomized clinical trial. Depress Anxiety. 2011;28:303–309. doi: 10.1002/da.20758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lauterbach E, Felber W, Muller-Oerlinghausen B, Ahrens B, Bronisch T, Meyer T, et al. Adjunctive lithium treatment in the prevention of suicidal behaviour in depressive disorders: a randomised, placebo-controlled, 1-year trial. Acta Psychiatr Scand. 2008;118:469–479. doi: 10.1111/j.1600-0447.2008.01266.x. [DOI] [PubMed] [Google Scholar]
- 183.Reeves H, Batra S, May RS, Zhang R, Dahl DC, Li X. Efficacy of risperidone augmentation to antidepressants in the management of suicidality in major depressive disorder: a randomized, doubleblind, placebo-controlled pilot study. J Clin Psychiatry. 2008;69:1228–1336. doi: 10.4088/jcp.v69n0805. [DOI] [PubMed] [Google Scholar]
- 184.Lauterbach E, Ahrens B, Felber W, Oerlinghausen BM, Kilb B, Bischof G, et al. Suicide prevention by lithium SUPLI--challenges of a multi-center prospective study. Arch Suicide Res. 2005;9:27–34. doi: 10.1080/13811110590512886. [DOI] [PubMed] [Google Scholar]
- 185.Verkes RJ, Van der Mast RC, Hengeveld MW, Tuyl JP, Zwinderman AH, Van Kempen GM. Reduction by paroxetine of suicidal behavior in patients with repeated suicide attempts but not major depression. Am J Psychiatry. 1998;155:543–547. doi: 10.1176/ajp.155.4.543. [DOI] [PubMed] [Google Scholar]
- 186.McCarthy MJ, Leckband SG, Kelsoe JR. Pharmacogenetics of lithium response in bipolar disorder. Pharmacogenomics. 2010;11:1439–1465. doi: 10.2217/pgs.10.127. [DOI] [PubMed] [Google Scholar]
- 187.Bonnin CM, Martinez-Aran A, Sanchez-Moreno J, Torrent C, Franco C, Pacchiarotti I, et al. Bipolar disorder, cognitive functioning and hypothalamic-pituitary-thyroid axis. Actas Esp Psiquiatr. 2010;38:223–228. [PubMed] [Google Scholar]
- 188.Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63:1121–1129. doi: 10.1001/archpsyc.63.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Drevets WC, Furey ML. Replication of scopolamine’s antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biol Psychiatry. 2010;67:432–438. doi: 10.1016/j.biopsych.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Machado-Vieira R. Purinergic system in the treatment of bipolar disorder: uric acid levels as a screening test in mania. J Clin Psychopharmacol. 2012;32:735–736. doi: 10.1097/JCP.0b013e318268391d. [DOI] [PubMed] [Google Scholar]
- 191.Sperlagh B, Csolle C, Ando RD, Goloncser F, Kittel A, Baranyi M. The role of purinergic signaling in depressive disorders. Neuropsychopharmacol Hung. 2012;14:231–238. [PubMed] [Google Scholar]


