Abstract
The establishment and validation of specific markers on the surfaces of pancreatic beta-cells would have a significant impact on the development of agents that specifically target these cells for imaging and/or image-guided therapy in diabetes patient samples. We have recently described unique, cholesterol-stabilized sphingomyelin (SM) patches on the surfaces of beta-cells using the IC2 antibody. To further investigate the utility of SM patches as a unique beta-cell biomarker, we embarked on the current study to correlate the expression of this antigen with the insulin secretory capacity of beta-cells in tissue samples from patients and animals with type 1 and type 2 diabetes and compared this with samples from normal subjects. We found that the locations of SM patches were consistent with the insulin status of islets in all tissues studied. Using immunohistochemistry and staining with an IC2 antibody, we demonstrated a direct correlation between the reduced expression of SM patches and insulin production in diabetic individuals, indicating that the former could potentially serve as a functional biomarker of beta-cells. We believe that our results have significant implications for the further development of ligands with SM specificity for the non-invasive functional assessment of beta-cells and/or for targeted therapeutic delivery in diabetic patients.
Keywords: beta-cells, diabetes, surface markers, sphingomyelin, antibody
Introduction
The gradual loss of insulin-producing beta cells results in hyperglycemia and diabetes ensues. In type 1 diabetes (T1D), the loss is due to an autoimmune attack on beta-cells (Eisenbarth 1986) whereas, in type-2 diabetes (T2D), increasing demand for insulin due to peripheral insulin resistance causes undue stress on beta cells and, ultimately, their apoptosis (Cnop et al. 2012). Currently, there are no clinical tests available to directly monitor beta-cell health. Routine tests such as measurement of insulin/c-peptide levels or blood glucose levels (fasting or after glucose challenge) do not provide adequate information about the mass and/or function of insulin-producing beta-cells. The development of new imaging technologies that allow for specific monitoring of beta-cell status, including evaluation of therapeutic efficacy and assistance in following transplanted islet grafts, would greatly aid in the clinical management of the disease. Similarly, therapeutic strategies that target beta-cells exclusively would overcome the issues with low bioavailability and significant toxicity of conventionally administered therapeutics. The wide implementation of these technologies is hindered by the lack of established and validated specific markers on the beta-cell surface. The several markers that have been described so far (GAD (Baekkeskov et al. 1990), carboxipeptidase H (Castano et al. 1991), ICA69 (Pietropaolo et al. 1993), VMAT-2 (Maffei et al. 2004; Pettibone et al. 1984), GLP-1R (Reiner et al. 2011; Wicki et al. 2007), SUR-1 (Schneider et al. 2005), FXYD2 (Flamez et al. 2010), TMEM27 (Vats et al. 2012) and scFv’s from phage display libraries (Ueberberg et al. 2009) still require validation in animal models (for review see (Kavishwar and Moore 2012)). Importantly, these markers do not reflect beta-cell function, which is a serious drawback when an evaluation of therapeutic efficacy is needed. A recent comprehensive study utilized zinc as a functional beta-cell biomarker, which could be used for non-invasive imaging (Lubag et al. 2011). However, the zinc sensor used by Lubag and colleagues cannot be used for the purposes of therapeutic delivery. Ideally, a beta-cell–specific biomarker, suitable for targeting for both imaging and therapy, should be located on the beta-cell surface and be indicative of beta-cell function.
To this end, we have recently described unique cholesterol-stabilized sphingomyelin (SM) patches on the surface of beta-cells defined by targeting with an IC2 antibody (Kavishwar et al. 2011). IC2 is a rat monoclonal IgM that was obtained by fusing splenocytes from diabetic BioBreeding (BB) rats with the Y.3 Ag 1.2.3 rat myeloma cell line. The resulting hybridomas were screened for the production of monoclonal antibodies specific to the surface of Rin5F rat insulinoma cells (Brogren et al. 1986). We have previously shown the utility of the IC2 antibody in determining beta cell mass ex vivo in an STZ model of diabetes (Moore et al. 2001). To fully exploit the value of this antibody or its fragments for imaging and/or targeted therapy we have recently elucidated the antigen for this antibody (Kavishwar et al. 2011). In particular, we showed that sphingomyelin stabilized with cholesterol forms unique patches on the surface of beta-cells that are recognized by the IC2 antibody. Furthermore, these patches were unique for beta-cells and were not expressed on any other types of islet cells or in any other tissue (Kavishwar et al. 2011). To further investigate the utility of SM patches as a unique beta-cell biomarker, we embarked on the current study. Specifically, we explored changes in the expressions of SM patches on the beta-cell surface during diabetes development and correlated it with changes in insulin secretion. We used pancreatic tissue samples from patients with type 1 and type 2 diabetes, as well as tissues from several animal models of diabetes. Using immunohistochemistry, we demonstrated direct correlation between reduced expression of SM patches and insulin production, indicating that the former could potentially serve as a functional biomarker of beta-cells. We believe that our results have significant implications for the further development of ligands with SM specificity for the non-invasive functional assessment of beta-cells and/or for targeted therapeutic delivery.
Materials and Methods
Cell Culture
RinM5f (CRL-11605, ATCC), a rat insulinoma cell line, was cultured in RPMI medium supplemented with 10% FBS. Media was changed every third day to keep the cultures in an exponential growth phase.
Antibody Purification
IC2 antibody was purified from hybridoma cell culture using the following procedure. First, 10 cm x 10 cm PVDF membrane was spray-coated with ~ 200 µl of porcine brain sphingomyelin (10 mg/ml, Avanti Polar Lipids Inc., Alabaster, AL), and allowed to air dry. IC2 hybridoma culture supernatant was cleared at 10,000 xg for 15 min and buffered 1:1 with 100 mM PBS. Then, 100 ml of this buffered supernatant was poured on top of the sphingomyelin-coated PVDF membrane and the membrane incubated overnight on a rocker at 4C. After extensive washing with PBS, the antibodies were eluted with IgG elution buffer (Thermo Fisher Scientific Inc., Rockford, IL). Purity of eluted antibody was analyzed by SDS-PAGE in the presence or absence of reducing agent (beta mercaptoethanol) following by staining with Coomassie blue.
Animals
The following animal models of diabetes were employed in this study.
NOD/ShiLtJ female mice were purchased and aged from The Jackson Laboratory (Bar Harbor, ME) and maintained in a germ-free environment at Massachusetts General Hospital animal facility. Mice were sacrificed at the ages of 4, 6, 10 and 17 weeks and their pancreata were isolated and analyzed by immunohistochemistry as described below. Onset of diabetes in mice was followed by blood glucose measurements (Bayer Contour, Tarrytown, NY) every other day. Animals with blood glucose levels of >250 mg/dL on two consecutive measurements were considered diabetic.
Both, multiple low-dose streptozotocin (MLDS) and single high-dose streptozotocin (SHDS) protocols were used to generate type 1 diabetic BALB/c mice. For MLDS, 8–10-week-old mice received freshly dissolved streptozotocin (STZ) 50 mg/kg body wt intraperitoneally every day for 5 days with concomitant blood glucose monitoring. The SHDS mice received one 300 mg/kg body wt STZ injection. Organs were harvested following euthanasia and stored frozen in OCT at -80C.
All animals were in a fed-state when the pancreata were collected. All animal experiments were approved by Institutional Animal Care and Use Committee at MGH and performed according to the guidelines of the committee.
Human Patient Samples
Frozen sections of human pancreata were obtained from the Network for Pancreatic Organ Donors with Diabetes (nPOD), a collaborative T1D research project sponsored by the Juvenile Diabetes Research Foundation International (JDRF). We used sample/CaseID as indicated on the nPOD website. A total of four T1D and five T2D samples were selected for this study based on their blood glucose, body mass index (BMI) values, and history of diabetes. Samples from healthy patients (n=5) were used as a control. All patient samples used in the study were excised from the tail of the pancreas.
Immunohistochemistry of Pancreatic Sections and Cells
Human and mouse pancreatic sections were co-immunostained with IC2, anti-insulin and anti-glucagon or anti-somatostatin antibodies. Frozen sections (5–7-µm thick) were fixed with 4% formaldehyde for 5 min, washed with PBS and blocked with 2% BSA in PBS for 1 hr at room temperature. IC2 (1 µg/ml) and guinea pig anti-insulin (Abcam, Cambridge, MA) and rabbit anti-glucagon antibodies (Abcam), diluted to 1:50 and 1:100, respectively, were mixed in 2% BSA, added to the sections and incubated at room temperature for 2 hr. Then, sections were washed with PBS and incubated in the mixture of goat anti-rat IgM-AF594 (1: 1000 dilution, Invitrogen, Carlsbad, CA), goat anti-guinea pig IgG (H+L)-FITC (1:200 dilution, Abcam) and goat anti-rabbit IgG (H+L) AF-680 (1:500 dilution, Invitrogen) for 2 hr. Next, the sections were washed with PBS, mounted in ProLong® Gold anti-fade reagent with DAPI (Invitrogen), and observed under fluorescence microscope. For pancreatic sections from human patients, the fluorescence intensity was scored on a scale of 1 to 5 by two blinded investigators. In all of these experiments, an irrelevant purified rat monoclonal IgM was used as control for IC2. Staining without primary antibodies was also used as a control.
For some experiments RinM5f cells were stained for sphingomyelin patches. First, cells grown on coverslips were washed three times with PBS at room temperature. Thereafter, all fixation and staining steps were similar to the procedures described above for tissue sections.
Staining of Purified Islets from T2D Patients
Purified islets from a T2D patient were obtained from Prodo Laboratories Inc., (Irvine, CA) through Integrated Islet Distribution Program. The patient was diagnosed with diabetes four years before death and was on oral medications for the treatment of diabetes and hypertension (medications were not specified in patient’s file). The islet preparation was more than 80% pure and had a viability of more than 80% at the time of the experiment. After receiving islets, they were allowed to recover overnight at 37C until the next morning. After fixing in 4% formaldehyde, the whole islet preparation (not islet sections) was stained with anti-insulin, anti-somatostatin and IC2 antibodies. After washing, islets were incubated with secondary goat anti-rat IgM-AF594, goat anti-guinea pig IgG (H+L)-FITC and goat anti-rabbit IgG (H+L) AF-680, as described above.
Results
IC2 Purification and Characterization
To obtain purified IC2 antibody, we utilized ligand affinity chromatography. We have previously identified sphingomyelin patches as an antigen for this antibody (Kavishwar et al. 2011). In this study, we used sphingomyelin-coated PVDF membranes as a stationary phase. The amount of antibody eluted after several rounds of purification was unchanged, suggesting that there was no loss of sphingomyelin coating from the PVDF membrane. Purified antibody preparation was found to be more than 90% pure by SDS-PAGE electrophoresis (Fig. 1A). Binding specificity of IC2 antibody was further confirmed by staining with RinM5f cells (Fig. 1B). Characteristic punctate staining was observed similar to that previously described (Kavishwar et al. 2011), indicating that the new purification method did not compromise antibody specificity.
Figure 1.

Characterization of ligand affinity purified antibody. (A) Purity of IC2 antibody was tested using SDS-PAGE electrophoresis under reducing conditions, followed by staining with Coomassie blue. (B) Purified IC2 antibody stained RinM5f insulinoma cells, producing a characteristic punctate staining (green, IC2; blue, DAPI nuclear stain). Bar = 50 µm.
Immunohistochemistry of SM Patches in Human Diabetic Pancreas
Sections of normal and diabetic pancreas were stained for IC2, insulin and glucagon and staining intensity was scored on a scale of 1 to 5 (Table 1). IC2 staining was concomitant with insulin staining and was localized within the islets. No IC2 staining of surrounding non-endocrine pancreatic islet tissue was observed (Figs. 2 and 3). In pancreatic tissue from normal individuals, the IC2 staining was intense, with a significant number of visible patches per cell compared with diabetic samples, where the staining intensity was low and the patches were not evident.
Table 1.
Description of patient samples.
| S. No. | CaseID | Group | AutoAb | Age (years) | Gender | Ethnicity | C-peptide (ng/ml) | HbA1c | BMI | Clinical History | Histopathology | Staining for IC2, Insulin and Glucagon |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6089 | T1D | mIAA+ | 14.3 (8 w/ diabetes) | Male | Caucasian | <0.05 | 10.4 | 26 | Not available | Ins-/Gluc+ (reduced) islets. Atrophied islets. CD3+ infiltrates, exocrine/periduct., occ. near Gluc+ islets. Ductular proliferation (Ki67+) multifocal, moderate. Acute pancreatitis-high Ki67+ multifocal, mild to moderate, region of duct rupture and exocrine necrosis. | IC2: None |
| Insulin: None | ||||||||||||
| Glucagon: +++ | ||||||||||||
| 2 | 6113 | T1D | mIAA+ | 13.1 (1 w/ diabetes) | Female | Caucasian | <0.05 | 24.5 | Placed on insulin pump within month. | Ins+ (reduced)/Gluc+ islets. Insulitis. Lobular islet heterogenity. Islet atrophy with mild acinar atrophy (heterogeneous). Glucagon cells as single cells and small clusters. | IC2: +++ | |
| Insulin:+++ | ||||||||||||
| Glucagon: +++++ | ||||||||||||
| 3 | 6138 | T1D | mIAA+ | 49.2 (41 w/diabetes) | Female | Caucasian | <0.05 | 33.7 | Coronary artery disease and hypertension. | Ins-/Gluc+, atrophy, decreased numbers. High acinar and duct Ki67. CD3+ infiltrates-acute and chronic pancreatitis with acinar necrosis, fat saponification, multifocal, severe, likely secondary to stones. | IC2: None | |
| Insulin: None | ||||||||||||
| Glucagon: +++++ | ||||||||||||
| No proper islets | ||||||||||||
| 4 | 6141 | T1D | GADA+ IA-2A+ ZnT8A+ mIAA+ | 36.7 (28 w/diabetes) | Male | Caucasian | <0.05 | 26 | Screening autoab testing showed IA-2A. Autoantibody core showed GADA+ slightly above cut off (22 versus assay cut off of 20). | Ins-/Gluc+ islets. Atrophy exocrine tissue with fatty replacement, mild. No infiltrates. Low Ki67. Nerve fibers prominent. Moderate atherosclerosis. | IC2: None | |
| Insulin: None | ||||||||||||
| Glucagon: ++++ | ||||||||||||
| 5 | 6110 | T2D | Negative | 20.7 (acute onset) | Female | African American | 0.58 | 40 | Gestational diabetes in past. Father had diabetes. | Ins+ (reduced)/Gluc+ islets various sizes, some atrophied. Low Ki67. No fatty infiltrates. No infiltrates except one small foci. | IC2: None | |
| Insulin: 0/1+ | ||||||||||||
| Glucagon: +++++ | ||||||||||||
| 6 | 6114 | T2D | Negative | 42.8 (2 w/ diabetes) | Male | Caucasian | 0.58 | 7.8 | 31 | Diabetes medication was oral Metformin (1 g/day) for 2 years but noncompliant. Multiple family members had diabetes on both maternal and paternal sides. Hypertension, hypercholesterolemia. | Ins+ (reduced)/Gluc+ islets with severe amyloidosis. Severe exocrine atrophy. Moderate CVD and fatty infiltration. No infiltrates. | IC2: +++ |
| Ins: +++ | ||||||||||||
| Glucagon:+++++ | ||||||||||||
| 7 | 6127 | T2D | mIAA+ | 44.8 (10 w/diabetes) | Female | Caucasian | 0.08 | 30.4 | Not available | Ins+ (reduced)/Gluc+ islets. Mild adiposity in exocrine regions. Low Ki67. | IC2: ++++ | |
| Insulin: ++++ | ||||||||||||
| Glucagon: +++++ | ||||||||||||
| 8 | 6132 | T2D | Negative | 55.8 (preclinical) | Female | Hispanic | 0.8 | 9.1 | 44.6 | Preclinical T2D. i.e., no diagnosis or medication for diabetes; Hypertension for at least 3 years and on anti-HTN medication. | Ins+ (reduced) islets, numerous, smaller. Low Ki67. Amyloid. Exocrine atrophy with moderate fatty infiltrates. | IC2: ++ |
| Insulin: +++ | ||||||||||||
| Glucagon: +++ | ||||||||||||
| Islets: small and few | ||||||||||||
| 9 | 6139 | T2D | Negative | 37.2 (1.5 w/diabetes) | Female | Hispanic | 0.6 | 45.4 | Mother had T2D. | Ins+ islets, plentiful with nuclear polymorphism. Minimal fibrosis. No pancreatitis. | IC2: ++++ | |
| Insulin: ++++ | ||||||||||||
| Glucagon: +++++ |
Figure 2.
Immunohistochemistry for pancreatic sections from normal and two type 1 diabetes (T1D) patients (case IDs: 6141 and 6113). Pancreatic tissue sections were stained for insulin (green), IC2 (red) and glucagon (white). DAPI nuclear stain is depicted in blue. Magnification bar = 20 µm. See Table 1 for patient sample description. Bar = 20 µm.
Figure 3.
Immunohistochemistry for pancreatic sections from three type 2 diabetes (T2D) patients (case IDs: 6127, 6132, 6110). Pancreatic tissue sections were stained for insulin (green), IC2 (red) and glucagon (white). DAPI nuclear stain is depicted in blue. Magnification bar = 20 µm.
Of the four T1D samples examined, three samples showed a lack of insulin staining. IC2 staining was also absent in those samples. These patients had a history of diabetes ranging from 8 to 41 years. On the contrary, a newly diagnosed case (1 year duration) exhibited insulin staining (Fig. 2 Case ID: 6113). This sample had a concomitant IC2 staining with clearly seen patches. In all T1D sections examined, we found that, although the islets showed significant atrophy, staining for glucagon was still present.
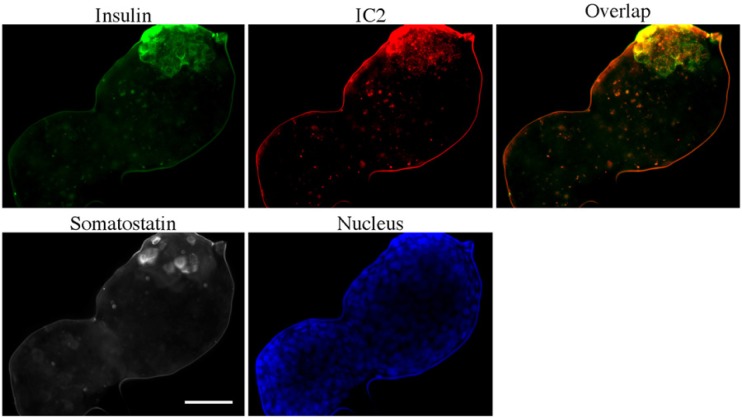
There was a significant variability in insulin staining among T2D patients, which mirrored IC2 staining (Fig. 3). Overall, IC2 staining was more pronounced in the samples with defined insulin production and lower or absent in the samples with poor insulin staining. Staining of purified islets from a type 2 diabetes patient showed the same trend with IC2 staining overlapping with the insulin staining (Fig. 4). In these islets, insulin staining was localized to the part of the islet where presumably the functional beta-cells were present. In samples from type 2 diabetes patients, we did not observe any correlation between the intensity of IC2/insulin staining and duration of the disease. We would like to point out that not all cells in the islet respond similarly to changes in blood glucose levels. Some cells release their content earlier than the others. Also, beta-cells never release all of their insulin granules at the same time and hence there will always be an inherent difference in insulin staining within the same islet, as seen in these figures. However, this would not interfere with our ultimate goal of developing beta-cell-specific imaging agents. This is because the overall imaging signal will be collected from the region of interest without the need to visualize a single cell.
Figure 4.
Immunohistochemical staining of purified islets from type 2 diabetes (T2D) patients. Islets from T2D patients were stained for insulin (green), IC2 (red) and somatostatin (white) DAPI nuclear stain is depicted blue. See Table 1 for patient sample description. Bar = 50 µm.
Changes in SM Patches Expression during T1D Development in NOD Mice
NOD, or non-obese diabetic, mice represent the most widely used mouse strain having T1D progression similar to that in humans (Anderson and Bluestone 2005). To establish and understand a possible relationship between insulin expression and the presence of SM patches observed after staining with IC2 antibody, we performed immunohistochemistry using pancreata from 4-, 6-, 10- and 17-week-old NOD mice. Our results indicated that insulin, IC2 and glucagon staining appeared normal in 4- and 5-week-old mice (data not shown for 5-week-old mice; Fig. 5). By 6 weeks of age, we observed some initial mononuclear cell infiltration in islets accompanied by a slight reduction in insulin and IC2 staining. By 9 weeks of age (not shown), almost all islets contained mononuclear cell infiltration to some degree. Parts of the islets infiltrated by mononuclear cells showed no insulin staining. Slightly reduced insulin staining was present in the areas devoid of infiltration. In all sections, IC2 staining followed the pattern of insulin staining. A slight reduction in intensity of staining or density of SM patches was seen in the areas of insulin staining. By 10 weeks of age, significant parts of the islets were taken over by infiltrating mononuclear cells but insulin and IC2 staining were still visible in some areas. Mice became severely diabetic by 17 weeks of age judging by their blood glucose levels. Pancreatic sections from these mice showed no insulin staining. As expected, IC2 staining was also absent. On some sections, T-cell infiltration could still be seen surrounding the islets and the destruction of insulin-producing beta-cells was complete (Fig. 5).
Figure 5.
Changes in insulin expression and sphingomyelin (SM) patches during type 1 diabetes (T1D) in non-obese diabetic (NOD) mice. Pancreatic tissue sections from 4-, 6-, 10- and 17-week-old NOD mice were stained for insulin (green) and IC2 (red). DAPI nuclear stain is depicted in blue. Magnification bar = 50 µm.
We need to mention that the bright spots observed in the images of 10-week-old NOD mice with IC2 staining represent non-specific staining with the secondary antibody against IgM. It is well known from the literature that IgM-positive cells have been found to be a major constituent of the mononuclear cell infiltrate in islets, forming follicular (nodular) cell aggregates (Kanazawa et al. 1984). Indeed, we performed control staining without primary antibody using the tissue from 10-week-old NOD mice, and observed these bright cell clusters that appear to resemble nodular cell aggregates (see Supplementary Information, Fig. 1). These nodules co-localized very well with mononuclear cell infiltrates observed in these islets. This was not seen in islets of mice without infiltrates of invading leukocytes.
Expression of SM Patches Is Affected by Streptozotocin
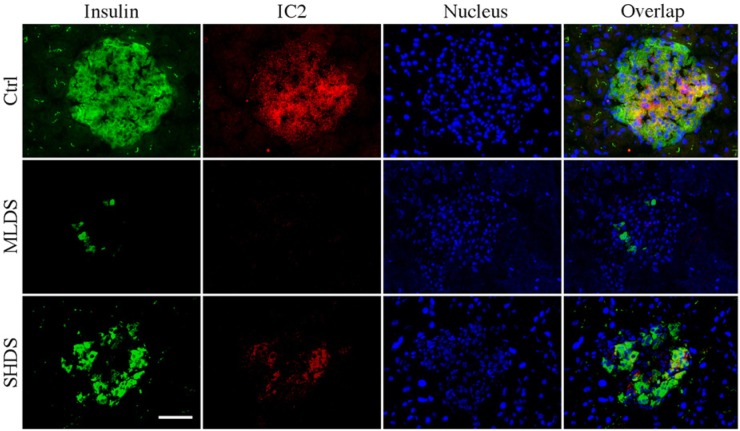
To confirm the interconnection between insulin staining and the expression of SM patches, we investigated the same in MLDS and SHDS mouse diabetes models. In MLDS-injected animals, a marked reduction in insulin staining (both in the number of cells and in the intensity per cell) was clearly visible already on the fifth day after STZ injection (Fig. 6). Insulin-positive cells that were still present appeared large. While the islets in these sections showed severe atrophy, no mononuclear cell infiltration was observed. Similar to our previous results in other animal models and human sections, IC2 staining correlated with insulin staining and was not observed if insulin staining was absent. Similar results were obtained for SHDS-injected mice. Islets in these mice had a slightly higher number of insulin-positive cells than that in the MLDS model. The results of these studies confirmed our previous findings in NOD mice and showed direct correlation between the reduction in insulin secretion upon STZ administration and the expression of SM patches.
Figure 6.
Changes in insulin and sphingomyelin (SM) patches expression in multiple low-dose streptozotocin (MLDS) and single high-dose streptozotocin (SHDS) models of diabetes. Pancreatic tissues from control, MLDS and SHDS mice were stained for insulin (green), IC2 (red) and glucagon (white). DAPI nuclear stain is depicted in blue. Bar = 50 µm.
Discussion
The determination of specific markers for pancreatic beta-cells has been a long sought after goal of diabetes research, as its realization would enable specific targeting of these cells for imaging and/or image-guided therapy. We recently reported the discovery of cholesterol-stabilized sphingomyelin patches on the surface of beta-cells that are targeted by IC2, a rat monoclonal antibody (Kavishwar et al. 2011). In this study, we investigated whether the expression of these patches vary during diabetes development. To this end, we examined pancreatic tissues from human subjects with various stages of type 1 and type 2 diabetes, as well as tissues from various animal models of diabetes. Our results with human samples pointed toward the direct correlation between insulin expression and the presence of SM patches as IC2 antibody staining. In all samples from patients with established diabetes for whom we observed a decrease or disappearance of insulin staining, IC2 staining was also compromised and accurately mirrored the former. To further investigate this phenomenon, we utilized three models of type 1 diabetes widely used in the laboratory setting. Reduced or absent staining for IC2 in NOD mice of various age as well as in SHDS- and MLDS-injected animals correlated well with insulin secretion in these animals. Interestingly, we found that disappearance of IC2-defined SM patches in NOD mice was gradual and was associated with the gradual loss of insulin staining, which started at 6 weeks of age along with emergence of mononuclear cell infiltrates.
We believe that this study is important for understanding the connection between SM and insulin secretion. Insulin is synthesized as preproinsulin and fed into rough endoplasmic reticulum (ER) where the signal peptide is immediately cleaved off releasing proinsulin into the lumen of the ER. Proinsulin is then shuttled into Golgi stacks where it is sorted for packaging into secretory vesicles. Condensation/crystallization of proinsulin is followed by the pinching-off of Golgi cisterna into a clathrin-coated secretory vesicle. Subsequent acidification of coated vesicle creates conditions for proteolytic cleavage of proinsulin into insulin and C-peptide. As these vesicles traverse toward the plasma membrane and begin to mature, the clathrin coat is lost and the insulin vesicles, now called insulin granules, are positioned against the plasma membrane for fusion and insulin release (Orci et al. 1987). Sphingomyelin synthase (SMS) 1 and 2 play important roles in the pinching-off of secretory vesicles from Golgi cisterna. Pharmacological blockage of SMS or the downregulation of SMS-1 or SMS-2 with siRNA inhibits this process. This causes repressed insulin secretion in response to glucose stimulation in Ins-1E cells (Subathra et al. 2011). Similarly, SMS-1 knockout animals display severe deficiencies in insulin secretion (Yano et al. 2011). These studies demonstrate that sphingomyelin metabolism is uniquely involved in insulin secretion. Our results are in agreement with these conclusions, demonstrating the reduction of SM expression through IC2 staining, which ran in parallel with the reduced insulin production. However, it is premature to make a conclusion about the correlation between sphingomyelin patch expression and insulin secretion. The only conclusion that we can make relates to the staining patterns of IC2 and insulin, which most likely reflect their expression. It would be extremely interesting to explore the possibility that such a correlation might exist in our future studies.
The significance of our study is underscored by the fact that SM patches not only serve as a marker for beta-cell surface, but also as an indicator of functionality of these cells. Importantly, we observed a similar trend in the correlation between insulin expression and the expression of SM patches in islets from both type 1 and type 2 diabetes pancreata; this points to the possibility of the universal biochemical mechanism connecting these events, which is independent of the mechanism that causes diabetes.
Supplementary Material
Footnotes
Supplementary material for this article is available on the Journal of Histochemistry & Cytochemistry Web site at http://jhc.sagepub.com/supplemental.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by the award from Juvenile Diabetes Research Foundation (JDRF 37-2009-30) to AM.
References
- Anderson MS, Bluestone JA. 2005. The NOD mouse: a model of immune dysregulation. Ann Rev Immunol. 23:447–485 [DOI] [PubMed] [Google Scholar]
- Baekkeskov S, Aanstoot HJ, Christgau S, Reetz A, Solimena M, Cascalho M, Folli F, Richter-Olesen H, De Camilli P. 1990. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 347:151–156 [DOI] [PubMed] [Google Scholar]
- Brogren CH, Hirsch F, Wood P, Druet P, Poussier P. 1986. Production and characterization of a monoclonal islet cell surface autoantibody from the BB rat. Diabetologia. 29:330–333 [DOI] [PubMed] [Google Scholar]
- Castano L, Russo E, Zhou L, Lipes MA, Eisenbarth GS. 1991. Identification and cloning of a granule autoantigen (carboxypeptidase-H) associated with type I diabetes. J Clin Endocrin Metabol. 73:1197–1201 [DOI] [PubMed] [Google Scholar]
- Cnop M, Foufelle F, Velloso LA. 2012. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol Med. 18:59–68 [DOI] [PubMed] [Google Scholar]
- Eisenbarth GS. 1986. Type I diabetes mellitus. A chronic autoimmune disease. The New Engl J Med. 314:1360–1368 [DOI] [PubMed] [Google Scholar]
- Flamez D, Roland I, Berton A, Kutlu B, Dufrane D, Beckers MC, De Waele E, Rooman I, Bouwens L, Clark A, Lonneux M, Jamar JF, Goldman S, Marechal D, Goodman N, Gianello P, Van Huffel C, Salmon I, Eizirik DL. 2010. A genomic-based approach identifies FXYD domain containing ion transport regulator 2 (FXYD2) gammaa as a pancreatic beta cell-specific biomarker. Diabetologia. 53:1372–1383 [DOI] [PubMed] [Google Scholar]
- Kanazawa Y, KOmeda K, Sato S, Mori S, Akanuma K, Takaku F. 1984. Non-obese-diabetic mice: immune mechanisms of pancreatic beta-cell destruction. Diabetologia. 27:113–115 [DOI] [PubMed] [Google Scholar]
- Kavishwar A, Medarova Z, Moore A. 2011. Unique sphingomyelin patches are targets of a beta-cell-specific antibody. J Lipid Res. 52:1660–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavishwar A, Moore A. 2012. Molecular Imaging of Diabetic Pancreas. Immunol Endocrine Metabol Agents Med Chem. 12:216–223 [Google Scholar]
- Lubag AJ, De Leon-Rodriguez LM, Burgess SC, Sherry AD. 2011. Noninvasive MRI of beta-cell function using a Zn2+-responsive contrast agent. Proc Nat Acad Sci U S A. 108:18400–18405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Liu Z, Witkowski P, Moschella F, Del Pozzo G, Liu E, Herold K, Winchester RJ, Hardy MA, Harris PE. 2004. Identification of tissue-restricted transcripts in human islets. Endocrinology. 145:4513–4521 [DOI] [PubMed] [Google Scholar]
- Moore A, Bonner-Weir S, Weissleder R. 2001. Noninvasive in vivo measurement of beta-cell mass in mouse model of diabetes. Diabetes. 50:2231–2236 [DOI] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Storch MJ, Anderson RG, Vassalli JD, Perrelet A. 1987. Proteolytic maturation of insulin is a post-Golgi event which occurs in acidifying clathrin-coated secretory vesicles. Cell. 49:865–868 [DOI] [PubMed] [Google Scholar]
- Pettibone DJ, Totaro JA, Pflueger AB. 1984. Tetrabenazine-induced depletion of brain monoamines: characterization and interaction with selected antidepressants. Eur J Pharm. 102:425–430 [DOI] [PubMed] [Google Scholar]
- Pietropaolo M, Castano L, Babu S, Buelow R, Kuo YL, Martin S, Martin A, Powers AC, Prochazka M, Naggert J. 1993. Islet cell autoantigen 69 kD (ICA69). Molecular cloning and characterization of a novel diabetes-associated autoantigen. J Clin Invest. 92:359–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner T, Thurber G, Gaglia J, Vinegoni C, Liew CW, Upadhyay R, Kohler RH, Li L, Kulkarni RN, Benoist C, Mathis D, Weissleder R. 2011. Accurate measurement of pancreatic islet beta-cell mass using a second-generation fluorescent exendin-4 analog. Proc Nat Acad Sci U S A. 108:12815–12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Feilen PJ, Schreckenberger M, Schwanstecher M, Schwanstecher C, Buchholz HG, Thews O, Oberholzer K, Korobeynikov A, Bauman A, Comagic S, Piel M, Schirrmacher E, Shiue CY, Alavi AA, Bartenstein P, Rosch F, Weber MM, Klein HH, Schirrmacher R. 2005. In vitro and in vivo evaluation of novel glibenclamide derivatives as imaging agents for the non-invasive assessment of the pancreatic islet cell mass in animals and humans. Exp Clin Endocrinol Diabetes. 113:388–395 [DOI] [PubMed] [Google Scholar]
- Subathra M, Qureshi A, Luberto C. 2011. Sphingomyelin synthases regulate protein trafficking and secretion. PloS One. 6:e23644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueberberg S, Meier JJ, Waengler C, Schechinger W, Dietrich JW, Tannapfel A, Schmitz I, Schirrmacher R, Koller M, Klein HH, Schneider S. 2009. Generation of novel single-chain antibodies by phage-display technology to direct imaging agents highly selective to pancreatic beta- or alpha-cells in vivo. Diabetes. 58:2324–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vats D, Wang H, Esterhazy D, Dikaiou K, Danzer C, Honer M, Stuker F, Matile H, Migliorini C, Fischer E, Ripoll J, Keist R, Krek W, Schibli R, Stoffel M, Rudin M. 2012. Multimodal imaging of pancreatic beta cells in vivo by targeting transmembrane protein 27 (TMEM27). Diabetologia. 55:2407–2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicki A, Wild D, Storch D, Seemayer C, Gotthardt M, Behe M, Kneifel S, Mihatsch MJ, Reubi JC, Macke HR, Christofori G. 2007. [Lys40(Ahx-DTPA-111In)NH2]-Exendin-4 is a highly efficient radiotherapeutic for glucagon-like peptide-1 receptor-targeted therapy for insulinoma. Clin Cancer Res. 13:3696–3705 [DOI] [PubMed] [Google Scholar]
- Yano M, Watanabe K, Yamamoto T, Ikeda K, Senokuchi T, Lu M, Kadomatsu T, Tsukano H, Ikawa M, Okabe M, Yamaoka S, Okazaki T, Umehara H, Gotoh T, Song WJ, Node K, Taguchi R, Yamagata K, Oike Y. 2011. Mitochondrial dysfunction and increased reactive oxygen species impair insulin secretion in sphingomyelin synthase 1-null mice. J Biol Chem. 286:3992–4002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.