Abstract
Stem cells participate in cervical carcinogenesis but their function and exact features are still not clear. One type of stem-like cells are endocervical reserve cells (RCs), and their association with other normal/altered cervical cells is not exactly known. Epithelial cells are attached to each other by tight junctions. Their dominant components are the claudin proteins, which show changed expression in cancer; however, no data are available on their pattern. Expressions of various claudins (1, 2, 3, 4, 7), occludin, cytokeratins 5/6 and 7, and p63 were analyzed in 60 paraffin-embedded cervical samples. Immunohistochemical reactions were evaluated semiquantitatively and statistically. Claudin 1 was as high in RCs as in cervical intraepithelial neoplasia (CIN) and higher than in suprabasal squamous epithelial cells, contrary to the negative glandular and squamous basal cells. Claudin 2 was positive in all cell types except parabasal cells, whereas claudins 4 and 7 were weakly positive and claudin 3 was negative in all cell types. Occludin was positive in RCs, basal/parabasal cells, and CIN, whereas glandular cells were negative. This is a first report that describes the intermediate claudin pattern of RCs, demonstrating that it differs from that of cervical glandular and squamous basal cells, but showing an expression similar to the strong claudin 1 expression detected in cervical neoplastic cells.
Keywords: reserve cells, claudin 1, tight junction
Introduction
Reserve cells (RCs) are located in the transformation zone of the cervix, under the endocervical columnar epithelium. They are thought to give rise to metaplastic squamous cells, may “qualify” as stem cells, and play a role in cervical carcinogenesis (Martens et al. 2004; Kerdraon et al. 2012). Evidence has been presented for the existence of two subpopulations of RCs, one giving rise to squamous, the other to glandular epithelium, and both of which can be differentiated by cytokeratin (CK) immunostaining (Regauer and Reich 2007; Martens et al. 2009). Data suggest that RCs have specific characteristics; however, their exact nature and role in cervical epithelium replacement, in cervical carcinogenesis, as well as their possibility of being a target for human papilloma virus (HPV) infection have still not been clearly clarified (Martens et al. 2004, 2009).
Polarized structure is highly important in epithelial cellular interactions, in both glandular and squamous epithelia (Balda and Matter 2008). Intercellular junctional complexes composed of tight junctions (TJs), adherens junctions, desmosomes, and gap junctions, play a role in epithelial organization and preservation of tissue integrity.
TJs structurally consist of transmembrane and membrane-associated proteins. The main constituents of TJ strands are a large family of transmembrane proteins—the claudins (20–27 kDa) (Tsukita et al. 2008; Furuse 2010)—thought to have 24 members (at least in mice and humans), but to which three subtypes have been recently added (Mineta et al. 2011). TJs of different cells are composed of several, highly specific claudins and the expression profile of individual claudins varies among tissues resulting in a characteristic claudin pattern.
Recently, several publications demonstrated alterations in the expression of different TJ proteins, the first of claudins in cancer cells. Up- or downregulation of different types of claudins has been previously described (for reviews, see Morin 2005; Hewitt et al. 2006; Oliveira and Morgado-Diaz 2007; Förster 2008; Tsukita et al. 2008; Szabó et al. 2009; Singh et al. 2010; Escudero-Esparza et al. 2011; Soini 2012). Changes in claudin expression during carcinogenesis were detected at gene and protein levels, suggesting the role of claudins as progression markers in several cancers. It was previously observed that the loss of TJs occurred in cancer in association with tumor progression, and a decrease in claudin expression was expected during cancer development. In contrast, however, upregulation of several individual claudins, such as claudin 4 in cholangiocarcinoma (Lódi et al. 2006), in pancreas carcinoma (Borka et al. 2007), and in ovarian cancer (for review, see Szabó et al. 2009), was observed and found in several cases to be associated with patient survival (Korompay et al. 2012). Increased claudin 1 expression was detected in cervical intraepithelial neoplasia (CIN) and invasive cervical cancer in our previous study, suggesting that upregulation of certain claudins does not necessarily mean increase in “tightness” of TJs but rather a malfunction (Sobel, Páska, et al. 2005; Sobel, Szabó, et al. 2005). Altered claudin expression has been described in other gynecological malignancies too, which besides having diagnostic significance, may have therapeutic consequences as well (Lee et al. 2005; Sobel et al. 2006; Choi et al. 2007; Gaetje et al. 2008; Kleinberg et al. 2008; Szabó et al. 2009). From this aspect, it might be important to analyze the expression of claudins and other TJ proteins, such as occludin, in normal and altered cervical cells and endocervical glands in RCs, further to compare the expression patterns with other normal mature and stem-like cervical cells, as well as the changes detected in neoplastic cells. The expression pattern of claudins might reflect the stage of development or the relationship between different cell types. For this reason, the current study focuses on a more indepth characterization of RCs with the aid of a TJ protein pattern as compared with already known markers, in order to better understand their role in cervical cellular differentiation and carcinogenesis.
Materials & Methods
Case Selection
A total of 60 cervical samples were studied, obtained from the archives of the 2nd Department of Pathology of Semmelweis University and Department of Pathology of St. Stephan Hospital, Budapest, with the permission of the Regional Ethical Committee. Samples were removed for diagnostic or therapeutic purposes and included 40 knife cone and loop excision samples of cervical intraepithelial neoplasias (CIN II–III) and 20 cases of normal cervical tissue (obtained from patients with myoma uteri). The samples were previously screened for the presence of RCs on hematoxylin-eosin (HE) stained slides by expert gynecopathologists (EB, AK).
Histology and Immunohistochemistry
Tissues were fixed in 10% neutral buffered formalin for 24 hr followed by paraffin embedding, then cut and stained using HE to establish a diagnosis.
The formalin-fixed paraffin-embedded (FFPE), 3- to 4-μm-thick sections were used for immunohistochemistry. After deparaffinization, slides were washed in PBS (pH 7.4) and then microwave oven treated for 3 min with 850 W, followed by 170 W for 30 min with antigen retrieval solution (Target Retrieval Solution; Dako, Glostrup, Denmark). Reactions were carried out in Ventana ES automatic immunostainer (Ventana Medical Systems Inc.; Tucson, AZ) using avidin-biotin peroxidase technique and diaminobenzidine as chromogen according to the manufacturer’s protocol (iView DAB Detection Kit; Ventana).
Well characterized primary antibodies against claudins (CLDNs) 1, 2, 3, 4, and 7, occludin, CK5/6, CK7, p63, and Ki67 were diluted (Table 1) and applied for 30 min at room temperature in the Ventana immunostainer. For negative controls, the specific antibodies were omitted and either the antibody diluent was used alone or isotype-matched IgG serum. Tissues recommended by manufacturers were used as positive controls.
Table 1.
Primary Antibodies and Dilutions Used.
| Antigen | Type | Dilution | Manufacturer |
|---|---|---|---|
| Claudin 1 | mouse monoclonal | 1:100 | Cell Marque (Rocklin, CA) |
| Claudin 2 | mouse monoclonal | 1:20 | Invitrogen (Carlsbad, CA) |
| Claudin 3 | rabbit monoclonal | 1:80 | Invitrogen (Carlsbad, CA) |
| Claudin 4 | mouse monoclonal | 1:100 | Invitrogen (Carlsbad, CA) |
| Claudin 7 | rabbit monoclonal | 1:100 | Invitrogen (Carlsbad, CA) |
| Occludin | rabbit monoclonal | 1:100 | Zymed (San Francisco, CA) |
| Cytokeratin 5/6 | mouse monoclonal | 1:600 | Dako (Glostrup, Denmark) |
| Cytokeratin 7 | mouse monoclonal | 1:2000 | Dako (Glostrup, Denmark) |
| p63 | mouse monoclonal | 1:100 | Izinta (Budapest, Hungary) |
| Ki67 | mouse monoclonal | 1:100 | Dako (Glostrup, Denmark) |
Evaluation and Statistical Analysis
Distribution, intensity, and subcellular localization (membranous, cytoplasmic, nuclear) of the immunostainings were recorded. Semiquantitative evaluation was applied for immunoreactions, analyzing five randomly selected areas per slide using ×40 objective and counting 100 cells in each field (Olympus BX50 microscope; Olympus Corporation, Tokyo, Japan). Scoring of cells showing positive immunoreaction was as follows: 0: ≤5%, 1: 6% to 30%, 2: 31% to 60%, and 3: 61% to 100%. Intensity was scored as 1 = weak, 2 = moderate, and 3 = strong staining reaction. A summary score multiplying percentage and intensity scores was created for each slide.
Kruskal-Wallis test was used to compare the expression of individual claudins and occludin detected by immunohistochemistry in the different groups. Two values were considered significantly different at p<0.05.
Immunoreactions for CK 5/6, CK7, and p63 were used to characterize RCs as described previously and for Ki67 to evaluate the rate of proliferation.
Results
Immunohistochemistry
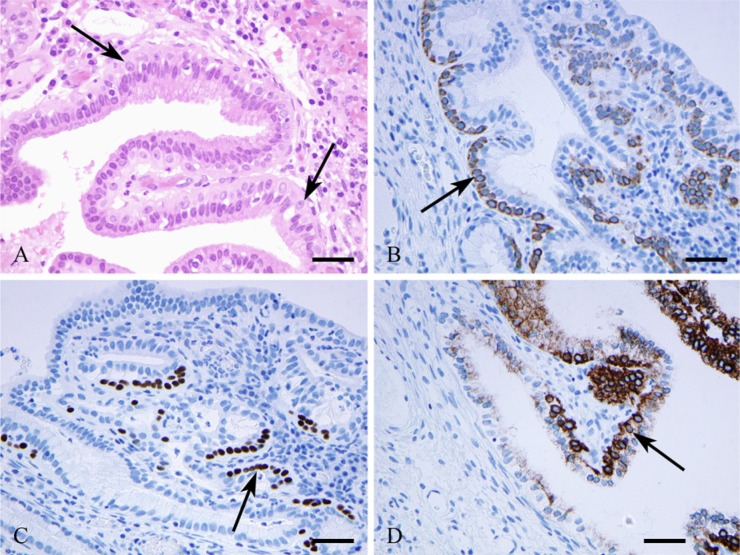
RCs were identified by HE staining along the columnar glandular cells (Fig. 1A) and identified further by CK5/6 (Fig. 1B), CK7 (Fig. 1D), and p63 (Fig. 1C) immunoreactions.
Figure 1.
Reserve cells (arrows) located alongside cervical glandular cells as seen by hematoxylin-eosin staining (A) and by immunohistochemical reactions using antibodies against cytokeratin (CK) 5/6 (B), p63 (C), and CK7 (D). Bars = 50 μm.
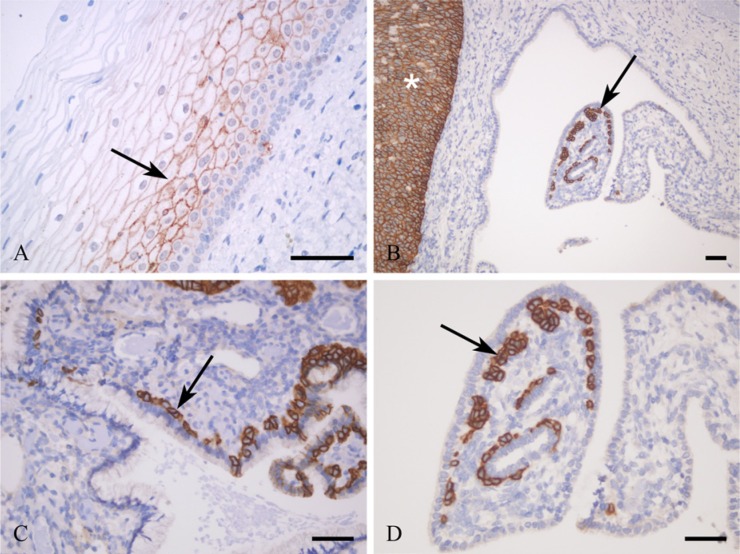
In normal cervical squamous epithelia, the expression of individual claudins and occludin was recognized in the same localization and distribution as described previously. Parabasal squamous epithelial cells expressed CLDNs1 (Fig. 2A), 4, and 7 (not shown) and occludin in membranous localization with moderate intensity (the latter two not shown), whereas the upper layers stained much less intensively or were negative. Contrarily, squamous basal cells were negative for CLDN1 (Fig. 2A) but positive for occludin (Fig. 3A) and CLDN2 (Fig. 3C) and stained weakly for CLDNs4 and 7 (not shown).
Figure 2.
Claudin 1 membranous immunoreaction in parabasal squamous epithelial cells is positive (A) (arrow) and negative in basal cells. The reserve cells are strongly positive (B, C, D) (arrows). Cervical intraepithelial neoplasia III is highly positive for claudin 1 (B) (asterisk). Bars = 50 μm.
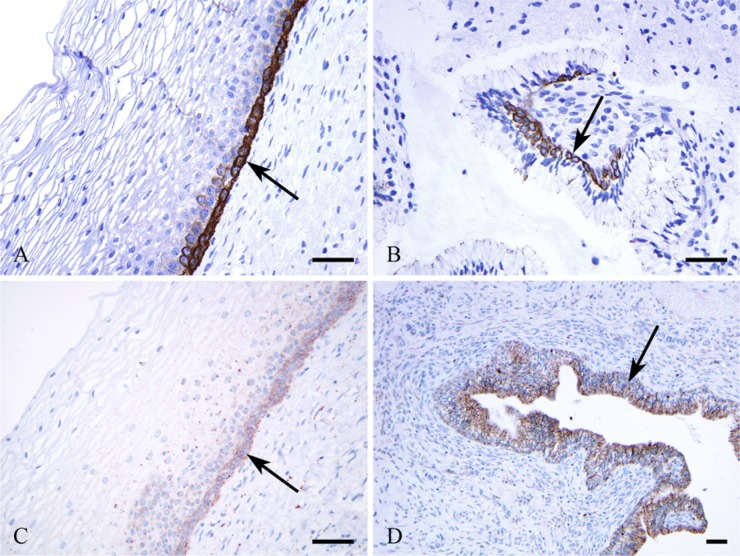
Figure 3.
Occludin (A, B) and claudin 2 (C, D) immunoreactions. The squamous epithelial basal cells (A) and reserve cells (B) are positive for occludin (arrows), whereas the columnar epithelial cells are negative (B). Claudin 2 is positive in squamous basal cells (C), in columnar glandular cells (D), and in reserve cells (D) (arrows). No differences are observable in the intensity of immunoreaction in claudin 2 between glandular and reserve cells (D). Bars = 50 μm.
Cervical columnar glandular cells were negative for CLDN1 (Fig. 2B), CLDN3, and occludin (Fig. 3B), positive for CLDN2 (Fig. 3C), and less intensively stained for CLDNs4 and 7 (not shown).
Endocervical RCs located along the columnar glandular cells facing the stroma (Fig. 1A) expressed CLDN1 (Figs. 2B, C, D) and CLDN2 (Fig. 3D) intensively and showed no CLDN3 and only weak CLND4 and CLDN7 expression (not shown). Occludin was detected in RCs, as well (Fig. 3B).
Strong CLDN1 expression was seen in CIN (Fig. 2B) as described earlier, with similar intensity as in the endocervical RCs.
Statistical Analysis
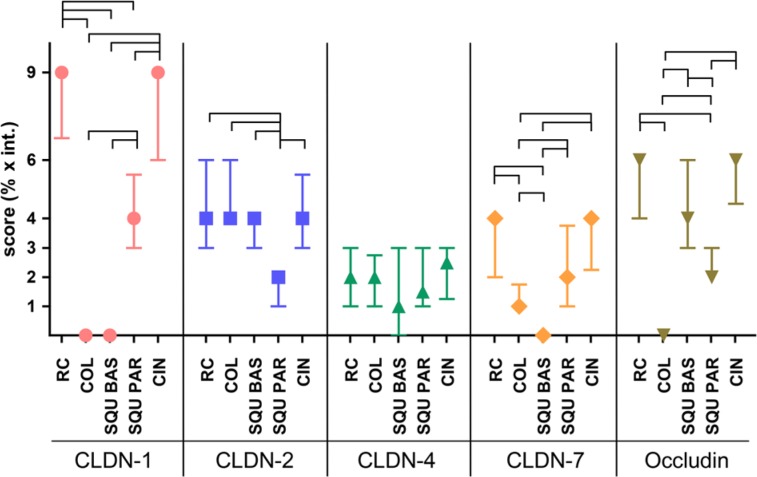
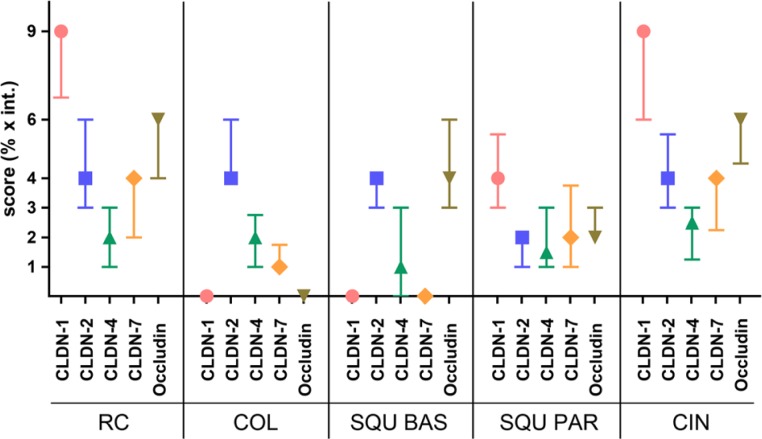
Claudin pattern in parabasal and basal cells of normal squamous epithelial cells, cervical glands, squamous metaplasia, and RCs differed significantly (Table 2). CLDN1 was notably higher in RCs than in glandular cells and squamous basal cells, and the latter two were found to be negative (Fig. 4). CLDN1 reaction in RCs was even higher than in the parabasal layer of squamous epithelia (p<0.0001); however, no differences were found in comparison to CIN lesions. In contrast, CLDN2 was high in columnar glandular cells, in basal cells of the squamous epithelia, and in RCs, with no significant differences observable. Parabasal squamous epithelial cells gave a reaction of lower intensity (Fig. 4). CLDN3 was negative in all cell types. CLDN4 gave a weak reaction, not differing significantly in the studied normal cell types. CLDN7 was positive in parabasal cells and RCs and negative in basal and columnar glandular cells (Fig. 4).
Table 2.
Results of Immunohistochemical Scoring and Statistical Comparison of Individual Groups According to Proteins.
|
p Value |
||||||||
|---|---|---|---|---|---|---|---|---|
| Median | IQR | K-W Test | vs. COL | vs. SQU BAS | vs. SQU PAR | vs. CIN | ||
| Claudin 1 | RC | 9 | 2.25 | <0.001* | <0.0001* | <0.0001* | <0.0001* | 1.0000 |
| COL | 0 | 0.00 | 1.0000 | <0.0001* | <0.0001* | |||
| SQU BAS | 0 | 0.00 | <0.0001* | <0.0001* | ||||
| SQU PAR | 4 | 2.50 | <0.0001* | |||||
| CIN | 9 | 3.00 | ||||||
| Claudin 2 | RC | 4 | 3.00 | <0.001* | 1.0000 | 1.0000 | <0.0001* | 1.0000 |
| COL | 4 | 2.00 | 0.8372 | <0.0001* | 1.0000 | |||
| SQU BAS | 4 | 1.00 | <0.0001* | 1.0000 | ||||
| SQU PAR | 2 | 1.00 | <0.0001* | |||||
| CIN | 4 | 2.50 | ||||||
| Claudin 4 | RC | 2 | 2.00 | 0.0488 | 1.0000 | 1.0000 | 1.0000 | 1.0000 |
| COL | 2 | 1.75 | 1.0000 | 1.0000 | 1.0000 | |||
| SQU BAS | 1 | 3.00 | 1.0000 | 0.0622 | ||||
| SQU PAR | 1.5 | 2.00 | 0.1681 | |||||
| CIN | 2.5 | 1.75 | ||||||
| Claudin 7 | RC | 4 | 2.00 | <0.001* | <0.0001* | <0.0001* | 0.0765 | 1.0000 |
| COL | 1 | 0.75 | 0.0012* | 0.0043* | <0.0001* | |||
| SQU BAS | 0 | 0.00 | <0.0001* | <0.0001* | ||||
| SQU PAR | 2 | 2.75 | 0.0714 | |||||
| CIN | 4 | 1.75 | ||||||
| Occludin | RC | 6 | 2.00 | <0.001* | <0.0001* | 0.1309 | <0.0001* | 1.0000 |
| COL | 0 | 0.00 | <0.0001* | <0.0001* | <0.0001* | |||
| SQU BAS | 4 | 3.00 | 0.0001* | 0.1439 | ||||
| SQU PAR | 2 | 1.00 | <0.0001* | |||||
| CIN | 6 | 1.50 | ||||||
Median and interquartile range values of immunohistochemical scoring are listed. Statistical comparison of horizontal versus vertical groups was performed using the Kruskal-Wallis test. Significant differences (p<0.05) are marked by asterisks. IQR, interquartile range (3 quartile–1 quartile); K-W Test, Kruskal-Wallis test; RC, reserve cell; COL, columnar glandular cell; SQU BAS, squamous basal cell; SQU PAR, squamous parabasal cell; CIN, cervical intraepithelial neoplasia.
Figure 4.
Results of immunohistochemistry scoring regarding claudin and occludin patterns among the different cervical cell types. The expression of claudin 1 (CLDN-1) is high in reserve cells (RC) and cervical intraepithelial neoplasia (CIN) and is negative in columnar glandular and basal cells. Median (symbol) and interquartile ranges are plotted. The links represent significant differences. COL, columnar glandular cell; SQU BAS, squamous basal cell; SQU PAR, squamous parabasal cell.
Comparing the claudin pattern of the different cell types, RCs and CIN lesions showed similar characteristics in expression of individual claudins (Fig. 5), whereas the characteristics of columnar epithelial cells were more similar to squamous basal cells at least in the expression of CLDNs1, 2, and 7 but were different regarding occludin (Fig. 5).
Figure 5.
The claudin (CLDN) and occludin patterns are similar in reserve cells (RC) and intraepithelial lesions (CIN). Median (symbol) and interquartile ranges are plotted. COL, columnar glandular cell; SQU BAS, squamous basal cell; SQU PAR, squamous parabasal cell.
Discussion
The endocervical canal is lined by columnar mucin-containing cells with villous processes, crypts, and tunnels, the latter referred to as glands (Malpica and Robboy 2009). The transitional, transformation zone between glandular and squamous epithelium of the endocervix is of special significance owing to its involvement in cervical alterations, mainly in squamous metaplasia, intraepithelial, and invasive cervical neoplasias.
Based on morphological and immunohistochemical studies, endocervical subcolumnar RCs were suggested as a potentional candidate for stem cells (Martens et al. 2004; Mak et al. 2012). Previous studies demonstrated the characteristic keratin pattern of RCs (Smedts et al. 1992; Martens et al. 2004, 2009; Kerdraon et al. 2012) and presented two subpopulations of these cells, one suggested to give rise to squamous, the other to glandular epithelium (Martens et al. 2009). Endocervical RCs showed strong expression for p63 and cytokeratins 5 and 7 and moderate expression for bcl-2 (Martens et al. 2009). There was, however, a difference in CK17 expression, which was strong in RCs closer to the squamo-columnar junction and less pronounced in the area closer to the endometrium (Martens et al. 2009). Kerdraon et al. (2012) specified the keratin profile of RCs as “intermediate” between squamous epithelium expressing high molecular-weight keratins (such as 4, 5, 6, 13, 14, and 17) and columnar epithelium expressing low molecular-weight keratins (such as 7, 8, 18, and 19).
Our study revealed differences in the expression of claudins between RCs and columnar and squamous epithelial cells, the RCs being intermediate as suggested by Kerdraon et al. (2012) for the keratin expression pattern. RCs expressed CLDN1 strongly, which was detected to a lesser degree in parabasal squamous cells and was found overexpressed in CIN (Sobel, Páska, et al. 2005; Sobel, Szabó, et al. 2005). The intensity of CLDN1 reaction in RCs was as high as previously found in CIN lesions (Sobel, Páska, et al. 2005; Sobel, Szabó, et al. 2005). In contrast, no CLDN1 was detected in the columnar glandular cells. On the other hand, RCs expressed CLDN2 with the same intensity as columnar epithelial cells and basal cells of squamous epithelium. These data support the notion that RCs “have a tendency towards bidirectional differentiation” as mentioned by Witkiewicz et al. (2005) for the keratin pattern of RCs.
P63 is expressed in cervical stem cell populations, in the basal cells of the ectocervical squamous epithelium, and in the endocervical subcolumnar RCs (Martens et al. 2004). In contrast, we could demonstrate only CLDN1 in the RCs, similar to CK17, which was found expressed only in RCs and not in basal cells by Martens et al. (2004).
Our data show that RCs are a distinct type of cell, with a TJ composition that differs from both the squamous and glandular epithelium. RCs have unique characteristics in the TJ pattern, expressing the highest CLDN1 among all normal epithelial cell types of the cervical epithelia, as high as in dysplastic cells in CIN lesions. This suggests a possible connection between the overexpression of CLDN1 during cervical carcinogenesis and the high expression of CLDN1 in RCs having stem-like cell characteristics.
Tight junctions are made up of a multi-gene protein family, the CLDNs (with 27 known subtypes currently), occludin, tricellulin, and so on (Tsukita et al. 2008). Epithelial cells and tissues have a highly characteristic CLDN pattern, composed of strictly confined individual CLDNs and other proteins forming the TJ strands (Mineta 2011). For example, CLDN4 is highly expressed in cholangiocytes, whereas no (or only low) expression is detected in hepatocytes (Lódi et al. 2006). A dynamic model of TJs has been created with the suggestion that a continuous remodeling of TJs occurs even during normal cell cycles, providing function of junctional complexes, which might mean polymerization of claudins with other components (Tsukita et al. 2008). During cellular differentiation or dedifferentiation, the CLDN pattern changes characteristically in association with the functional needs. For example, CLDN1 cannot be demonstrated in basal cells of the squamous epithelium but can be detected in normal suprabasal squamous epithelial cells; this shows that, during maturation, the TJs are remodeled and the morphological appearance of the cells has changed together with TJ structure and function. However, there is no clear explanation for the overexpression of CLDN1 in CINl lesions and invasive squamous cell carcinomas and other cancers (Morin 2005; Sobel et al. 2005). There are data suggesting that an increase or upregulation of individual CLDNs in TJs does not mean that an those TJs are “tighter” or function better (Turksen and Troy 2011). It shows only that the proportion of the different protein components has changed with the probability of dysfunction of TJs, such as paracellular barrier functions, selective permeabilities, and altered cellular signaling pathways that are associated with TJ alterations (Mineta 2011). The assumption that cancer cells lose their TJs and are associated with dyscohesion might be true for the morphological appearance but not for the proportion of the protein components.
The role and significance of stem/progenitor cells in gynecological malignancies are not clear. Basal cells and RCs are thought to be stem or progenitor cells in the cervix, the main function of which is to maintain tissue integrity in the normal cervix (Mak et al. 2012). During cervical cancer development, however, stem cells might participate in carcinogenesis. Despite this, the characteristics of normal and cancer stem cells in the cervix have not been well described yet. To understand the changes in TJ composition, the mechanism of remodeling of individual proteins that compose the TJ strands of RCs might help to better clarify changes that could be associated with cancer development.
Further investigations are needed to determine whether the increased CLDN1 expression in dysplastic cervical cells, even in the early stages of carcinogenesis, is a sign of dedifferentiation. These studies would also promote our attempts to gain more insight into the “stemness” characteristics or expansion of the stem cell compartment.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Hungarian National Scientific Research Fund (OTKA PD105019) and the Hungarian Ministry of National Development (KMR-12-A) and by funding from L’Oreal Hungary.
References
- Balda MS, Matter K. 2008. Tight junctions at a glance. J Cell Sci. 121:3677–3682 [DOI] [PubMed] [Google Scholar]
- Borka K, Kaliszky P, Szabó E, Lotz G, Kupcsulik P, Schaff Z, Kiss A. 2007. Claudin expression in pancreatic endocrine tumors as compared with ductal adenocarcinomas. Virchows Arch. 450:549–557 [DOI] [PubMed] [Google Scholar]
- Choi YL, Kim J, Kwon MJ, Choi JS, Kim TJ, Bae DS, Koh SS, In YH, Park YW, Kim SH, et al. 2007. Expression profile of tight junction protein claudin 3 and claudin 4 in ovarian serous adenocarcinoma with prognostic correlation. Histol Histopathol. 22:1185–1195 [DOI] [PubMed] [Google Scholar]
- Escudero-Esparza A, Jiang WG, Martin TA. 2011. The claudin family and its role in cancer and metastasis. Front Biosci. 16:1069–1083 [DOI] [PubMed] [Google Scholar]
- Förster C. 2008. Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol. 130:55–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M. 2010. Molecular basis of the core structure of tight junctions. Cold Spring Harb Perspect Biol. 2:a002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetje R, Holtrich U, Engels K, Kissler S, Rody A, Karn T, Kaufmann M. 2008. Differential expression of claudins in human endometrium and endometriosis. Gynecol Endocrinol. 24:442–449 [DOI] [PubMed] [Google Scholar]
- Hewitt KJ, Agarwal R, Morin PJ. 2006. The claudin gene family: expression in normal and neoplastic tissues. BMC Cancer. 6:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerdraon O, Cornélius A, Farine MO, Boulanger L, Wacrenier A. 2012. Adenoid basal hyperplasia of the uterine cervix: a lesion of reserve cell type, distinct from adenoid basal carcinoma. Hum Pathol. 43:2255–2265 [DOI] [PubMed] [Google Scholar]
- Kleinberg L, Holth A, Trope CG, Reich R, Davidson B. 2008. Claudin upregulation in ovarian carcinoma effusions is associated with poor survival. Hum Pathol. 39:747–757 [DOI] [PubMed] [Google Scholar]
- Korompay A, Borka K, Lotz G, Somorácz Á, Törzsök P, Erdélyi-Belle B, Kenessey I, Baranyai Z, Zsoldos F, Kupcsulik P, et al. 2012. Tricellulin expression in normal and neoplastic human pancreas. Histopathology. 60:E76–E86 [DOI] [PubMed] [Google Scholar]
- Lee JW, Lee SJ, Seo J, Song SY, Ahn G, Park CS, Lee JH, Kim BG, Bae DS. 2005. Increased expressions of claudin-1 and claudin-7 during the progression of cervical neoplasia. Gynecol Oncol. 97:53–59 [DOI] [PubMed] [Google Scholar]
- Lódi C, Szabó E, Holczbauer Á, Batmunkh E, Szijártó A, Kupcsulik P, Kovalszky I, Paku S, Illyés G, Kiss A, et al. 2006. Claudin-4 differentiates biliary tract cancers from hepatocellular carcinomas. Mod Pathol. 19:460–469 [DOI] [PubMed] [Google Scholar]
- Mak VCY, Siu MKY, Wong OGW, Chan KKL, Ngan HYS, Cheung ANY. 2012. Dysregulated stemness-related genes in gynecological malignancies. Histol Histopathol. 27:1121–1130 [DOI] [PubMed] [Google Scholar]
- Malpica A, Robboy SJ. 2009. Cervical benign and non-neoplastic conditions. In: Robboy SJ, Mutter GL, Prat J, Bentley RC, Russel P, Anderson MC, editors. Robboy’s pathology of the female reproductive tract, 2nd ed. Edinburgh, UK: Churchill Livingstone Elsevier Ltd; p. 141–172 [Google Scholar]
- Martens JE, Arends J, van der Linden PJQ, De Boer BAG, Helmerhorst TJM. 2004. Cytokeratin 17 and p63 are markers of the HPV target cell, the cervical stem cell. Anticancer Res. 24:771–776 [PubMed] [Google Scholar]
- Martens JE, Smedts FMM, Ploeger D, Helmerhorst TJM, Ramaekers FCS, Arends JW, Hopman AHN. 2009. Distribution pattern and marker profile show two subpopulations of reserve cells in the endocervical canal. Int J Gynecol Pathol. 28:381–388 [DOI] [PubMed] [Google Scholar]
- Mineta K, Yamamoto Y, Yamazaki Y, Tanaka H, Tada Y, Saito K, Tamura A, Igarashi M, Endo T, Takeuchi K, et al. 2011. Predicted expansion of the claudin multigene family. FEBS Lett. 585:606–612 [DOI] [PubMed] [Google Scholar]
- Morin PJ. 2005. Claudin proteins in human cancer: promising new targets for diagnosis and therapy. Cancer Res. 65:9603–9606 [DOI] [PubMed] [Google Scholar]
- Oliveira SS, Morgado-Diaz JA. 2007. Claudins: multifunctional players in epithelial tight junctions and their role in cancer. Cell Mol Life Sci. 64:17–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regauer S, Reich O. 2007. CK17 and p16 expression patterns distinguish (atypical) immature squamous metaplasia from high-grade cervical intraepithelial neoplasia (CIN III). Histopathology. 50:629–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AB, Sharma A, Dhawan P. 2010. Claudin family of proteins and cancer: an overview. J Oncol. 10.1155/2010/541957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedts F, Ramaeker F, Troyanovsky S, Pruszczynski M, Robben H, Lane B, Leigh I, Plantema F, Yooijs P. 1992. Basal-cell keratins in cervical reserve cells and a comparison to their expression in cervical intraepithelial neoplasia. Am J Pathol. 140:601–612 [PMC free article] [PubMed] [Google Scholar]
- Sobel G, Németh J, Kiss A, Lotz G, Szabó I, Udvarhelyi N, Schaff Z, Páska C. 2006. Claudin 1 differentiates endometrioid and serous papillary endometrial adenocarcinoma. Gynecol Oncol. 103:591–598 [DOI] [PubMed] [Google Scholar]
- Sobel G, Páska C, Szabó I, Kiss A, Kádár A, Schaff Z. 2005. Increased expression of claudins in cervical squamous intraepithelial neoplasia and invasive carcinoma. Hum Pathol. 36:162–169 [DOI] [PubMed] [Google Scholar]
- Sobel G, Szabó I, Páska C, Kiss A, Kovalszky I, Kádár A, Paulin F, Schaff Z. 2005. Changes of cell adhesion and extracellular matrix (ECM) components in cervical intraepithelial neoplasias. Pathol Oncol Res. 11:26–31 [DOI] [PubMed] [Google Scholar]
- Soini Y. 2012. Tight junctions in lung cancer and lung metastasis: a review. Int J Clin Exp Pathol. 5:126–136 [PMC free article] [PubMed] [Google Scholar]
- Szabó I, Kiss A, Schaff Z, Sobel G. 2009. Claudins as diagnostic and prognostic markers in gynecological cancer. Review. Histol Histopathol. 24:1607–1615 [DOI] [PubMed] [Google Scholar]
- Tsukita S, Yamazaki Y, Katsuno T, Tamura A, Tsukita S. 2008. Tight junction-based epithelial microenvironment and cell proliferation. Oncogene. 27:6930–6938 [DOI] [PubMed] [Google Scholar]
- Turksen K, Troy TC. 2011. Junctions gone bad: claudins and loss of the barrier in cancer. Biochim Biophys Acta. 1816:73–79 [DOI] [PubMed] [Google Scholar]
- Witkiewicz AK, Hecht JL, Cviko A, McKeon FD, Ince TA, Crum CP. 2005. Microglandular hyperplasia: a model for the de novo emergence and evolution of endocervical reserve cells. Hum Pathol. 36:154–161 [DOI] [PubMed] [Google Scholar]