Abstract
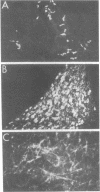
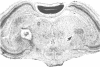
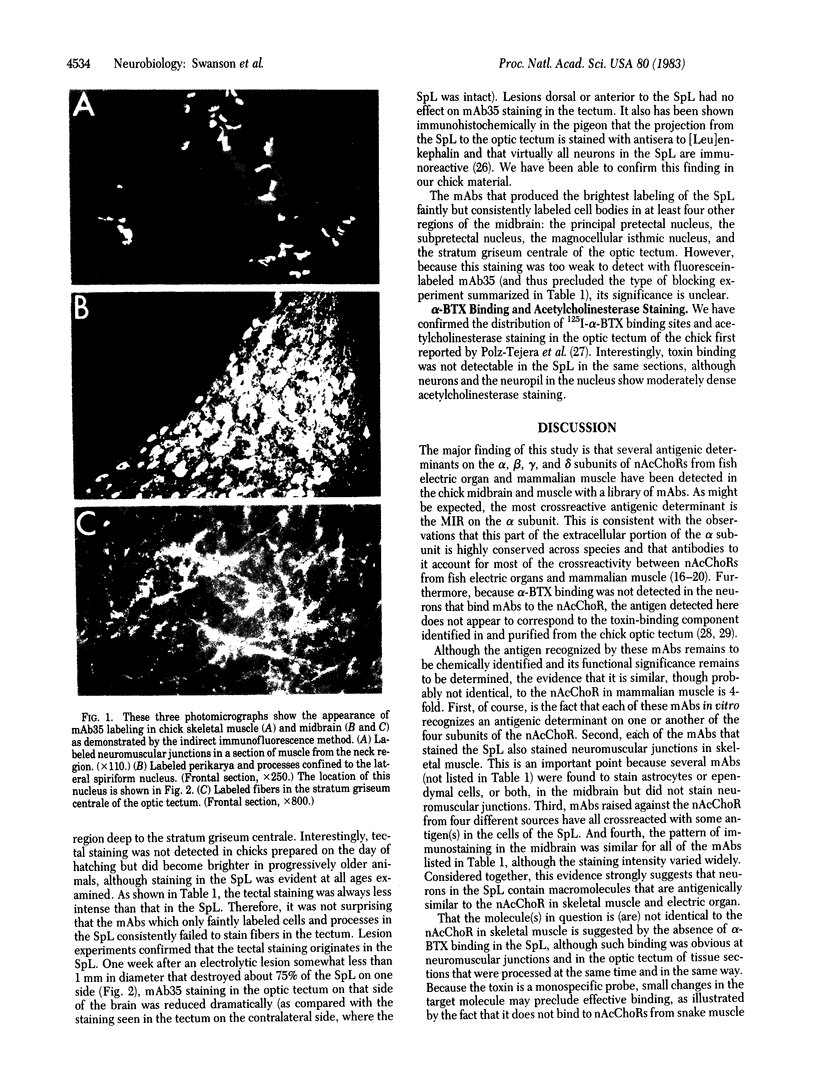
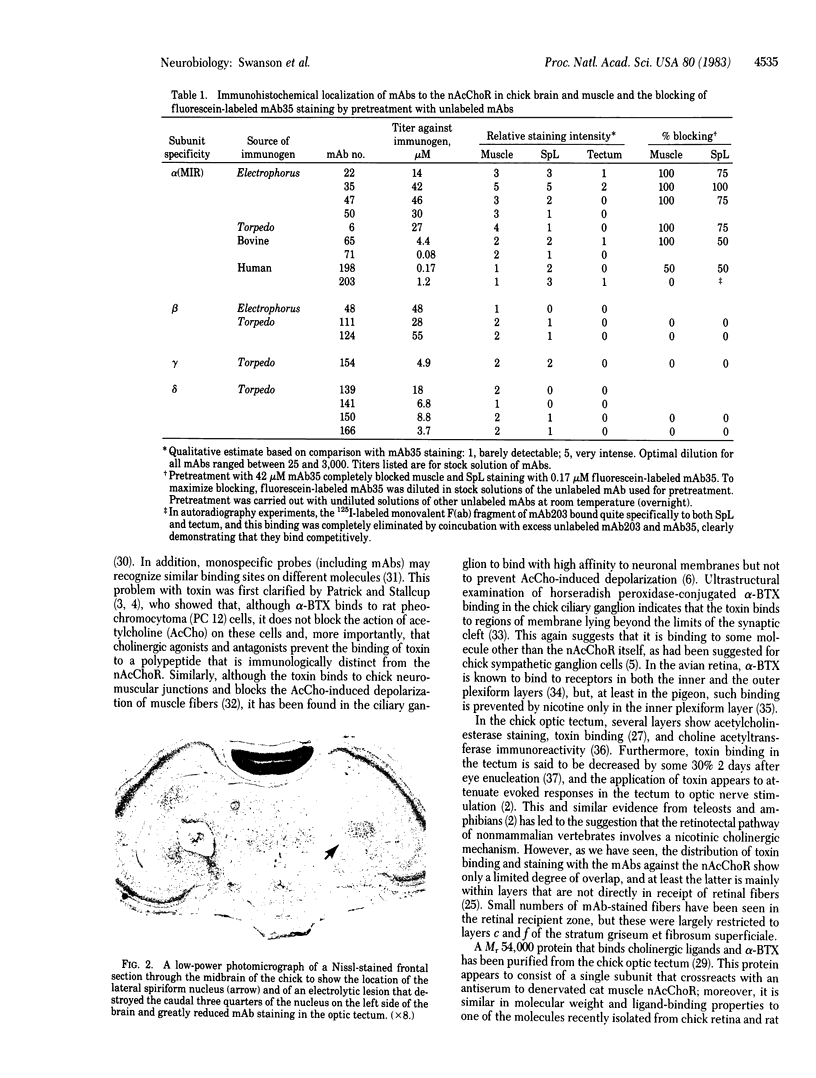
We used the indirect immunofluorescence method to determine the crossreactivity of a library of 57 monoclonal antibodies (mAbs) against each of the subunits of the nicotinic acetylcholine receptor (nAcChoR) isolated from Torpedo and Electrophorus electric organs or from fetal calf and human muscle, with specific neural elements in the midbrain of the chick. Out of 17 mAbs that recognized motor end plates on chick muscle, 14 produced a similar pattern of labeling in the midbrain: the neuronal perikarya and dendrites in the lateral spiriform nucleus (SpL) were intensely labeled, and there was moderate labeling of fibers in certain of the deeper layers of the optic tectum, which disappeared after the SpL was destroyed electrolytically. Two lines of evidence suggest that the mAbs may be crossreacting with nAcChoRs in the midbrain. First, all of the mAbs that stained the SpL also stained neuromuscular junctions in skeletal muscle, whereas none of the 40 mAbs that failed to stain end plates crossreacted with the SpL; second, in vitro immunological studies and blocking experiments on tissue sections (in which unlabeled mAbs were used to block the staining of a directly fluorescein-treated mAb) indicated the presence of mAbs specific for unique antigenic determinants on all four of the subunits (alpha, beta, gamma, and delta) from Torpedo nAcChoR in chick midbrain and muscle. On the other hand, the distribution of mAb staining in the optic tectum does not closely parallel that of either acetylcholinesterase staining or of 125I-labeled alpha-bungarotoxin binding; no toxin binding has been observed autoradiographically in the SpL, but the nucleus does contain moderately dense acetylcholinesterase staining. Take together, our observations suggest that there may be a cholinergic input to the SpL and that the projection fibers from the SpL to the optic tectum (which are also stained with an antiserum to [Leu]enkephalin) may contain presynaptic nAcChoRs. It is clear, however, that the distribution of the putative nAcChoRs, alpha-bungarotoxin binding sites, and acetylcholinesterase staining in the avian midbrain are quite different, although they do overlap to some degree in the deeper layers of the optic tectum.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betz H., Graham D., Rehm H. Identification of polypeptides associated with a putative neuronal nicotinic acetylcholine receptor. J Biol Chem. 1982 Oct 10;257(19):11390–11394. [PubMed] [Google Scholar]
- Brecha N., Francis A., Schechter N. Rapid loss of nicotine-cholinergic receptor binding activity in the deafferented avian optic lobe. Brain Res. 1979 May 11;167(2):273–280. doi: 10.1016/0006-8993(79)90822-9. [DOI] [PubMed] [Google Scholar]
- COWAN W. M., ADAMSON L., POWELL T. P. An experimental study of the avian visual system. J Anat. 1961 Oct;95:545–563. [PMC free article] [PubMed] [Google Scholar]
- Carbonetto S. T., Fambrough D. M., Muller K. J. Nonequivalence of alpha-bungarotoxin receptors and acetylcholine receptors in chick sympathetic neurons. Proc Natl Acad Sci U S A. 1978 Feb;75(2):1016–1020. doi: 10.1073/pnas.75.2.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti-Tronconi B. M., Gotti C. M., Hunkapiller M. W., Raftery M. A. Mammalian muscle acetylcholine receptor: a supramolecular structure formed by four related proteins. Science. 1982 Dec 17;218(4578):1227–1229. doi: 10.1126/science.7146904. [DOI] [PubMed] [Google Scholar]
- Conti-Tronconi B. M., Hunkapiller M. W., Lindstrom J. M., Raftery M. A. Subunit structure of the acetylcholine receptor from Electrophorus electricus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6489–6493. doi: 10.1073/pnas.79.21.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Belleroche J., Bradford H. F. Biochemical evidence for the presence of presynaptic receptors on dopaminergic nerve terminals. Brain Res. 1978 Feb 17;142(1):53–68. doi: 10.1016/0006-8993(78)90176-2. [DOI] [PubMed] [Google Scholar]
- Einarson B., Gullick W., Conti-Tronconi B., Ellisman M., Lindstrom J. Subunit composition of bovine muscle acetylcholine receptor. Biochemistry. 1982 Oct 12;21(21):5295–5302. doi: 10.1021/bi00264a027. [DOI] [PubMed] [Google Scholar]
- Fischbach G. D., Cohen S. A. The distribution of acetylcholine sensitivity over uninnervated and innervated muscle fibers grown in cell culture. Dev Biol. 1973 Mar;31(1):147–162. doi: 10.1016/0012-1606(73)90326-6. [DOI] [PubMed] [Google Scholar]
- Geneser-Jensen F. A. Distribution of acetyl cholinesterase in the hippocampal region of the guinea pig. 3. The dentate area. Z Zellforsch Mikrosk Anat. 1972;131(4):481–495. doi: 10.1007/BF00306966. [DOI] [PubMed] [Google Scholar]
- Gullick W. J., Lindstrom J. M. Structural similarities between acetylcholine receptors from fish electric organs and mammalian muscle. Biochemistry. 1982 Sep 14;21(19):4563–4569. doi: 10.1021/bi00262a008. [DOI] [PubMed] [Google Scholar]
- Henke H., Fonnum F. Topographical and subcellular distribution of choline acetyltransferase and glutamate decarboxylase in pigeon optic tectum. J Neurochem. 1976 Aug;27(2):387–391. doi: 10.1111/j.1471-4159.1976.tb12258.x. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y., Shimada Y. Fluorescent staining of neuromuscular junctions by using the antibody against acetylcholine receptors of Narke japonica, and double staining with the antibody and erabutoxin b. Brain Res. 1981 Nov 9;224(1):45–54. doi: 10.1016/0006-8993(81)91115-x. [DOI] [PubMed] [Google Scholar]
- Jacob M. H., Berg D. K. The ultrastructural localization of alpha-bungarotoxin binding sites in relation to synapses on chick ciliary ganglion neurons. J Neurosci. 1983 Feb;3(2):260–271. doi: 10.1523/JNEUROSCI.03-02-00260.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom J., Einarson B., Tzartos S. Production and assay of antibodies to acetylcholine receptors. Methods Enzymol. 1981;74(Pt 100):432–460. doi: 10.1016/0076-6879(81)74031-x. [DOI] [PubMed] [Google Scholar]
- Lindstrom J., Merlie J., Yogeeswaran G. Biochemical properties of acteylcholine receptor subunits from Torpedo californica. Biochemistry. 1979 Oct 16;18(21):4465–4470. doi: 10.1021/bi00588a003. [DOI] [PubMed] [Google Scholar]
- Lindstrom J., Walter B., Einarson B. Immunochemical similarities between subunits of acetylcholine receptors from Torpedo, Electrophorus, and mammalian muscle. Biochemistry. 1979 Oct 16;18(21):4470–4480. doi: 10.1021/bi00588a004. [DOI] [PubMed] [Google Scholar]
- Morley B. J., Kemp G. E. Characterization of a putative nicotinic acetylcholine receptor in mammalian brain. Brain Res. 1981 Aug;228(1):81–104. doi: 10.1016/0165-0173(81)90013-8. [DOI] [PubMed] [Google Scholar]
- Nigg E. A., Walter G., Singer S. J. On the nature of crossreactions observed with antibodies directed to defined epitopes. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5939–5943. doi: 10.1073/pnas.79.19.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman R. I., Mehraban F., Barnard E. A., Dolly J. O. Nicotinic acetylcholine receptor from chick optic lobe. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1321–1325. doi: 10.1073/pnas.79.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald R. E., Freeman J. A. Alpha-bungarotoxin binding and central nervous system nicotinic acetylcholine receptors. Neuroscience. 1981;6(1):1–14. doi: 10.1016/0306-4522(81)90239-6. [DOI] [PubMed] [Google Scholar]
- Patrick J., Stallcup B. alpha-Bungarotoxin binding and cholinergic receptor function on a rat sympathetic nerve line. J Biol Chem. 1977 Dec 10;252(23):8629–8633. [PubMed] [Google Scholar]
- Patrick J., Stallcup W. B. Immunological distinction between acetylcholine receptor and the alpha-bungarotoxin-binding component on sympathetic neurons. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4689–4692. doi: 10.1073/pnas.74.10.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polz-Tejera G., Schmidt J., Karten H. J. Autoradiographic localisation of alpha-bungarotoxin-binding sites in the central nervous system. Nature. 1975 Nov 27;258(5533):349–351. doi: 10.1038/258349a0. [DOI] [PubMed] [Google Scholar]
- Raftery M. A., Hunkapiller M. W., Strader C. D., Hood L. E. Acetylcholine receptor: complex of homologous subunits. Science. 1980 Jun 27;208(4451):1454–1456. doi: 10.1126/science.7384786. [DOI] [PubMed] [Google Scholar]
- Ravdin P. M., Berg D. K. Inhibition of neuronal acetylcholine sensitivity by alpha-toxins from Bungarus multicinctus venom. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2072–2076. doi: 10.1073/pnas.76.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A., Brecha N. C., Karten H. J. Basal ganglia pathways to the tectum: the afferent and efferent connections of the lateral spiriform nucleus of pigeon. J Comp Neurol. 1982 Jun 10;208(1):16–36. doi: 10.1002/cne.902080103. [DOI] [PubMed] [Google Scholar]
- Reiner A., Karten H. J., Brecha N. C. Enkephalin-mediated basal ganglia influences over the optic tectum: immunohistochemistry of the tectum and the lateral spiriform nucleus in pigeon. J Comp Neurol. 1982 Jun 10;208(1):37–53. doi: 10.1002/cne.902080104. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Karlin A. Molecular weight in detergent solution of acetylcholine receptor from Torpedo californica. Biochemistry. 1978 May 30;17(11):2035–2038. doi: 10.1021/bi00604a001. [DOI] [PubMed] [Google Scholar]
- Sakurai Y., Takano Y., Kohjimoto Y., Honda K., Kamiya H. O. Enhancement of [3H]dopamine release and its [3H]metabolites in rat striatum by nicotinic drugs. Brain Res. 1982 Jun 17;242(1):99–106. doi: 10.1016/0006-8993(82)90499-1. [DOI] [PubMed] [Google Scholar]
- Sumikawa K., Barnard E. A., Dolly J. O. Similarity of acetylcholine receptors of denervated, innervated and embryonic chicken muscles. 2. Subunit compositions. Eur J Biochem. 1982 Sep 1;126(3):473–479. doi: 10.1111/j.1432-1033.1982.tb06804.x. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Sawchenko P. E., Rivier J., Vale W. W. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36(3):165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Tzartos S. J., Lindstrom J. M. Monoclonal antibodies used to probe acetylcholine receptor structure: localization of the main immunogenic region and detection of similarities between subunits. Proc Natl Acad Sci U S A. 1980 Feb;77(2):755–759. doi: 10.1073/pnas.77.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzartos S. J., Rand D. E., Einarson B. L., Lindstrom J. M. Mapping of surface structures of electrophorus acetylcholine receptor using monoclonal antibodies. J Biol Chem. 1981 Aug 25;256(16):8635–8645. [PubMed] [Google Scholar]
- Tzartos S. J., Seybold M. E., Lindstrom J. M. Specificities of antibodies to acetylcholine receptors in sera from myasthenia gravis patients measured by monoclonal antibodies. Proc Natl Acad Sci U S A. 1982 Jan;79(1):188–192. doi: 10.1073/pnas.79.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel Z., Nirenberg M. Localization of acetylcholine receptors during synaptogenesis in retina. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1806–1810. doi: 10.1073/pnas.73.6.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. K., Molinaro S., Schmidt J. Ligand responses of alpha-bungarotoxin binding sites from skeletal muscle and optic lobe of the chick. J Biol Chem. 1978 Dec 10;253(23):8507–8512. [PubMed] [Google Scholar]
- Yazulla S., Schmidt J. Two types of receptors for alpha-bungarotoxin in the synaptic layers of the pigeon retina. Brain Res. 1977 Dec 9;138(1):45–57. doi: 10.1016/0006-8993(77)90783-1. [DOI] [PubMed] [Google Scholar]