Abstract
Background
The lack of structural support remains a challenge in the treatment of comminuted distal radius fractures. Calcium phosphate and calcium sulfate bone cement has been used in other fracture locations in addition to fixation and has been shown to allow for retention of reduction in difficult cases.
Methods
A case-control retrospective review of 34 consecutive distal radius fractures treated with surgery was performed with the patients classified by Arbeitsgemeinschaft für Osteosynthesefragen (AO) classification. Complications and postoperative radiographs were evaluated.
Results
Cement was used in the most difficult cases. Radial height was retained in both groups. Volar tilt was significantly better in the cement group. There were no significant differences between the case and control groups for any complication. No complications related to the use of the cement were found.
Conclusions
The use of bone cement as an adjunct to fixation of distal radius fractures seems to include minimal risks and may afford a technical advantage in maintaining reduction during surgery for difficult fractures. Since there is an aspect of fracture difficulty that we cannot control for by using radiographic assessment alone, cement may provide an advantage over fixation without cement, despite similar outcomes. Bone cement can be part of the "tool box" for difficult distal radius fractures. Further study is necessary to define the technical advantages and limitations of each particular cement product.
Keywords: Bone cement, Distal radius fractures, Arbeitsgemeinschaft für Osteosynthesefragen Osteosynthesefragen (AO) classification
Background and Purpose
The treatment of comminuted, intra-articular distal radius fractures has been evolving to include a multitude of techniques for internal fixation [2, 3]. While long-term outcomes and complications are still being evaluated, a significant challenge in the treatment of these fractures is structural support in the presence of soft and sometimes osteoporotic metaphyseal bone. This is especially true for already healing fractures where an osteotomy is necessary prior to reduction (Fig. 1). Current techniques utilized to overcome this problem have included the use of adjunct external fixation, locking, and bridging plates. Bone grafting has been used for support, but requires cortical bone, which may not provide immediate or delayed maintenance during the healing process [12]. Furthermore, bone grafting includes donor site morbidity in autologous grafts and potential infection in allografts [5]. Bone cement has been used for scaffolding in spine surgery, but also in metaphyseal and intra-articular fractures such as in the tibial plateau, proximal humerus, and distal radius [4, 8, 11, 13, 16].
Fig. 1.

An example of loss of reduction despite internal fixation without bone graft. Lateral radiograph of a distal radius fracture immediately after surgery for open reduction and fixation and after 3 weeks. There is loss of reduction of the dorsal cortex with dorsal subluxation of the carpus
We retrospectively compared distal radius fractures treated with open reduction and internal fixation using bone cement to those without the use of cement in an attempt to define the role of bone cement in the surgery of distal radius fractures.
Materials and Methods
All distal radius fractures treated over a period of 30 months by a single surgeon with open reduction and internal fixation were retrospectively reviewed in a case-control study. Institutional review board (IRB) approval was obtained prior to study commencement. Exclusion criteria included patients with missing radiographs, radiographs of poor quality, and patients with incomplete follow-up. Information regarding age, background disease, smoking status, surgical approach, the use of bone graft, and bone cement was recorded. All complications were documented including nonunion, malunion, chronic regional pain syndrome (CRPS), and infection. Fracture classification was assessed using the Arbeitsgemeinschaft für Osteosynthesefragen (AO) classification system for the distal radius [1, 9].
Posteroanterior (PA) radiographs from the first post-operative visit and from the latest available visit within 3 months were used to evaluate radial height and ulnar variance, volar tilt, radial incline and intra-articular step off and gap. The measurements were performed by an independent observer using a digital measurement tool (Stentor iSite(TM) Picture Archive and Communications System [PACS]). Radial height was measured between two parallel lines perpendicular to the long axis of the radius. One line was drawn on the articular surface of the radius, and the other at the tip of the radial styloid (normal values between 9.9 nd 17.3 mm).
Radial inclination angle was measured by drawing a line along the articular surface of the radius perpendicular to the long axis of the radius, and a tangent from the radial styloid (normal value between 15° and 25°). Volar tilt was measured on the lateral view as the angle between a line perpendicular to the long axis of the radius and a tangent line drawn along the slope of the dorsal-to-palmar surface of the radius (normal angle between 10° and 25°). Ulnar variance was described as being zero, minus, or plus [14].
All distal radius fractures using cement were included. These cases were controlled with retrospective distal radius fractures not using cement by AO fracture classification. Radial height loss, ulnar variance, volar tilt, radial incline and intra-articular step off and gap were compared between groups using a t-test, and complications were compared between groups using Chi-square tests. A multivariate general linear model was used to account for variance from all other factors. A p value smaller or equal to 0.05 was considered significant.
Technique
The distal radius fractures were treated operatively under general or block anesthesia. The reduction and fixation was obtained using volar fixed angle plates or plating systems that included fragment specific fixation according to the fracture type. The cement could not be used prior to fixation. If there was a big bony void or an unstable reduction after insertion of the hardware that necessitated additional support (such as an external fixator or bridging plate), the calcium phosphate cement was used to fill all voids.
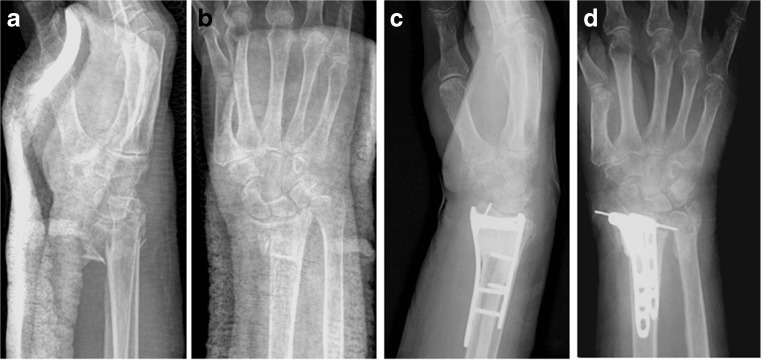
HydroSet (Stryker, Kalamazoo, MI, USA), a mixture of calcium phosphate and sulfate bone cement, was used in all of the cement cases. Calcium phosphate hardens within minutes and is expected to remain at least in part several years after implantation. Because of its high calcium content it is radiopaque. The calcium phosphate forms a solid structure that is mechanically stable and will have a compressive strength between cancellous and cortical bone. The calcium sulfate is highly biocompatible but resorbs quickly and has low compressive strength. The mixture of the two allows for better handling, curing at body temperature and replacement by bone at a time that is between that of calcium sulfate and phosphate [17]. Complications have been reported using bone cement in the spine but not in the extremities. The bone cement is mixed for 45 s, drawn into a syringe and injected through an 8-gauge cannula (diameter 4.2 mm) into the area of bone loss. The injection is performed through the cortical defect, we injected through the dorsal cortical defect more often simply because there is usually more cortical loss dorsally, but if only a volar approach is used, the injection is performed volarly. It was injected following completion of the reduction and fixation in all cases since the authors' experience is that it is not possible to drill through the cement or to manipulate the cement without interfering with the hardening process. Since the cement hardens at body temperature there is no need to protect the soft tissue but injection when the construct is complete usually prevents the cement from extruding into the soft tissue. Following injection, soft tissue closure was completed (Fig. 2). The cement was not used to promote healing of the fractures since closed distal radius fractures usually unite, but rather for structural support.
Fig. 2.
Before and after radiographs of a distal radius fracture treated with open reduction internal fixation and cement bone graft
Following surgery, all patients were immobilized in a splint that allowed for finger motion but limited wrist motion for a period of 6 weeks. Hand therapy was instituted for range of motion (ROM) and gradual strengthening. Patients were followed in clinic every 2 weeks for the first 3 months. At 6 weeks, radiographs were obtained to evaluate healing of the fractures.
Results
The population consisted of 36 distal radius fractures that were treated with open reduction and internal fixation using cement. Of these, 34 patients had adequate follow-up. Therefore, 34 cases were controlled with 26 retrospective distal radius fractures not using cement by AO fracture classification. The controls were cases in the last 30 months that were operated on without the use of cement and were matched to cases by AO classification. We were unable to control for eight C3 fracture cases. One patient in the cement group was treated with an external fixator in addition to internal fixation and cement (Table 1).
Table 1.
The distribution of fractures by AO stage in the two populations
| AO stage | A2 | B1 | B2 | C1 | C2 | C3 | Total |
|---|---|---|---|---|---|---|---|
| No cement | 1 | 2 | 2 | 3 | 13 | 5 | 26 |
| Cement | 1 | 2 | 2 | 3 | 13 | 13 | 34 |
| Total | 2 | 4 | 4 | 6 | 26 | 18 | 60 |
The case and control populations were not shown to be significantly different and could be compared (Table 2). The average follow-up period was 56.4 (67.7) days with no significant difference between the cement and control groups. Fifteen cases and nine control patients had follow-up radiographs of sufficient quality to assess radial height measurements. Volar tilt, radial inclination, and ulnar variance were measured on radiographs of 30 cases and 23 controls. Both cement and non-cement patients averaged less than 1 mm in radial height loss. The difference between the groups was not significant. Volar tilt measurements differed significantly between the two groups (Table 3). Articular gapping and step-off were not apparent on any of the post- operative radiographs and therefore the results in the cement and control groups did not differ.
Table 2.
Factors that may have an effect on fracture healing; comparison between the two groups
| Hydroset (n=34) | No hydroset (n=26) | |
|---|---|---|
| Age, years | 46.9 (18.5) | 42.6 (16.4) |
| Males | 59% | 54% |
| Background disease | 24% | 0% |
| smoking | 32% | 35% |
| Diabetes | 18% | 4% |
| Immunocompromised | 9% | 0% |
| Dominant=Injured | 50% | 58.3% |
Table 3.
Measurement results; comparison between cement and controls mean (standard deviation)
| Radial height loss | Radial inclination (°s) | Volar tilt (°) | Ulnar variance (mm) | |
|---|---|---|---|---|
| Cement | 0.8 (0.3) | 22.4 (1.2) | 3.5 (0.6) | 0.5 (0.6) |
| No cement | 0.7 (0.8) | 23.6 (1.0) | −2.7 (2.0) | 2.6 (0.4) |
| p value | 0.8 | 0.5 | 0.002 | 0.3 |
Radial height loss was the measured change in radial height between the immediate post-fixation radiographs and the final films once healed. A significant difference was found for volar tilt between the cemented and non-cemented groups
There was one nonunion in the cement group at 6 weeks post-surgery and one malunion in the same group. Overall complications, pin site infection, nonunion, malunion, chronic pain, and CRPS did not significantly differ between the case and control groups for any complication. The cement group initially had a significant occurrence of chronic pain and CRPS but when controlled for AO grade, the association was not significant (Table 4).
Table 4.
Comparison of complication rates between the groups
| Cement (n=34) | No cement (n=26) | |
|---|---|---|
| Complications — overall | 5 (15%) | 3 (12%) |
| Pin site infection | 1 (3%) | 0 (0%) |
| Nonunion | 1 (3%) | 0 (0%) |
| Malunion | 1 (3%) | 0 (0%) |
| Chronic pain | 3 (9%) | 3 (12%) |
| RSD | 2 (6%) | 1 (4%) |
Discussion
Kim et al. concluded that adding bone cement does not change the amount of collapse in distal radial fractures treated with internal fixation [6]. We did find a significant difference between the groups in the measurement of volar tilt. Overall, there was an average difference of 6° between the groups. It is not clear if this difference is clinically significant. However, since height retention was similar in both groups, and volar tilt was improved, it is possible that the use of cement prevents height loss in fractures that otherwise would have collapsed.
While minimal complications did exist in the cement group such as infection, nonunion, and malunion, we were unable to find a clear association between the use of cement and these complications. These complications could be accounted for by the greater complexity of cases for which cement was used (e.g., the nonunion was in a patient who fell from a height with significant associated soft tissue injury due to the high energy of the injury). We could not control for eight fractures, which were all C3 fractures. This is logical since we used the cement only in fractures with significant intra-articular or metaphyseal bone loss. Since a higher fracture grade is associated with complications such as chronic pain and CRPS, it was necessary to correct for this bias in our analysis when evaluating complications by comparing the cases and controls within each individual AO classification for each complication.
Since our study is a retrospective review with a small population, the conclusions from the results are limited. The measurement of radial height, ulnar variance, articular step-off, gapping, and radial inclination are all dependent on the quality of the radiograph and the precision of the PA view. Many of these radiographs were taken before the patients regained maximal ROM, and therefore many of the views were suboptimal and were excluded from the study. Moreover the differences in angle and height were small. Radial height measurements were all <1mm, and possibly within the range of our measurement error. The study by Knirk and Jupiter [7] that was also performed on similar radiographs found that the clinically significant step-off was 2 mm, so it is probably valid to say there was no clinically significant loss of height in the population as a whole.
A limitation of the study is that we were unable to extract significant data regarding the patients' clinical outcomes. This is likely due to the fact that this was a retrospective study performed in a clinic in a level I trauma center with poor follow up. Further study to correlate the measurements with clinical outcome is needed. Furthermore, our relatively short follow up period does not provide long-term information. However, radiographs were evaluated after healing of the fractures and the beginning of mobilization, after which the fracture configuration should remain unchanged [10].
In general the cases that included the use of cement were the more difficult ones. Even though we controlled for fracture comminution using the AO radiographic system, this probably does not completely control for actual instability and technical difficulty encountered during surgery. More specifically, not all fractures in a certain radiographic category will be equally difficult/easy to stabilize, and sometimes during actual surgery we encounter more bone loss or instability than we expect from looking at the radiographs.
When dealing with difficult distal radius fractures and significant articular comminution and bone loss, it is helpful to have a variety of technical options so that the fixation methods can be tailored to the fracture. Different plating systems, Kirschner wires, and external fixators all include inherent advantages and disadvantages. After reviewing our results, we found that the use of calcium phosphate/calcium sulfate bone cement as an adjunct to internal fixation in comminuted distal radius fractures allows for retention of reduction in difficult cases and includes no significant additional adverse effects as found in previous similar studies [15]. Though cement use does not seem to confer a clear advantage in the final outcome of surgery, we feel that there is an aspect of fracture difficulty and instability that we are not able control for using radiographic assessment alone. It is our opinion that bone cement should be available since it provides immediate stability thereby enabling the surgeon in certain cases to reduce the need for adjunct fixators while accumulating minimal complications. It seems to be most helpful in the very difficult subset of fractures including "old" partially healed fractures and those with the most significant articular involvement. The cost of cement use needs to be taken into account but may be compensated for in those cases where its use allows the surgeon to abandon using an external fixator or other adjunct stabilization. Further study is necessary to define the technical limitations of each particular cement product, and to better delineate the indications for cement use in fractures of the distal radius.
Acknowledgments
The study was approved by the IRB and was conducted in accordance with the Helsinki declaration.
Conflict of interest
The authors have no conflict of interest to disclose
The need for informed consent was waived by the IRB since this was a retrospective study.
References
- 1.Andersen DJ, Blair WF, Steyers CM, Jr, et al. Classification of distal radius fractures: an analysis of interobserver reliability and intraobserver reproducibility. J Hand Surg [Am] 1996;21(4):574–82. doi: 10.1016/S0363-5023(96)80006-2. [DOI] [PubMed] [Google Scholar]
- 2.Cherubino P, Bini A, Marcolli D. Management of distal radius fractures: treatment protocol and functional results. Injury. 2010;41(11):1120–6. doi: 10.1016/j.injury.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 3.Gruber G, Zacherl M, Giessauf C, et al. Quality of life after volar plate fixation of articular fractures of the distal part of the radius. J Bone Joint Surg Am. 2010;92(5):1170–8. doi: 10.2106/JBJS.I.00737. [DOI] [PubMed] [Google Scholar]
- 4.Handoll HH, Watts AC. Bone grafts and bone substitutes for treating distal radial fractures in adults. Cochrane Database Syst Rev. 2008;2:CD006836. doi: 10.1002/14651858.CD006836.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heneghan HM, McCabe JP. Use of autologous bone graft in anterior cervical decompression: morbidity and quality of life analysis. BMC Musculoskelet Disord. 2009;10:158. doi: 10.1186/1471-2474-10-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JK, Koh YD, Kook SH. Effect of calcium phosphate bone cement augmentation on volar plate fixation of unstable distal radial fractures in the elderly. J Bone Joint Surg Am. 2011;93(7):609–14. doi: 10.2106/JBJS.J.00613. [DOI] [PubMed] [Google Scholar]
- 7.Knirk JL, Jupiter JB. Intra-articular fractures of the distal end of the radius in young adults. J Bone Joint Surg Am. 1986;68(5):647–59. [PubMed] [Google Scholar]
- 8.Kopylov P, Jonsson K, Thorngren KG, Aspenberg P. Injectable calcium phosphate in the treatment of distal radial fractures. J Hand Surg (Br) 1996;21(6):768–71. doi: 10.1016/S0266-7681(96)80184-7. [DOI] [PubMed] [Google Scholar]
- 9.Kreder HJ, Hanel DP, McKee M, et al. Consistency of AO fracture classification for the distal radius. J Bone Joint Surg Br. 1996;78(5):726–31. [PubMed] [Google Scholar]
- 10.Lozano-Calderón SA, Brouwer KM, Doornberg JN, Goslings JC, Kloen P, Jupiter JB. Long-term outcomes of corrective osteotomy for the treatment of distal radius malunion. J Hand Surg Eur. 2010;35(5):370–80. [DOI] [PubMed]
- 11.Robinson CM, Page RS. Severely impacted valgus proximal humeral fractures. Results of operative treatment. J Bone Joint Surg Am. 2003;85-A(9):1647–55. doi: 10.2106/00004623-200309000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Schatzker J, Haeri GB, Chapman M. Methylmethacrylate as an adjunct in the internal fixation of intertrochanteric fractures of the femur. J Trauma. 1978;18:732–735. doi: 10.1097/00005373-197810000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Simpson D, Keating JF. Outcome of tibial plateau fractures managed with calcium phosphate cement. Injury. 2004;35(9):913–8. doi: 10.1016/S0020-1383(03)00109-8. [DOI] [PubMed] [Google Scholar]
- 14.Spence LD, Savenor A, Nwachuku I. MRI of fractures of the distal radius: comparison with conventional radiographs. Skeletal Radiol. 1998;27(5):244–9. doi: 10.1007/s002560050375. [DOI] [PubMed] [Google Scholar]
- 15.Suhm N, Gisep A. Injectable bone cement augmentation for the treatment of distal radius fractures: a review. J Orthop Trauma. 2008;22(8 Suppl):S121–5. doi: 10.1097/BOT.0b013e3181830d13. [DOI] [PubMed] [Google Scholar]
- 16.Vlad MD, Sindilar EV, Marinoso ML, et al. Osteogenic biphasic calcium sulphate dihydrate/iron-modified alpha-tricalcium phosphate bone cement for spinal applications: In vivo study. Acta Biomater 2009. [DOI] [PubMed]
- 17.Yetkinler DN, Ladd AL, Poser R, Constantz BR, Carter D. Biomechanical evaluation of fixation of intraarticular fractures of the distal part of the radius in cadavera: Kirschner wires compared with calcium-phosphate bone cement. J Bone Joint Surg. 1999;81-B(3):391–399. doi: 10.2106/00004623-199903000-00012. [DOI] [PubMed] [Google Scholar]