Abstract
Background
By promoting salt and water excretion, the corin and the atrial natriuretic peptide (ANP) system should help to maintain fluid balance in heart failure. Yet, the development of fluid retention despite high levels of ANP-related peptides, suggests that this compensatory system is limited.
Methods and Results
Levels of circulating corin (the pro-ANP converting enzyme) and pro-ANP were measured in hospitalized patients with heart failure, using novel immunoassays. Patients (n = 14) had severe heart failure (NYHA class III–IV) with a median ejection fraction of 18 % and median BNP levels of 1940 pg/ml. In heart failure, median plasma corin levels were 7.6-fold lower than measured in plasma from 16 normal controls (180 vs. 1368 pg/ml, p<0.01). In contrast, in heart failure patients, levels of plasma N-terminal ANP peptides (N-ANP and pro-ANP) levels were markedly elevated (42.0 vs. 7.5 ng/ml, p<0.01). Levels of uncleaved pro-ANP, measured by novel immunoassays, were significantly higher in heart failure patients (p < 0.01) suggesting that corin cleavage of pro-ANP was impaired. Median plasma levels of cGMP were elevated in heart failure patients (150.0 vs. 7.6 pmol/ml, p<0.01) and plasma cGMP levels positively correlated with the fractional amount of cleaved pro-ANP (rs = 0.59, p < 0.03) but not with levels of uncleaved pro-ANP, implying that the cellular response to ANP remained intact.
Conclusions
Taken together these data suggest that there may be patients for whom low corin levels and impaired pro-ANP cleavage contribute to acute decompensation.
Keywords: atrial natriuretic peptide, natriuretic peptides, heart failure
Heart failure is a major public health problem that affects more than 4.5 million Americans; about 550,000 new cases are diagnosed each year1, 2. Despite treatment advances, heart failure is associated with high morbidity and, half of heart failure patients die within 5 years3. For unknown reasons, individuals with the same degree of impaired heart function can show marked differences in the development of heart failure symptoms,4, 5 suggesting that factors other than cardiac function contribute to this condition.
Corin is a serine protease in the heart6 that is primarily responsible for cleavage-activation of pro-atrial natriuretic peptide (pro-ANP) to ANP7. Although corin expression has not been quantified in humans with heart failure, a previous study reported that cardiac tissue levels of corin were not altered in an autopsy specimen from a patient with total anomalous pulmonary venous return8. Corin transcripts and protein are abundantly expressed in cardiomyocytes of both the atrium and ventricle, often in cells that also contain pro-ANP6, 8, 9. Corin gene expression is increased in the hypertrophied regions of the ventricle of the heart after coronary ligation in rats10 but corin is decreased in the atrium after the development of experimental heart failure in rats11. This suggests that the activity of corin and, the subsequent cleavage of pro-ANP, may be altered in humans who develop heart failure and that corin may be involved in the pathogenesis of heart failure.
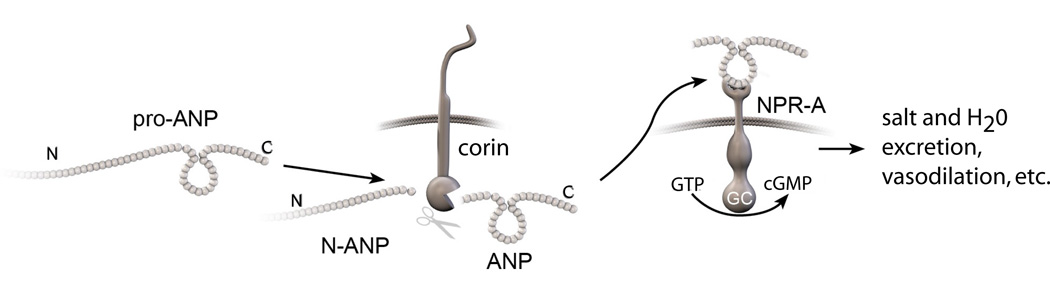
In individuals with diminished heart function there is increased production of pro-ANP12–16. Pro-ANP is a pro-hormone that is secreted from heart and cleaved to an N-terminal fragment (N-ANP) and a C-terminal, biologically active peptide (ANP) by the heart-specific serine protease corin (Figure 1)7,17. ANP, but not pro-ANP, binds to the natriuretic peptide-A receptor (NPR-A) to initiate signaling through 3'-5'-cyclic guanosine monophosphate (cGMP)18, 19. Through this mechanism ANP triggers sodium excretion (natriuresis), causes vasodilation, interferes with the renin-angiotensin system, inhibits mitogenesis, and mediates other effects14, 20, 21. These effects of the ANP can be beneficial in patients with impaired heart function and heart failure22 and there is evidence that infusions of ANP ameliorate heart failure23, 24.
Figure 1. The corin-ANP system.
Pro-ANP is cleaved by the serine protease corin to N-ANP and ANP9. ANP activates natriuretic peptide receptor-A (NPR-A), a transmembrane guanylyl cyclase. NPR-A catalyzes the synthesis of cGMP, which mediates salt & water excretion, vasodilation and other effects19.
It is an apparent paradox that the highest levels of ANP-related peptides (pro-ANP, N-ANP and ANP) occur in patients with severe heart failure who have the worse prognosis25–28. Why high levels of ANP-related peptides fail to avert heart failure is poorly understood. We hypothesized that defects in the corin-ANP system may occur in heart failure patients that lead to impaired cleavage of pro-ANP to the active peptide ANP. Unfortunately, current immunoassays can’t separately measure pro-ANP, N-ANP or ANP peptides, because they use antibodies directed to the N-terminal portion or C-terminal portion of the molecule. For example, immunoassays directed against N-terminal sequences in pro-ANP detect both pro-ANP and N-ANP; immunoassays directed against C-terminal sequences in ANP detect both pro-ANP and ANP. To examine if the corin-ANP system was altered in humans with heart failure, we developed novel assays to measure plasma levels of pro-ANP, N-ANP and corin.
Methods
Study Design
The study protocol and consent form were approved by IRB of the Medical College of Georgia, Augusta, Georgia, USA. Plasma samples were obtained from healthy normal blood donors (16 total) and from 14 patients admitted to the hospital with a diagnosis of acute decompensated heart failure due to impaired heart systolic function (ejection fraction < 35%). Heart failure patients provided baseline demographics and medical history such as age, history of hypertension, hyperlipidemia, smoking, and family history of premature coronary heart disease. Heart failure patients were excluded who had conditions that might also affect the natriuretic system such as chronic kidney disease (estimated glomerular filtration rate <60ml/min/1.73m2), pulmonary hypertension (pulmonary artery pressure >55mmHg by transthoracic echocardiography), myocardial infarction within the last 6 weeks, critical valvular heart disease, metastatic or terminal cancer. Demographic data on controls were obtained from the Medical College of Georgia Blood Bank.
Blood Sample Collection
Venous blood samples were collected from consenting subjects on admission using standard EDTA-aprotinin tubes and immediately stored on ice. These inhibitors block enzymes in the blood known to affect the degradation of pro-ANP29. The blood samples were centrifuged at 3000×g for 20 min at 4°C, aliquoted, and stored at −80°C until analysis.
Heart failure Biomarkers Measurement
Assays were performed by personnel unaware of the patient’s identity and outcome. BNP-related peptides were measured by the standard clinical laboratory method. Elevated BNP levels were defined as those values above the 95th percentile of normal30. The cGMP was measured with an enzyme immunoassay (R&D Systems, Inc., Minneapolis, MN).
Plasma N-terminal-related ANP peptides (pro-ANP + N-ANP) were measured using an enzyme immunoassay directed against an N-terminal amino acid sequence (26–55 of pre-pro ANP, or 1–30 of pro-ANP, EK-005-19, Phoenix Pharm., Inc.). This ELISA measures pro-ANP and N-ANP but has no reactivity with C-ANP-related peptides such as ANP. In order to separately measure pro-ANP and N-ANP we altered the assay process to immunodeplete all C-ANP-related peptides (pro-ANP and C-ANP but not N-ANP) from samples using an antibody directed against C-ANP (G-005-06, ANP1-28, Phoenix Pharm, Inc.). This process then allowed direct measurement of the N-ANP remaining in the plasma. Plasma samples were diluted with 0.05% Tween 20 in PBS and applied into a 96-well, U-bottom flexible microplate (Becton Dickinson Labware, Franklin Lakes, NJ) pre-coated with either purified rabbit anti-human C-ANP (1–28) IgG (5 µg/ml PBS, Phoenix Pharm., Inc) or with control rabbit IgG (5 µg/ml PBS) sealed and incubated for 1 h at room temperature. Subsequently we measured the N-ANP in the plasma depleted of C-ANP-related peptides or, both N-ANP and pro-ANP in control-depleted plasma samples (after pretreatment with control IgG) using the N-terminal enzyme immunoassay (EK-005-19, Phoenix Pharm., Inc.). In our assays, N-ANP (26–55) or purified recombinant pro-ANP (see below) was used as a control. Pro-ANP values in the plasma samples were determined by the difference between the measured N-ANP and the total N-ANP-related peptides (pro-ANP and N-ANP). To confirm the complete depletion of C-ANP related peptides (pro-ANP and C-ANP) in these assays we used an enzyme immunoassay directed against an C-terminal amino acid sequence (ANP 1–28) contained in C-ANP (EK-005-06, Phoenix Pharm., Inc.) and recombinant pro-ANP31 proteins. Under these assay conditions the specific anti-C-ANP IgG, but not the control rabbit IgG, successfully depleted recombinant proteins at concentrations that exceed the highest values measured in all our plasma samples. When the ANP-related peptides were measured using this immunodepletion method the sensitivity of these assays increased32.
Immunoblots were performed to identify pro-ANP in plasma. Plasma (1 ml) was incubated with immobilized antibody directed against full-length pro-ANP antibody (FL-153, Santa Cruz Biotechnology) for 2–4 hours at 4 deg. After washing, bound proteins were eluted with sample buffer (100° C) and subjected to SDS-PAGE under reducing conditions. After electrophoretic transfer, blots were probed with antibodies directed to sequences in the N-terminus (prepro-atrial natriuretic peptide (26–55), Bachem Americas) and the C-terminus of pro-ANP (ANP alpha (1–28), Phoenix Pharm.). Blots were probed with IRDye-680 goat-anti-rabbit secondary antibodies and exposed on Li-Cor Odyssey Infrared Imaging System (Li-Cor Biosciences).
Enzyme Immunoassay for human corin in plasma
A sandwich ELISA assay was developed to measure human corin in plasma samples using DuoSet ELISA Development kit (R&D Systems). Plasma samples were diluted 2–3 fold with ELISA assay buffer, 0.05% Tween 20 in PBS, pH 7.2–7.4. To precipitate ballast proteins and amplify corin binding to the capture antibody32, diluted plasma samples were applied for 40 min. to a 96-well, U-bottom flexible microplate (Becton Dickinson Labware, Franklin Lakes, NJ) pre-coated with non-specific rabbit IgG (5 µg/ml PBS) purified by protein A-Sepharose (Pierce, Rockford, IL). For the ELISA assay wells of a flat-bottom microplate (R&D Systems) were pre-coated with the capture anti-human corin rat monoclonal antibody (4 µg/ml PBS) produced against the recombinant extracellular domain of corin 67–1042 amino acids (R&D Systems). The plate was sealed and incubated over night. Non-specific binding sites were block with 1% BSA in PBS for 1 h. Recombinant human corin protein was used as a standard for this assay. All assay steps were performed at room temperature and with orbital shaking. Plasma samples after pretreatment with control rabbit IgG or standards were applied to the plate pre-coated with capture antibody and incubated 2 h. After multiple washing with 0.05% Tween 20 in PBS, pH 7.2–7.4, captured corin was detected with biotinylated polyclonal goat anti-human corin antibody (raised against recombinant extracellular domain of corin spanning amino acids 67–1042 (R&D Systems). After incubation for 2 h followed by 4 washes, the wells were incubated with streptavidin-horseradish peroxidase for 30 min protected from light. After repeat washing, horse-radish peroxidase substrate solution (1:1 mixture of H2O2 and tetramethylbenzidine, R&D Systems) was added to the plate, and the reaction was stopped after 30 min by 2N HCl. Optical density was measured immediately at 450 nm, using a microplate reader (Bio-Tek Instruments Inc.). The assay sensitivity for corin was 30–50 pg/ml.
Recombinant pro-ANP protein
Pro-ANP cDNA was amplified from a Human Adult 8 Tissue GenePools cDNA library (NT Omics, Inc San Mateo, CA) by PCR31. The pro-ANP cDNA was sequenced for confirmation, cloned into the pcDNA 3.1/V5-His-TOPO vector (Invitrogen, Carlsbad, CA) using the pcDNA3.1/V5-His-TOPO TA Expression Kit (Invitrogen) to yield the plasmid, pcDNA-pro-ANP. Recombinant pro-ANP was expressed in human embryonic kidney (HEK) 293 cells (American Type Culture Collection, Manassas, VA) using Lipofectin (Invitrogen) according to the manufacturer’s protocol. The pro-ANP in the conditioned medium (serum-free Opti-MEM, Invitrogen) was measured with C-ANP enzyme immunoassay (Phoenix Pharm., Inc.).
Statistical Analysis
Unless otherwise indicated, continuous data are presented in tabular form or in box plots indicating the medians with upper and lower quartile values. Data were analyzed by non-parametric methods (Mann Whitney tests and Spearman’s rank correlation-rs). A two-tailed p-value < 0.05 was considered statistically significant.
Results
For the heart failure patients, the mean BNP level on hospital admission was 1940 pg/ml (Table 1) which is markedly higher than the upper limit of normal in our hospital (<100 pg/ml). The mean age of heart failure patients was 49 years and 57% were males. Half of the heart failure patients had ischemic cardiomyopathy and half had dilated cardiomyopathy. Most (93%) had severe heart failure symptoms (NYHA class III and IV). The median ejection fraction of 18% was significantly reduced.
Table.
Characteristics of Heart Failure Patients and Controls
| Heart Failure Patients | Controls | ||
| Age (years) | Median* | 49 (35,56) | 45 (35,55) |
| Range | 26–74 | 16–69 | |
| Gender | Male | 57% | 51% |
| Female | 43% | 49% | |
| Etiology | Ischemic Cardiomyopathy | 50% | - |
| Dilated Cardiomyopathy | 50% | - | |
| Admission BNP (pg/ml)* | 1940 (967,2725) | - | |
| LVEF (%)* | 18% (15,28) | - | |
| NYHA Class | II | 7.1 % | - |
| III | 42.9% | - | |
| IV | 50% | - | |
median (25%ile, 75%ile) of demographic data on heart failure patients and control group. Samples were obtained from 14 patients and 16 controls.
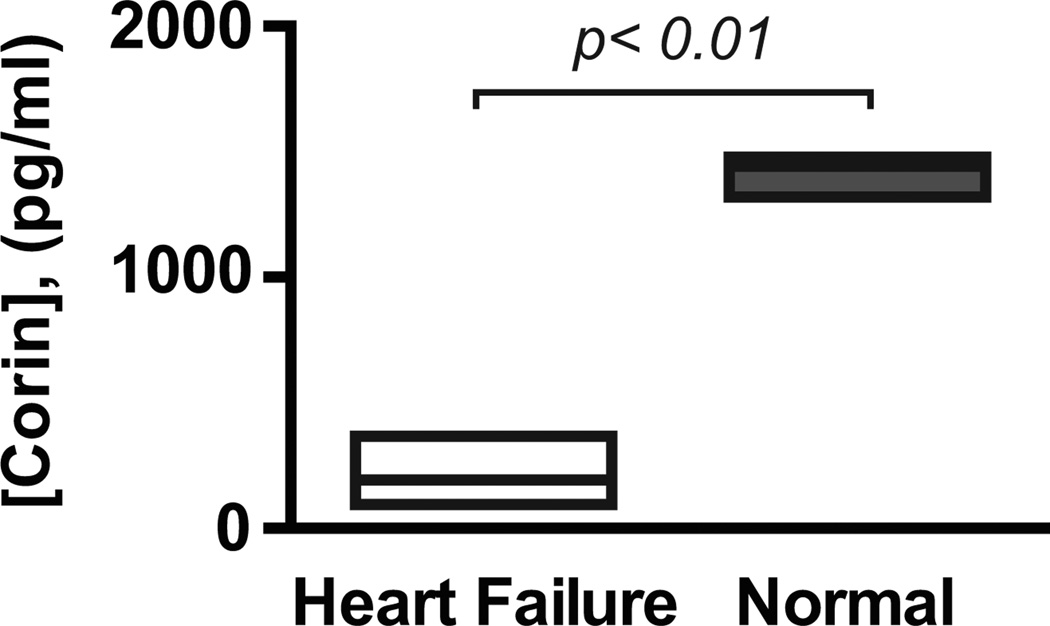
In the initial immunoassay, corin was undetectable in the plasma from several heart failure patients, although it was readily measured in normal plasma samples. To increase the sensitivity of the test, we reconfigured the immunoassay to remove ballast proteins32. Subsequently corin was measurable in all subjects. The median plasma corin level was 7.6-fold lower in heart failure patients than normal controls (180 pg/ml vs. 1368 pg/ml, p<0.01; Figure 2). In immunoassays using our previously reported anti-corin monoclonal antibodies6 we also found that corin levels were markedly lower in patients with heart failure (data not shown). There were no significant differences in median corin levels between men and women (286 vs. 99 pg/ml, p =0.35).
Figure 2. Plasma levels of corin.
Box plots showing corin concentrations (median, upper and lower quartiles) in plasma of individuals with acute heart failure (n = 14) and in pooled normal human plasmas (from 16 donors). p<0.01 by Mann Whitney analysis.
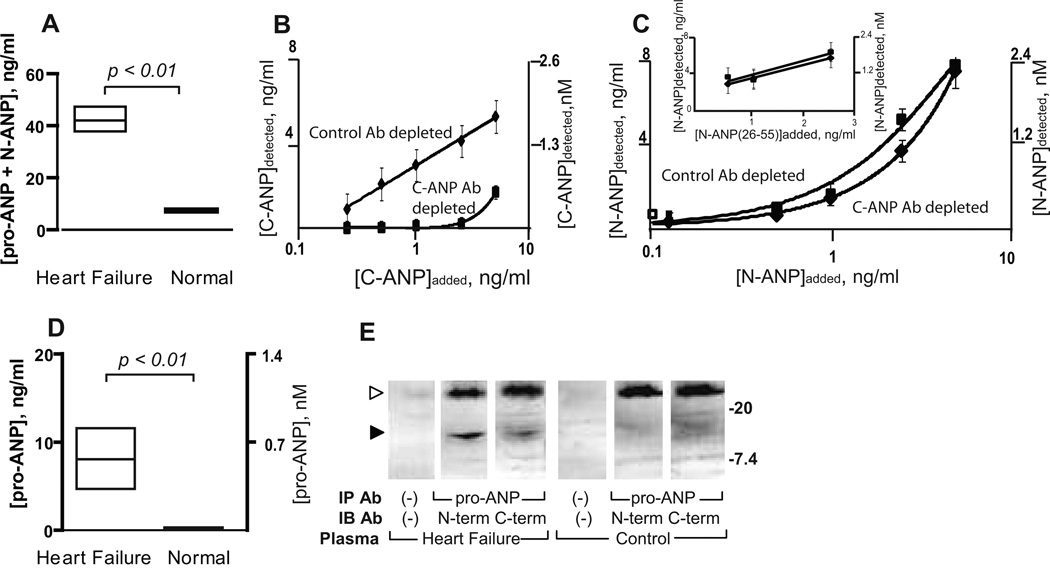
Lower corin levels may signify a reduced capacity to cleave pro-ANP in patients with heart failure. An immunoassay, which detects the N-terminal ANP peptides N-ANP and pro-ANP, showed that these median peptide levels were significantly higher in patients with heart failure than in the plasma from normal individuals (42.0 vs. 7.5 ng/ml, p<0.01, Figure 3A). To assess the cleavage of pro-ANP, we developed an assay that allowed us to measure separately the levels of pro-ANP and N-ANP, a biologically stable cleavage product. Plasma samples were treated with a control, non-specific antibody or with an antibody directed against C-ANP related peptides to immunodeplete or remove ANP and pro-ANP. The C-ANP antibody completely removed ~1.4 nM of C-ANP related peptides from plasma (which exceeded the pro-ANP in our samples-see below), but the control antibody did not (Figure 3B). Pre-treatment with the C-antibody was specific in that it did not affect levels of N-ANP peptides measured by comparison to pre-treatment with the control antibody (Figure 3C). The remaining N-ANP peptide was then measured in the plasma immunodepleted of C-ANP-related peptides. In the heart failure patients the amount of cleaved pro-ANP (N-ANP) rose as the total levels of pro-ANP and N-ANP increased (rs =0.58, p < 0.03). In normal plasma, essentially all pro-ANP was cleaved to N-ANP and the circulating pro-ANP was not significantly different from 0 ng/ml (p =0.18). However, in the patients with heart failure, the levels of uncleaved pro-ANP were significantly higher than in normal plasma (Figure 3D, p<0.01) indicating that corin cleavage of pro-ANP was impaired in these patients. Pro-ANP could be detected in heart failure plasma but not normal plasma by immunoblotting (Figure 3E).
Figure 3. Pro-ANP cleavage in plasma from patients with acute heart failure in comparison with normal plasma.
(A) The levels of N-terminal ANP-related peptides (pro-ANP + N-ANP) in the plasmas of individuals with acute heart failure and that of normal individuals (box plot showing median, upper and lower quartiles). (B) Depletion with C-ANP antibody but not control, non-specific antibody quantitatively removes C-ANP from samples. The mean level of C-ANP (ANP) peptide in samples after preincubation with anti-C-ANP antibody or with control antibody was determined by an immunoassay for C-ANP. (C) Depletion with C-ANP antibody does not affect N-ANP levels in samples. The mean level of N-ANP after preincubation with anti-C-ANP antibody or control antibody in assay buffer was determined by an immunoassay for N-ANP. Inset, the level of N-ANP after pre-incubation with anti-C-ANP or control antibody in plasma. (D) The levels of uncleaved pro-ANP in plasma of individuals with acute heart failure and in normal plasma (box plot showing median, upper and lower quartiles). (E) Pro-ANP is detected by immunoblotting in heart failure plasma but not normal plasma. Pro-ANP was immunoprecipitated (IP) from heart failure or normal plasma by a pro-ANP antibody or no antibody and subjected to SDS-PAGE under reducing conditions. Membranes were immunoblotted (IB) with antibodies directed to the N-terminal sequence of pro-ANP (N-term), the C-terminal sequence of pro-ANP (C-term) or no antibody. Filled arrow identifies pro-ANP staining with both N-terminal and C-terminal antibodies; unfilled arrow identifies the light chain of the immunoprecipitating antibodies. Relative migration of molecular standards in kilo-Daltons is shown at right.
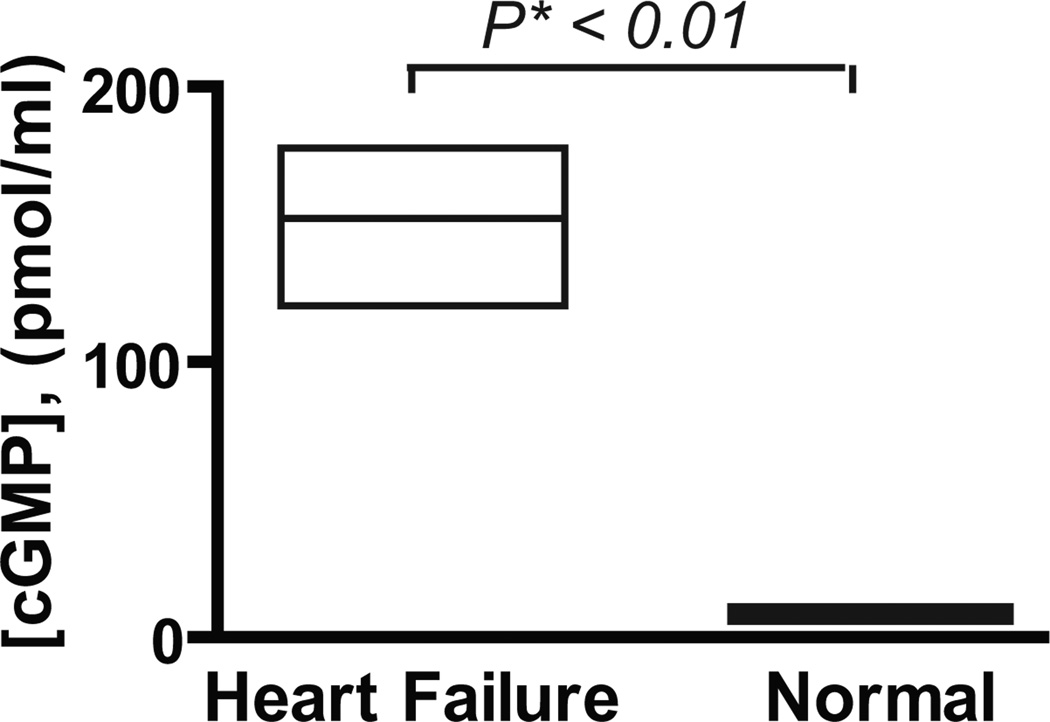
After cleavage of pro-ANP, ANP acts on cells through the natriuretic peptide receptor A to increase cGMP levels and execute its biological effects (Figure 1)18. Plasma levels of cGMP were much higher (20-fold) in patients with heart failure than in normal individuals (150 vs. 7.6 pmol/ml, p<0.01, Figure 4). Still, in these heart failure patients there was no significant correlation between the levels of cGMP and levels of N-terminal ANP-related peptides as measured by a conventional immunoassay (rs = −0.47, p =0.09). In addition there was no significant relationship between levels of cGMP and levels of pro-ANP (rs = −0.42, p =0.14). However, there was a significant positive correlation between the fractional amount of cleaved pro-ANP and cGMP levels (rs = 0.59, p <0.03).
Figure 4. Levels of cGMP and cleaved pro-ANP in patients with heart failure.
Box plot showing the median, upper and lower quartiles of cGMP level in plasma of individuals with heart failure and normal human plasma.
Discussion
ANP is an important regulator of physiologic volume in heart failure through its effects on salt and water excretion33. The observation that patients develop worsening salt and water overload despite very high levels of ANP-related peptides has been perplexing. Indeed, the severity of heart failure is linked to levels of ANP-related peptides34–36. While patients with severe heart failure develop high levels of ANP-related peptides and high levels of cGMP, our study and others have shown that there is no correlation between cGMP levels and total ANP-related peptides5, 37. We postulated that one explanation for the loss of the relationship between levels of ANP-related peptides and cGMP could be that corin levels and pro-ANP cleavage were reduced in severe heart failure. Consistent with this notion, in normal plasma corin was readily detectable (Figure 2) and pro-ANP was absent, because it was fully cleaved to N-ANP (Figure 3D). By comparison, in plasma from heart failure patients, corin was markedly decreased (Figure 2) and pro-ANP was readily detected because it was not all cleaved to N-ANP (Figure 3D). Still, in the heart failure patients, cGMP levels were positively correlated with the levels of cleaved pro-ANP, which suggests that at the cellular level, patients retain some degree of responsiveness to biologically active forms of ANP. Supporting this notion, recent studies in patients with severe heart failure have shown that infusions of ANP (carperitide) increase cGMP levels, which indicates that these patients retain responsiveness to biologically active forms of ANP at the cellular and receptor level.38
In addition to decreased corin levels and decreased pro-ANP cleavage, other defects not examined in this study, could occur in the natriuretic peptide system that may contribute to heart failure such as alterations in NPR clearance and signaling, the bioavailability of natriuretic peptides and, the degradation of natriuretic peptides.39, 40 While this paper was in review, a retrospective study by Rame et al. found that a polymorphism in corin I555 (P568) was associated with lower BNP and pro-BNP levels as well as worse outcomes in the A-HeFT study.41 It has been suggested that in some patients with severe heart failure, the responsiveness of the kidney to increasing levels of ANP-related peptides is diminished, although vasodilatory effects persist.42, 43 Renal hyporesponsiveness to ANP has been attributed to many potential factors including the counter-regulatory effects of the renin-angiotensin-aldosterone-sympathetic system.
There are limitations to the conclusions that can be drawn from this study. Since our study focused on acute heart failure by comparison to normal controls (blood donors), we are unable to determine whether changes in circulating corin levels and altered natriuretic processing are also seen in individuals with chronic heart failure, impaired systolic function without heart failure, or other cardiomyopathies or conditions. The relatively small sample in this study may have reduced our statistical power to detect correlations between levels of corin expression and age, sex or other variables. Due to limited information on the controls we are unable to determine whether other factors besides heart failure account for the differences we observed. In the absence of myocardial expression studies, we are unable to determine whether reduced corin levels reflect diminished cardiac production, cleavage, etc.
These studies demonstrate a plausible link between levels of uncleaved pro-ANP and low circulating corin in these patients with heart failure. Corin is a transmembrane protease originally identified in cardiomyocytes by homology to serine proteases and LDL-like molecules9, 44. Although corin can cleave pro-ANP and pro-BNP in vitro, there is only strong evidence that corin cleaves pro-ANP45 in vivo. In contrast, pro-BNP appears to be cleaved in secretory granules in the heart46–49 and the relationship between corin cleavage of pro-BNP and heart failure is unknown. Mice lacking corin have mild hypertension and lack detectable ANP45. Polymorphisms in the corin gene have been associated with increased risk of hypertrophy in the setting of systolic hypertension50, 51. These polymorphisms are associated with decreased activation of corin (zymogen) and thereby decreased corin activity52. Although there are no reports of circulating corin levels in humans, other transmembrane and matrix proteins such as P-selectin, matrix metalloproteases (1, 2, 9) and their endogenous tissue inhibitors circulate in the blood and have pathophysiologic significance in cardiovascular disease53–59. Many of these molecules retain biological activity. Although we were unable to assess the proteolytic activity of corin in our samples (due to the addition of inhibitors to prevent proteolysis of pro-ANP and corin), it has been shown that soluble forms of recombinant corin cleave recombinant pro-ANP molecules in human plasma60. Since the heart is the primary site for the production of corin, decreased plasma corin levels may reflect myocardial disease, but additional studies will be required to determine the linkage between myocardial corin expression, plasma corin levels and corin activity. The reduced plasma levels of corin may be due to diminished cardiac expression of this protein, enhanced protein cleavage or, production of splice variants that lack transmembrane domains.
This small study is the first to evaluate the corin-ANP system in humans and, larger studies, in more diverse populations, will be necessary to generalize our findings.61 Still, the ability to accurately measure both the cleaved and uncleaved forms of pro-ANP should provide further insights into the physiologic and pathologic regulation of the corin-natriuretic system. These assays may identify patients for whom acute decompensated heart failure is the outcome of impaired pro-ANP cleavage. They may also provide a means for phenotyping individuals for personalized treatment with ANP or other therapies. In addition, levels of circulating corin may have additional diagnostic and prognostic value as a surrogate marker in individuals with suspected heart disease.
Clinical Summary.
Levels of atrial and B-type natriuretic peptides (ANP, BNP) are elevated in patients with heart failure with fluid retention. However, ANP and BNP should help prevent fluid retention because they facilitate sodium excretion (natriuresis), cause vasodilation and interfere with the renin-angiotensin system. Corin, a heart-specific enzyme plays an important role in this system by cleaving and activating pro-ANP to ANP. In a cohort of patients with reduced systolic function and decompensated heart failure, we found markedly reduced levels of circulating corin and evidence that cleavage of pro-ANP to ANP was impaired. Taken together, these findings suggest that low corin levels and impaired pro-ANP cleavage may contribute to fluid retention in some patients with heart failure.
Acknowledgments
We thank Michael Konomos for the diagram preparation.
Sources of Funding
This work was supported in part by the NIH (HL58496, HL78562 to G.L.R.) and by an American Heart Association grant (SDG 0835376N) and a start up grant from the Medical College of Georgia (to I.P.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A portion of this study was reported in abstract and oral form for the 2009 Young Investigator’s Award of the American College of Cardiology
Disclosures
MCG has recently applied for patents related to this research. Otherwise none.
References
- 1.Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358:2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 2.Collins SP, Ronan-Bentle S, Storrow AB. Diagnostic and prognostic usefulness of natriuretic peptides in emergency department patients with dyspnea. Ann Emerg Med. 2003;41:532–545. doi: 10.1067/mem.2003.113. [DOI] [PubMed] [Google Scholar]
- 3.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33:1948–1955. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 4.Franciosa JA, Park M, Levine TB. Lack of correlation between exercise capacity and indexes of resting left ventricular performance in heart failure. Am J Cardiol. 1981;47:33–39. doi: 10.1016/0002-9149(81)90286-1. [DOI] [PubMed] [Google Scholar]
- 5.Chati Z, Mertes PM, Aliot E, Zannad F. Plasma levels of atrial natriuretic peptide and of other vasoconstricting hormones in patients with chronic heart failure: relationship to exercise capacity. International journal of cardiology. 1996;57:135–142. doi: 10.1016/s0167-5273(96)02822-7. [DOI] [PubMed] [Google Scholar]
- 6.Gladysheva IP, Robinson BR, Houng AK, Kovats T, King SM. Corin is co-expressed with pro-ANP and localized on the cardiomyocyte surface in both zymogen and catalytically active forms. J Mol Cell Cardiol. 2008;44:131–142. doi: 10.1016/j.yjmcc.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci U S A. 2000;97:8525–8529. doi: 10.1073/pnas.150149097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hooper JD, Scarman AL, Clarke BE, Normyle JF, Antalis TM. Localization of the mosaic transmembrane serine protease corin to heart myocytes. Eur J Biochem. 2000;267:6931–6937. doi: 10.1046/j.1432-1033.2000.01806.x. [DOI] [PubMed] [Google Scholar]
- 9.Yang J, Furie BC, Furie B. The biology of P-selectin glycoprotein ligand-1: its role as a selectin counterreceptor in leukocyte-endothelial and leukocyte-platelet interaction. Thrombosis and haemostasis. 1999;81:1–7. [PubMed] [Google Scholar]
- 10.Tran KL, Lu X, Lei M, Feng Q, Wu Q. Upregulation of corin gene expression in hypertrophic cardiomyocytes and failing myocardium. Am J Physiol Heart Circ Physiol. 2004;287:H1625–H1631. doi: 10.1152/ajpheart.00298.2004. [DOI] [PubMed] [Google Scholar]
- 11.Langenickel TH, Pagel I, Buttgereit J, Tenner K, Lindner M, Dietz R, Willenbrock R, Bader M. Rat corin gene: molecular cloning and reduced expression in experimental heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1516–H1521. doi: 10.1152/ajpheart.00947.2003. [DOI] [PubMed] [Google Scholar]
- 12.de Bold AJ. Atrial natriuretic factor: a hormone produced by the heart. Science. 1985;230:767–770. doi: 10.1126/science.2932797. [DOI] [PubMed] [Google Scholar]
- 13.Burnett JC, Jr, Kao PC, Hu DC, Heser DW, Heublein D, Granger JP, Opgenorth TJ, Reeder GS. Atrial natriuretic peptide elevation in congestive heart failure in the human. Science. 1986;231:1145–1147. doi: 10.1126/science.2935937. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto K, Burnett JC, Jr, Redfield MM. Effect of endogenous natriuretic peptide system on ventricular and coronary function in failing heart. Am J Physiol. 1997;273:H2406–H2414. doi: 10.1152/ajpheart.1997.273.5.H2406. [DOI] [PubMed] [Google Scholar]
- 15.Wilkins MR, Redondo J, Brown LA. The natriuretic-peptide family. Lancet. 1997;349:1307–1310. doi: 10.1016/S0140-6736(96)07424-7. [DOI] [PubMed] [Google Scholar]
- 16.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339:321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 17.Wu F, Yan W, Pan J, Morser J, Wu Q. Processing of pro-atrial natriuretic peptide by corin in cardiac myocytes. J Biol Chem. 2002;277:16900–16905. doi: 10.1074/jbc.M201503200. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz D, Geller DM, Manning PT, Siegel NR, Fok KF, Smith CE, Needleman P. Ser-Leu-Arg-Arg-atriopeptin III: the major circulating form of atrial peptide. Science. 1985;229:397–400. doi: 10.1126/science.3160114. [DOI] [PubMed] [Google Scholar]
- 19.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 20.Chang MS, Lowe DG, Lewis M, Hellmiss R, Chen E, Goeddel DV. Differential activation by atrial and brain natriuretic peptides of two different receptor guanylate cyclases. Nature. 1989;341:68–72. doi: 10.1038/341068a0. [DOI] [PubMed] [Google Scholar]
- 21.Burnett JC, Jr, Granger JP, Opgenorth TJ. Effects of synthetic atrial natriuretic factor on renal function and renin release. Am J Physiol. 1984;247:F863–F866. doi: 10.1152/ajprenal.1984.247.5.F863. [DOI] [PubMed] [Google Scholar]
- 22.Chen HH, Burnett JC., Jr The natriuretic peptides in heart failure: diagnostic and therapeutic potentials. Proc Assoc Am Physicians. 1999;111:406–416. doi: 10.1111/paa.1999.111.5.406. [DOI] [PubMed] [Google Scholar]
- 23.Saito Y, Nakao K, Nishimura K, Sugawara A, Okumura K, Obata K, Sonoda R, Ban T, Yasue H, Imura H. Clinical application of atrial natriuretic polypeptide in patients with congestive heart failure: beneficial effects on left ventricular function. Circulation. 1987;76:115–124. doi: 10.1161/01.cir.76.1.115. [DOI] [PubMed] [Google Scholar]
- 24.Lee CY, Burnett JC., Jr Natriuretic peptides and therapeutic applications. Heart Fail Rev. 2007;12:131–142. doi: 10.1007/s10741-007-9016-3. [DOI] [PubMed] [Google Scholar]
- 25.Gottlieb SS, Kukin ML, Ahern D, Packer M. Prognostic importance of atrial natriuretic peptide in patients with chronic heart failure. J Am Coll Cardiol. 1989;13:1534–1539. doi: 10.1016/0735-1097(89)90344-6. [DOI] [PubMed] [Google Scholar]
- 26.Wallen T, Landahl S, Hedner T, Hall C, Saito Y, Nakao K. Atrial natriuretic peptides predict mortality in the elderly. J Intern Med. 1997;241:269–275. doi: 10.1046/j.1365-2796.1997.128149000.x. [DOI] [PubMed] [Google Scholar]
- 27.Omland T, Bonarjee VV, Nilsen DW, Sundsfjord JA, Lie RT, Thibault G, Dickstein K. Prognostic significance of N-terminal pro-atrial natriuretic factor (1–98) in acute myocardial infarction: comparison with atrial natriuretic factor (99–126) and clinical evaluation. Br Heart J. 1993;70:409–414. doi: 10.1136/hrt.70.5.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall C, Rouleau JL, Moye L, de Champlain J, Bichet D, Klein M, Sussex B, Packer M, Rouleau J, Arnold MO. N-terminal proatrial natriuretic factor. An independent predictor of long-term prognosis after myocardial infarction. Circulation. 1994;89:1934–1942. doi: 10.1161/01.cir.89.5.1934. [DOI] [PubMed] [Google Scholar]
- 29.Gibson TR, Shields PP, Glembotski CC. The conversion of atrial natriuretic peptide (ANP)-(1–126) to ANP-(99–126) by rat serum: contribution to ANP cleavage in isolated perfused rat hearts. Endocrinology. 1987;120:764–772. doi: 10.1210/endo-120-2-764. [DOI] [PubMed] [Google Scholar]
- 30.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC., Jr Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol. 2002;40:976–982. doi: 10.1016/s0735-1097(02)02059-4. [DOI] [PubMed] [Google Scholar]
- 31.Gladysheva IP, King SM, Houng AK. N-glycosylation modulates the cell-surface expression and catalytic activity of corin. Biochem Biophys Res Commun. 2008;373:130–135. doi: 10.1016/j.bbrc.2008.05.181. [DOI] [PubMed] [Google Scholar]
- 32.Zolotarjova N, Martosella J, Nicol G, Bailey J, Boyes BE, Barrett WC. Differences among techniques for high-abundant protein depletion. Proteomics. 2005;5:3304–3313. doi: 10.1002/pmic.200402021. [DOI] [PubMed] [Google Scholar]
- 33.Lee ME, Miller WL, Edwards BS, Burnett JC., Jr Role of endogenous atrial natriuretic factor in acute congestive heart failure. J Clin Invest. 1989;84:1962–1966. doi: 10.1172/JCI114385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson JV, Woodruff PW, Bloom SR. The effect of treatment of congestive heart failure on plasma atrial natriuretic peptide concentration: a longitudinal study. British heart journal. 1988;59:207–211. doi: 10.1136/hrt.59.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berglund H, Bevegard S, Carlens P, Hedner J, Hedner T. Clinical physiology. Vol. 8. Oxford, England: 1988. Atrial natriuretic peptide during acute treatment of congestive heart failure; pp. 155–162. [DOI] [PubMed] [Google Scholar]
- 36.Marumo F, Kurosawa T, Takeda S, Katoh Y, Hasegawa N, Ando K. Changes of molecular forms of atrial natriuretic peptide after treatment for congestive heart failure. Klinische Wochenschrift. 1988;66:675–681. doi: 10.1007/BF01726925. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi M, Takeda S, Kurokawa S, Kubo T, Fukuda N, Izumi T. Cyclic GMP production by ANP, BNP, and NO during worsening and improvement of chronic heart failure. Japanese heart journal. 2003;44:713–724. doi: 10.1536/jhj.44.713. [DOI] [PubMed] [Google Scholar]
- 38.Hata N, Seino Y, Tsutamoto T, Hiramitsu S, Kaneko N, Yoshikawa T, Yokoyama H, Tanaka K, Mizuno K, Nejima J, Kinoshita M. Effects of carperitide on the long-term prognosis of patients with acute decompensated chronic heart failure: the PROTECT multicenter randomized controlled study. Circ J. 2008;72:1787–1793. doi: 10.1253/circj.cj-08-0130. [DOI] [PubMed] [Google Scholar]
- 39.Andreassi MG, Del Ry S, Palmieri C, Clerico A, Biagini A, Giannessi D. Up-regulation of 'clearance' receptors in patients with chronic heart failure: a possible explanation for the resistance to biological effects of cardiac natriuretic hormones. Eur J Heart Fail. 2001;3:407–414. doi: 10.1016/s1388-9842(01)00161-1. [DOI] [PubMed] [Google Scholar]
- 40.Charloux A, Piquard F, Doutreleau S, Brandenberger G, Geny B. Mechanisms of renal hyporesponsiveness to ANP in heart failure. European journal of clinical investigation. 2003;33:769–778. doi: 10.1046/j.1365-2362.2003.01222.x. [DOI] [PubMed] [Google Scholar]
- 41.Rame JE, Tam SW, McNamara D, Worcel M, Sabolinski ML, Wu AH, Dries DL. Dysfunctional corin i555(p568) allele is associated with impaired brain natriuretic peptide processing and adverse outcomes in blacks with systolic heart failure: results from the Genetic Risk Assessment in Heart Failure substudy. Circ Heart Fail. 2009;2:541–548. doi: 10.1161/CIRCHEARTFAILURE.109.866822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cody RJ, Atlas SA, Laragh JH, Kubo SH, Covit AB, Ryman KS, Shaknovich A, Pondolfino K, Clark M, Camargo MJF, Scarborough RM, Lewicki JA. Atrial natriuretic factor in normal subjects and heart failure patients. Plasma levels and renal, hormonal, and hemodynamic responses to peptide infusion. J Clin Invest. 1986;78:1362–1374. doi: 10.1172/JCI112723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crozier IG, Nicholls MG, Ikram H, Espiner EA, Gomez HJ, Warner NJ. Haemodynamic effects of atrial peptide infusion in heart failure. Lancet. 1986;2:1242–1245. doi: 10.1016/s0140-6736(86)92675-9. [DOI] [PubMed] [Google Scholar]
- 44.Tomita Y, Kim DH, Magoori K, Fujino T, Yamamoto TT. A novel low-density lipoprotein receptor-related protein with type II membrane protein-like structure is abundant in heart. J Biochem (Tokyo) 1998;124:784–789. doi: 10.1093/oxfordjournals.jbchem.a022180. [DOI] [PubMed] [Google Scholar]
- 45.Chan JC, Knudson O, Wu F, Morser J, Dole WP, Wu Q. Hypertension in mice lacking the proatrial natriuretic peptide convertase corin. Proc Natl Acad Sci U S A. 2005;102:785–790. doi: 10.1073/pnas.0407234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mukoyama M, Nakao K, Hosoda K, Suga S, Saito Y, Ogawa Y, Shirakami G, Jougasaki M, Obata K, Yasue H. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest. 1991;87:1402–1412. doi: 10.1172/JCI115146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aburaya M, Suzuki E, Minamino N, Kangawa K, Tanaka K, Matsuo H. Concentration and molecular forms of brain natriuretic peptide in rat plasma and spinal cord. Biochem Biophys Res Commun. 1991;177:40–47. doi: 10.1016/0006-291x(91)91945-9. [DOI] [PubMed] [Google Scholar]
- 48.Ogawa Y, Nakao K, Mukoyama M, Shirakami G, Itoh H, Hosoda K, Saito Y, Arai H, Suga S, Jougasaki M, Itoh H, Hosoda K, Yamada T, Kambayashi Y, Inouye K, Imura H. Rat brain natriuretic peptide--tissue distribution and molecular form. Endocrinology. 1990;126:2225–2227. doi: 10.1210/endo-126-4-2225. [DOI] [PubMed] [Google Scholar]
- 49.Kambayashi Y, Nakao K, Itoh H, Hosoda K, Saito Y, Yamada T, Mukoyama M, Arai H, Shirakami G, Suga S, Ogawa Y, Jougasaki M, Minamino N, Kangawa K, Matsuo H, Inouye K, Imura H. Isolation and sequence determination of rat cardiac natriuretic peptide. Biochem Biophys Res Commun. 1989;163:233–240. doi: 10.1016/0006-291x(89)92126-8. [DOI] [PubMed] [Google Scholar]
- 50.Rame JE, Drazner MH, Post W, Peshock R, Lima J, Cooper RS, Dries DL. Corin I555(P568) allele is associated with enhanced cardiac hypertrophic response to increased systemic afterload. Hypertension. 2007;49:857–864. doi: 10.1161/01.HYP.0000258566.95867.9e. [DOI] [PubMed] [Google Scholar]
- 51.Dries DL, Victor RG, Rame JE, Cooper RS, Wu X, Zhu X, Leonard D, Ho SI, Wu Q, Post W, Drazner MH. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation. 2005;112:2403–2410. doi: 10.1161/CIRCULATIONAHA.105.568881. [DOI] [PubMed] [Google Scholar]
- 52.Wang W, Liao X, Fukuda K, Knappe S, Wu F, Dries DL, Qin J, Wu Q. Corin variant associated with hypertension and cardiac hypertrophy exhibits impaired zymogen activation and natriuretic peptide processing activity. Circ Res. 2008;103:502–508. doi: 10.1161/CIRCRESAHA.108.177352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woollard KJ, Suhartoyo A, Harris EE, Eisenhardt SU, Jackson SP, Peter K, Dart AM, Hickey MJ, Chin-Dusting JP. Pathophysiological levels of soluble P-selectin mediate adhesion of leukocytes to the endothelium through Mac-1 activation. Circ Res. 2008;103:1128–1138. doi: 10.1161/CIRCRESAHA.108.180273. [DOI] [PubMed] [Google Scholar]
- 54.Deschamps AM, Spinale FG. Matrix modulation and heart failure: new concepts question old beliefs. Curr Opin Cardiol. 2005;20:211–216. doi: 10.1097/01.hco.0000162397.44843.83. [DOI] [PubMed] [Google Scholar]
- 55.Yan AT, Yan RT, Spinale FG, Afzal R, Gunasinghe HR, Arnold M, Demers C, McKelvie RS, Liu PP. Plasma matrix metalloproteinase-9 level is correlated with left ventricular volumes and ejection fraction in patients with heart failure. J Card Fail. 2006;12:514–519. doi: 10.1016/j.cardfail.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 56.Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, Leonardi AH, McClure CD, Spinale FG, Zile MR. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006;113:2089–2096. doi: 10.1161/CIRCULATIONAHA.105.573865. [DOI] [PubMed] [Google Scholar]
- 57.Kramer F, Sandner P, Klein M, Krahn T. Plasma concentrations of matrix metalloproteinase-2, tissue inhibitor of metalloproteinase-1 and osteopontin reflect severity of heart failure in DOCA-salt hypertensive rat. Biomarkers. 2008;13:270–281. doi: 10.1080/13547500801903123. [DOI] [PubMed] [Google Scholar]
- 58.Vorovich EE, Chuai S, Li M, Averna J, Marwin V, Wolfe D, Reilly MP, Cappola TP. Comparison of matrix metalloproteinase 9 and brain natriuretic peptide as clinical biomarkers in chronic heart failure. Am Heart J. 2008;155:992–997. doi: 10.1016/j.ahj.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gonzalez A, Lopez B, Ravassa S, Beaumont J, Arias T, Hermida N, Zudaire A, Diez J. Biochemical markers of myocardial remodelling in hypertensive heart disease. Cardiovasc Res. 2009;81:509–518. doi: 10.1093/cvr/cvn235. [DOI] [PubMed] [Google Scholar]
- 60.Knappe S, Wu F, Masikat MR, Morser J, Wu Q. Functional analysis of the transmembrane domain and activation cleavage of human corin: design and characterization of a soluble corin. J Biol Chem. 2003;278:52363–52370. doi: 10.1074/jbc.M309991200. [DOI] [PubMed] [Google Scholar]
- 61.Ibebuogu UN, Gladysheva IP, Reed GL. Is heart failure due to impaired cleavage and activation of atrial natriuretic peptide? J Am Coll Cardiol. 2009;53:A468–A469. [Google Scholar]