Abstract
The present research work focused on the comparative assessment of porous versus nonporous films in order to develop a suitable buccoadhesive device for the delivery of glibenclamide. Both films were prepared by solvent casting technique using the 32 full factorial design, developing nine formulations (F1–F9). The films were evaluated for ex vivo mucoadhesive force, ex vivo mucoadhesion time, in vitro drug release (using a modified flow-through drug release apparatus), and ex vivo drug permeation. The mucoadhesive force, mucoadhesion time, swelling index, and tensile strength were observed to be directly proportional to the content of HPMC K4M. The optimized porous film (F4) showed an in vitro drug release of 84.47 ± 0.98%, ex vivo mucoadhesive force of 0.24 ± 0.04 N, and ex vivo mucoadhesion time of 539.11 ± 3.05 min, while the nonporous film (NF4) with the same polymer composition showed a release of 62.66 ± 0.87%, mucoadhesive force of 0.20 ± 0.05 N, and mucoadhesive time of 510 ± 2.00 min. The porous film showed significant differences for drug release and mucoadhesion time (p < 0.05) versus the nonporous film. The mechanism of drug release was observed to follow non-Fickian diffusion (0.1 < n < 0.5) for both porous and nonporous films. Ex vivo permeation studies through chicken buccal mucosa indicated improved drug permeation in porous films versus nonporous films. The present investigation established porous films to be a cost-effective buccoadhesive delivery system of glibenclamide.
KEY WORDS: buccoadhesive drug delivery, glibenclamide, in vitro release and ex vivo permeation, porous film
INTRODUCTION
The aim of any drug delivery system is to provide a therapeutic amount of drug to the appropriate site in the body so as to rapidly achieve and then maintain the desired concentration (1). Owing to the ease of administration, the oral cavity is an attractive site for the delivery of therapeutic agents. It is possible to not only achieve a site-specific drug effect on the mucosa (local effect) but to also attain drug absorption through the mucosal barrier (systemic effect) (2). The major disadvantages associated with the oral route are extensive hepatic first-pass metabolism and presystemic enzymatic drug degradation (3,4).
Buccal delivery of drugs is an attractive alternative to circumvent high hepatic first-pass metabolism and degradation in the harsh gastrointestinal environment (5–7). Moreover, the retentive buccoadhesive formulations can be readily attached to the mucosa, can be retained for a longer period of time, and can be easily removed (8). Mucoadhesive formulations may also be used to achieve the controlled release of drugs having a short half-life (9,10). Drugs exhibiting poor aqueous solubility and those which are sensitive to enzymatic degradation may be delivered successfully across the buccal mucosa (11).
Glibenclamide (5-chloro-N-[2-[4-cyclohexylcarbamoylsulfamoyl]phenyl]ethyl)-2-methoxybenzamide; having a molecular weight of 494.0 Da), a potent derivative of the second-generation sulfonylureas, is used in the treatment of type II diabetes mellitus. It has a short biological half-life (3 to 5 h), with a log p value of 4.7 (o/w); thus, it has poor aqueous solubility and undergoes oxidative hepatic first-pass metabolism to yield metabolites having no hypoglycemic activity (12). All these factors contribute to low oral bioavailability (about 45%). Hence, it was hypothesized that delivering glibenclamide through the buccal route would help to improve the oral bioavailability of the drug (13).
Literature review reveals that buccoadhesive tablets, gels, strips, and films have been investigated for the delivery of glibenclamide. Marikanti developed a bilayered buccoadhesive tablet for systemic delivery of glibenclamide by the direct compression method (14). Buccoadhesive tablets, besides causing discomfort to the patients because of their thickness, have low area of contact and lack flexibility with the buccal mucosa. Philip and coworkers reported a buccoadhesive gel of glibenclamide using the solution polymerization technique (12). Buccoadhesive gels have short retention time on the buccal mucosa since they can be easily washed away by saliva (15). Ilango and coworkers developed chitosan-based buccal strips of glibenclamide using the solvent casting technique (16). Gaudanavar and coworkers and Muzib and coworkers reported mucoadhesive buccal films of glibenclamide developed using the solvent casting technique (13,17). The formulation of strips and films as buccal delivery systems involves the use of an additional hydrophilic polymer to facilitate drug release and a permeation enhancer to facilitate permeation of the drug across the buccal mucosa. This not only adds to the cost of the developed formulation but also leads to a decrease in mucoadhesive strength and time.
Hence, the present research work attempted to compare porous versus nonporous mucoadhesive films as a potential cost-effective and patient-compliant delivery system for glibenclamide using the 32 full factorial design as the statistical optimization tool. HPMC K4M was employed as the mucoadhesive polymer and EC as the controlled release polymer. EC was chosen since it is one of the most widely used water-insoluble polymers in pharmaceutical film coating due to its convenient film formability, good physicochemical property, and minimal toxicity. HPMC K4M was selected since it acts as a mucoadhesive polymer and swells in water to produce a clear to opalescent, viscous, colloidal dispersion. The interpolymer complexation of these two polymers may help to control the release of the drug from the formulation (18).
MATERIALS AND METHODS
Materials
Glibenclamide was obtained as a gift sample from Sun Pharmaceutical Industries Ltd. (Sikkim, India). HPMC K4M and EC [with an ethoxyl content of 48.0–49.5% by weight and a viscosity of 14 cps in 5% (w/w) toluene/ethanol (80:20) solution at 25°C] were procured from Central Drug House Pvt. Ltd. (New Delhi, India). Potassium chloride, agar–agar, dichloromethane, and glycerol were procured from Qualigens Fine Chemicals Pvt. Ltd. (Mumbai, India). All other reagents used were of analytical grade.
Methods
Preparation of Polymeric Porous Films
Films were prepared by solvent casting technique. Backing layer was prepared by dissolving EC (2%, w/v) in ethanol and adding 2.5% (v/v) of glycerol as plasticizer. The plasticized EC solution was poured into a Petri dish and the solvent was allowed to evaporate at room temperature in a controlled fashion by covering the Petri dish with an inverted glass funnel so as to avoid the blistering effect on dried films. The mucoadhesive layer was prepared by using EC and HPMC K4M in the ratio as stated in Table I. Glibenclamide was dissolved in a small amount of methanol, and 2.5% (v/v) of glycerol was added as plasticizer. EC was added to this solution by dissolving it in 10 ml of dichloromethane, followed by the addition of HPMC K4M. The polymeric dispersion was then poured into the Petri dish containing preformed baking layer and covered with an inverted glass funnel to allow controlled evaporation of the solvent for 24 h at room temperature.
Table I.
Evaluation of Pharmacotechnical Parameters of Buccoadhesive Porous Films of Glibenclamide
| Film code | Weight (mg) | Surface pH | Thickness (mm) | Percentage swelling | Folding endurance | Tensile strength (dyn/cm2) | Drug content (%) |
|---|---|---|---|---|---|---|---|
| F1 | 24.24 ± 1.21 | 6.96 ± 0.05 | 0.19 ± 0.01 | 45.33 ± 0.61 | 230 ± 5.13 | 150.14 ± 0.21 | 92.28 ± 0.44 |
| F2 | 26.55 ± 1.28 | 6.95 ± 0.05 | 0.27 ± 0.01 | 35.75 ± 0.54 | 233 ± 4.16 | 144.52 ± 0.55 | 91.31 ± 0.25 |
| F3 | 29.42 ± 1.12 | 6.86 ± 0.07 | 0.25 ± 0.01 | 32.86 ± 0.97 | 221 ± 3.60 | 123.05 ± 0.11 | 93.65 ± 0.51 |
| F4 | 25.80 ± 0.78 | 6.90 ± 0.13 | 0.23 ± 0.02 | 51.26 ± 0.70 | 286 ± 6.00 | 175.20 ± 0.56 | 95.38 ± 0.44 |
| F5 | 30.00 ± 0.95 | 6.83 ± 0.15 | 0.25 ± 0.01 | 46.73 ± 1.51 | 230 ± 3.60 | 166.32 ± 0.34 | 94.95 ± 0.89 |
| F6 | 32.14 ± 1.55 | 7.05 ± 0.08 | 0.27 ± 0.01 | 41.44 ± 1.52 | 242 ± 5.00 | 160.45 ± 0.83 | 93.92 ± 0.92 |
| F7 | 28.50 ± 1.05 | 6.81 ± 0.02 | 0.20 ± 0.01 | 57.43 ± 0.22 | 292 ± 4.00 | 178.40 ± 0.67 | 92.02 ± 0.67 |
| F8 | 31.75 ± 1.18 | 6.78 ± 0.02 | 0.29 ± 0.05 | 51.30 ± 0.30 | 250 ± 2.86 | 170.07 ± 0.12 | 93.30 ± 1.09 |
| F9 | 34.10 ± 1.35 | 6.68 ± 0.02 | 0.34 ± 0.02 | 52.55 ± 1.00 | 261 ± 5.56 | 171.95 ± 0.71 | 94.96 ± 1.27 |
Preparation of Polymeric Nonporous Films
Nonporous films were prepared using the methodology as described previously, but the solvent system used was a mixture of dichloromethane and ethanol (50:50, v/v).
Design of Experiments
A 32 randomized full factorial design was applied for the statistical optimization of the experiments. Two factors, i.e., amounts of HPMC K4M and EC, were selected as the independent variables and the responses obtained, such as mucoadhesion time, mucoadhesive strength, and percentage of cumulative drug release (%CDR) at 8 h, were selected as the dependent variables. Each independent variable was evaluated at three levels, and a total of nine experimental runs were performed. Formulations F1 to F9 were prepared using three different levels of HPMC K4M and EC, as shown in Table II. Polynomial equations were generated for each response using the Design Expert Software (version 8.0.5; Stat-Ease, Inc., Minneapolis, MN, USA). An extra-design checkpoint formulation was prepared using the amount of independent variables not included in the formulation design and was used to validate the obtained polynomial equations. These equations were then utilized to select the optimized formulation.
Table II.
Experimental Design: Coded Factors, Levels, and Responses of Porous Films of Glibenclamide
| Film code | Drug (mg) | EC (X1) (% w/v) | HPMC (X2) (% w/v) | Glycerol (% v/v) | Response |
|---|---|---|---|---|---|
| F1 | 12 | 1.0 (−1) | 1.0 (−1) | 5 | Mucoadhesion time (Y 1) |
| F2 | 12 | 2.0 (0) | 1.0 (−1) | 5 | |
| F3 | 12 | 3.0 (+1) | 1.0 (−1) | 5 | |
| F4 | 12 | 1.0 (−1) | 2.0 (0) | 5 | Mucoadhesive force (Y 2) |
| F5 | 12 | 2.0 (0) | 2.0 (0) | 5 | |
| F6 | 12 | 3.0 (+1) | 2.0 (0) | 5 | |
| F7 | 12 | 1.0 (−1) | 3.0 (+1) | 5 | |
| F8 | 12 | 2.0 (0) | 3.0 (+1) | 5 | %CDR at 8 h (Y 3) |
| F9 | 12 | 3.0 (+1) | 3.0 (+1) | 5 | |
| F10a | 12 | 1.5 | 1.5 | 5 |
aExtra-design checkpoint formulation
Evaluation of Prepared Mucoadhesive Buccal Porous Films
Uniformity of Weight
The individual weight of 10 samples of each batch of formulation was determined, and the average weight was calculated. The results obtained were reported as the mean ± standard deviation (SD).
Thickness
Three films of each formulation were taken and the film thickness was measured by using a micrometer screw gauge (Mitutoyo Corporation, Kawasaki, Japan) at three different places. The results obtained were reported as the mean ± SD.
Endurability/Flexibility
Flexibility of the films was measured in terms of folding endurance. Three films from each formulation were cut into 2 × 2 cm2 sizes, and the folding endurance was determined by repeatedly folding the film at the same place till it broke. The number of times the film could be folded at the same place without breaking gave the value of the folding endurance (19). The results were analyzed for the mean and SD.
Surface pH
The surface pH of the films was determined in order to rule out the possibility of any irritation to the buccal mucosa due to the acidic or alkaline pH of the films. The buccal films were left to swell for 2 h on the surface of the agar plate which was prepared by dissolving 2% (w/v) agar in warmed isotonic phosphate buffer (pH 6.8) under constant stirring and then pouring into a Petri dish and allowing it to gel at room temperature. The surface pH was measured by means of a pH paper placed on the surface of the swollen film (19). A mean of three readings was recorded.
Drug Content
Drug content was determined by homogenization of 1 × 1 cm2 film in 100 ml simulated saliva (pH 6.8), filtered through 0.45 μ filter, and the resultant solution was diluted suitably with simulated saliva (pH 6.8) and analyzed spectrophotometrically at 300.2 nm (UV spectrophotometer 1700; Shimadzu, Kyoto, Japan). The assay values were determined from the calibration curve of the drug in phosphate buffer, pH 6.8 (y = 0.004x + 0.039; r2 = 0.9997).The experiments were carried out in triplicate, and the average value was reported.
Swelling Behavior
The swelling property of the films was evaluated by determining the percentage of hydration. Each film was cut (2 × 2 cm), weighed (W1), and immersed in simulated saliva (pH 6.8) for 2 h. After every 15 min, the surface of the films was wiped using a filter paper to remove excess simulated saliva and again weighed (W2). The experiment was performed in triplicate (n = 3, α = 0.05). Percentage swelling (%S) was calculated by the following expression:
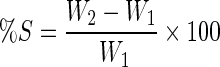 |
1 |
where W2 is the weight of the swollen film after time t and W1 is the original weight of the film at time 0.
Tensile Strength
The tensile strength of the buccal film refers to the tension or force required to tear the film apart into two pieces. It measures the strength of the film in terms of diametric tension or tearing force and can be measured by using simple calibrated vertical spring balance based on the American Standard for Testing Material tests principles (Fig. 1a). The film, in dimensions of 25 × 10 mm with uniform thickness, was held between two clamps positioned at a distance of 15 mm. One clamp was fixed to the solid support and the other clamp was attached to the pan balance. Weight was loaded into the other pan till the film broke apart. The force needed to break the film was determined by measuring the total weight loaded in the pan. The weight required to break the film was used to calculate the tensile strength using the following expression:
 |
2 |
where m is the weight in grams loaded in the pan, g is the acceleration due to gravity, and A is the initial cross-sectional area of the film.
Fig. 1.
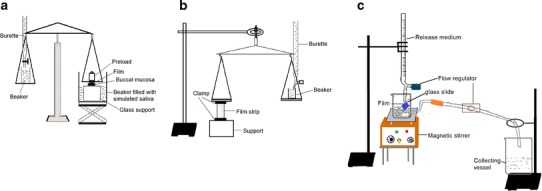
a Modified tensile strength apparatus, b modified apparatus for ex vivo determination of mucoadhesive force, and c in vitro release study in simulated saliva (pH 6.8) using a novel modified flow-through apparatus
Determination of the In Vitro Residence Time/Mucoadhesion Time
The ex vivo mucoadhesion time was determined by application of the films (n = 3) on freshly cut chicken buccal mucosa procured from a local slaughterhouse. The buccal tissue was fixed on the internal side of a beaker, about 2.5 cm from the bottom, with the help of cyanoacrylate adhesive. Each film was cut into 2 × 2 cm. The mucoadhesive side of the film was wet with 50 μl of simulated saliva (pH 6.8) and pasted to the chicken buccal tissue by applying light force with figure tip for 20 s. The beaker was then filled with 200 ml of simulated saliva (pH 6.8) and kept at 37 ± 1°C. A stirring rate of 150 rpm was applied to simulate the buccal cavity environment, and film adhesion was monitored. The time taken by the film to detach from the buccal tissue was recorded as the mucoadhesion time.
Ex Vivo Mucoadhesive Force
Ex vivo mucoadhesive strength was assessed by the modified balance method using chicken buccal mucosa (Fig. 1b). The mucosal membrane was separated by removing underlying fat and loose tissues and washed with distilled water, followed by washing with simulated saliva (pH 6.8). A piece of buccal mucosa was tied to a lower Teflon block which was placed in a beaker filled with simulated saliva (pH 6.8) such that the simulated saliva (pH 6.8) touched the mucosal surface and was maintained at 37 ± 1°C. A film of 4 cm2 was stuck to the upper Teflon block with cyanoacrylate adhesive. The two sides of the balance were made equal prior to initiation of the study by keeping a weight of 5 g on both sides. The 5-g weight was removed from the right-hand side pan, which lowered the left-hand pan along with the film adhered to the upper block over the buccal mucosa tied to the lower block. The balance was kept in this position for 5 min. Then, water was added slowly at 100 drops/min to the other pan until the film detached from the mucosal surface. The weight in grams required to detach the film from the mucosal surface provided the measure of mucoadhesive strength. The experiments were performed in triplicate, and average values were reported.
In Vitro Drug Release
An in-house fabricated flow-through apparatus (Fig. 1c) was employed to evaluate the drug release which simulated the continuous flow of saliva in the buccal cavity environment. A portion of 4 cm2 (2 × 2 cm) of the film was used. The release medium consisted of simulated saliva (pH 6.8). The side facing the backing layer of the film was attached to a glass slide with double adhesive tape and kept at an angle of 60° in a modified flow-through beaker (100 ml). The release medium kept in the reservoir was allowed to fall on the film at a flow rate of 2 ml/min (maintained with the help of flow regulators). The temperature of the release medium was maintained at 37 ± 0.5°C, and a stirring speed of 50 rpm was employed. Five milliliters of the sample was collected at predetermined time intervals from the collecting beaker and analyzed at 300.2 nm using a spectrophotometer (PharmaSpec1700, Shimadzu, Tokyo, Japan) and reported as an average of three measurements (n = 3, α = 0.05).
Ex Vivo Drug Permeation Across Buccal Mucosa
Permeation of the drug from optimized formulations across the buccal mucosa was studied using fresh chicken buccal mucosa, which was procured from a local slaughterhouse, as a barrier membrane. Chicken buccal mucosa was used due to its similarity to the nonkeratinized human buccal mucosa. Moreover, it is inexpensive and convenient to handle and maintain. The buccal mucosa was excised and trimmed evenly from the sides and used within 2 h of this treatment. A modified Franz diffusion cell was used for permeation studies which consisted of two compartments, one was the donor compartment and the other was the receptor compartment having 7 ml capacity. The receptor compartment was surrounded by a water jacket to maintain the temperature at 37 ± 0.5°C. The excised buccal mucosa was mounted in between the two compartments, with the mucosal side facing the donor compartment and the receptor compartment filled with phosphate buffer (pH 7.4). The buccal mucosa was allowed to stabilize for 1 h and then a film (2 × 2 cm) was kept on the mucosa with the backing membrane facing the donor compartment. The contents of the receptor compartment were stirred with a magnetic stirrer. One milliliter sample was withdrawn at predetermined time intervals, and sink conditions were maintained throughout the study. Aliquots of samples were withdrawn, filtered, diluted suitably, and then analyzed spectrophotometrically at 300.2 nm.
Data Analysis
The following statistical model incorporating interactive and polynomial terms was used to evaluate the responses:
 |
3 |
where β0, the intercept, is the arithmetic average of all the outcomes of nine runs, β1 to β8 are the coefficients computed from the observed experimental values of Y (dependent variables), and X1 and X2 are the coded levels of the independent variables. The terms X1X2 and X2i (i = 1, 2) are the interactions and polynomial terms, respectively.
Statistical and Kinetic Analysis
The in vitro release profiles were tested for their kinetic behavior so as to establish the probable release mechanism involved in glibenclamide release from the film matrix. Data was analyzed by fitting it into various release models, namely, zero, first, Higuchi, and Korsmeyer–Peppas equations (20).
Scanning Electron Microscopy
Surface morphology of the optimized porous and nonporous films (before and after release) was examined by scanning electron microscopy (SEM). The samples were coated with gold ions under argon atmosphere using a gold sputter in a high vacuum evaporator (Sputter Coater Unit VG; Microtek, West Sussex, UK). The coated samples were then placed in the scanning electron microscope (JEOL 5400, Tokyo, Japan) chamber. The samples were randomly scanned and photomicrographs were taken.
Stability in Simulated Saliva
Stability of the optimized film was investigated in simulated saliva (pH 6.8). The film was placed in a Petri dish containing 5 ml of simulated saliva and put in a temperature-controlled oven at 37 ± 2°C. At predetermined time intervals of 0, 1, 2, 3, 4, 5, 6, 7, and 8 h, the film was observed for change in color, thickness, and drug content.
RESULTS AND DISCUSSION
Evaluation of Mucoadhesive Porous Films
Film Weight
The average film weight was found to be within the range of 24.24 ± 1.21 to 34.10 ± 1.35 mg (Table I). The film weight varied, depending upon the amount of polymer. Formulation F1 was found to have the minimum average weight, while F9 showed the maximum average weight.
Thickness
No particular pattern was found in the thickness of the films F1 to F9. This observation may be attributed to the different degrees of interpolymer complexation between the two polymers (18) used for the preparation of the buccal film. Thickness was higher in F2, F6, F8, and F9, which could be due to the higher quantity of HPMC and EC (Table I).
Flexibility/Folding Endurance
Folding endurance was found to be highest for F7 (292 ± 4.0) and lowest for F3 (221 ± 3.60). It was found that the folding endurance of films increased with an increase in concentration of hydrophilic polymer, whereas it decreased with an increase in concentration of hydrophobic polymer (Table I). The higher folding endurance leads to a more flexible film, which aids in adherence of the film to the buccal mucosa.
Surface pH
Considering the fact that acidic or alkaline pH may cause irritation to the buccal mucosa and influence the degree of hydration of polymers, the surface pH of the buccal films was determined to optimize avoid any irritation of the buccal mucosa by the films (21). The surface pH of all the films was found to be within the range of pH 6.8–7.0, i.e., close to neutral, and hence, safe for the buccal mucosa. No significant difference (p > 0.05) was observed in the surface pH of different formulations (Table I).
Drug Content
The drug content of each formulation was determined and was found to be within the range of 91.31 ± 0.25% to 95.38 ± 0.44% (Table I). This indicates that the drug was uniformly distributed throughout the films.
Swelling Behavior
Hydration is required for a mucoadhesive polymer to expand and create a macromolecular mesh of sufficient size as well as to induce mobility in the polymer chains to increase the interpenetration process between polymer and mucin (22). Swelling is an important parameter for uniform and prolonged release of the drug as well as effective mucoadhesion (23). Polymer swelling permits a mechanical entanglement by exposing the bioadhesive sites for hydrogen bonding or electrostatic interaction between the polymer and the mucous network. EC is water-insoluble and less hydrophilic. This could be the reason that the films with higher amount of EC showed less swelling upon hydration (24). Glibenclamide-loaded films had higher swelling values as compared to plain films because the addition of water-insoluble drug increased the water uptake by the dosage form. This may be due to the presence of micronized drug particles between the polymer chains, which allows each chain to hydrate freely, leading to the development of areas with weak hydrogen bonding around the glibenclamide molecules. These areas may increase the strength of the swollen matrix, followed by an obvious increase in the amount of penetrated water. Percentage swelling was found to be within the range of 32.86 ± 0.97% to 57.43 ± 0.22%. Percentage swelling increased with increasing hydrophilic polymer (HPMC), and for a given concentration of HPMC, the increase in concentration of the hydrophobic polymer (EC) resulted in diminutive effect on swelling (Table I). Careful observation of swelling profiles (Fig. 2) revealed a time-dependent increase in swelling. Though the graphs do not indicate a perfect zero-order kinetics (r2 = 0.9192–0.9605), an almost linear increase in swelling could be deduced. This is attributable to the porous nature of the film. The film interspersed with numerous pores provided numerous 3D windows (sites) for water uptake in addition to the regular film surface. These pores acted as channels for free water movement across them and facilitated uptake of water molecules by the interpolymerized polymeric buccal film. This is expected to promote drug release from the buccal film.
Fig. 2.
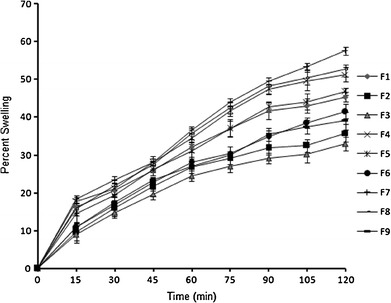
In vitro swelling behavior of porous films of glibenclamide in simulated saliva, pH 6.8
Tensile Strength
Tensile strength gives an indication of the strength and elasticity of the film. A weak and soft polymer is characterized by a low tensile strength; a hard and brittle polymer shows a moderate tensile strength, whereas a hard and tough polymer shows a high tensile strength (24). Formulation F7 showed the highest tensile strength (178.40 ± 0.67 dynes/cm2), while F3 had the lowest value of tensile strength (123.05 ± 0.11 dynes/cm2). As the amount of mucoadhesive polymer (HPMC K4M) in the formulation increased, the tensile strength at break also increased, whereas an increase in the amount of EC made the films more brittle and weak with a lower tensile strength (Table I).
Ex Vivo Mucoadhesion Time
Ex vivo mucoadhesion time was found to be highest for F7 (548.03 ± 2.88 min) and lowest for F1 (494 ± 8.14 min). The mucoadhesion time was found to increase with an increase in concentration of HPMC K4M. This could be due to the higher quantity of HPMC K4M which provided better interaction with the mucous membrane (Table III) because HPMC K4M is a long-chain nonionic polymer containing a large number of hydroxyl groups that are responsible for the formation of hydrogen bonds with a mucus component. On increasing the concentration of HPMC K4M, the number of hydroxyl group which aid in mucoadhesion increases (25). Additionally, due to its high viscosity following hydration, it can sustain the drug release.
Table III.
Mucoadhesive Characteristics of the Porous Films
| Film code | Mucoadhesion time (min) | Mucoadhesive strength (gm) | Mucoadhesive force (N) |
|---|---|---|---|
| F1 | 494.00 ± 8.14 | 12.20 ± 0.26 | 0.12 ± 0.02 |
| F2 | 516.51 ± 2.12 | 07.90 ± 0.11 | 0.07 ± 0.01 |
| F3 | 490.01 ± 4.50 | 10.45 ± 0.20 | 0.10 ± 0.02 |
| F4 | 539.11 ± 3.05 | 24.11 ± 0.42 | 0.24 ± 0.04 |
| F5 | 520.16 ± 2.51 | 19.62 ± 0.25 | 0.19 ± 0.02 |
| F6 | 517.23 ± 1.73 | 18.24 ± 0.05 | 0.18 ± 0.01 |
| F7 | 548.00 ± 2.88 | 25.23 ± 0.60 | 0.25 ± 0.06 |
| F8 | 535.42 ± 2.64 | 23.69 ± 0.37 | 0.23 ± 0.03 |
| F9 | 538.03 ± 3.21 | 23.48 ± 0.28 | 0.23 ± 0.02 |
Ex Vivo Mucoadhesive Force
The value of the mucoadhesive force (Table III) was found to range between 0.07 ± 0.011 and 0.25 ± 0.060 N. Mucoadhesive force was found to increase with an increase in the amount of mucoadhesive polymer (26). Mucoadhesive force was observed to be highest for formulation F7 (0.25 ± 0.060 N). This could be due to the fact that F7 contained the maximum amount of mucoadhesive polymer, i.e., HPMC K4M. Formulations F4, F7, F8, and F9 had higher mucoadhesive strength among the developed formulations due to the higher amount of HPMC K4M in these formulations. EC has no mucoadhesive property due to its hydrophobic character, and hence, an increase in the concentration of EC resulted in a decrease in mucoadhesive strength and force.
In Vitro Drug Release
The comparative in vitro drug release profiles of formulations F1 to F9 (Fig. 3) demonstrated an initial slow release of glibenclamide, probably as a result of the combined effect of the hydrophobicity of EC and gel-forming property of HPMC K4M. Incorporation of a gel-forming polymer like HPMC K4M retards drug release because an increase in tortuosity of the polymer as a result of swelling upon contact with aqueous fluid increases the path length available for the drug to diffuse out from the swollen matrix (27). Later on, beyond 2 h, the release of glibenclamide enhanced from all formulations at variable rates. Formulation F4, made of HPMC K4M and EC in a 2:1 ratio, had the highest drug release (84.47 ± 0.94%), followed by F7 (80.85 ± 1.71%) constituted in the polymeric ratio of 3:1. When both polymers were in a 1:1 ratio, the %CDR was lowered to 77.70 ± 0.61% that is attributable to the hydrophobicity of EC. This is also evident in the formulations made with higher levels of EC for a given HPMC concentration. Thus, F9 made with the highest levels of HPMC and EC displayed the least %CDR of 61.23 ± 0.86%. In addition to behavior toward water, the variation in release may be attributed to the nature of the network formed within the porous film. A looser network in the case of F4 perhaps led to the ease of penetration of the release medium and diffusion of the drug from the matrix than the rest of the formulations. As explained by Pathak (28), mucoadhesion based on water absorption lowers the water activity and imposes a larger gradient in water activity over the mucosa. This means that the formulation may also induce a mucosal response, as dehydration can affect the structure and barrier properties of mucosa in counterproductive ways. Thus, the applied formulation with low water activity that favors mucoadhesion would also induce a mucosal response detrimental to drug release and, consequently, absorption. It is noteworthy that, during the entire period of the release test, the formulation(s) remained adhered to the angular slide, conforming to the mucoadhesive properties of the films in the experimental setup. This setup, to the best of our knowledge, has not been utilized previously for the evaluation of drug release from porous buccal films. The release of glibenclamide from formulations F1, F2, F3, F4, and F9 almost conformed to zero-order release kinetics, whereas formulations F5, F6, F7, and F8 showed sustained release behavior. The release data were analyzed using Korsmeyer–Peppas equation to obtain values of k (kinetic constant), n (diffusional exponent), and r2 (coefficient of determination) and are depicted in Table IV. For non-Fickian release, the value of n falls between 0.5 and 1.0, while in case of Fickian diffusion, n = 0.5; for zero-order release (case II transport), n = 1 and, for supercase II transport, n > 1. The values of n were estimated by linear regression of log (Mt/M∞) versus log t and were found to be between 0.6 and 1.0, indicating that the release of glibenclamide from porous films followed non-Fickian diffusion.
Fig. 3.
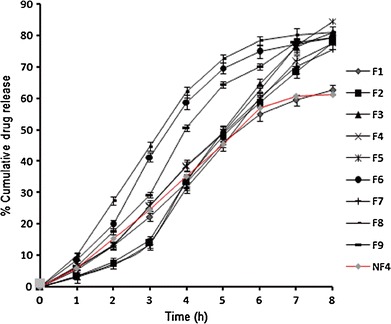
In vitro glibenclamide release from porous films (F1–F9) and nonporous film (NF4) in simulated saliva, pH 6.8
Table IV.
Model Dependent Parameters of the Formulations (F1–F9)
| Film code | Evaluation parameters | Zero order | First order | Matrix model | Korsmeyer–Peppas model |
|---|---|---|---|---|---|
| F1 | r 2 | 0.9819 | 0.9017 | 0.9322 | 0.9754 |
| k | 6.3037 | −0.1822 | 1.2874 | 0.1231 | |
| n | – | – | – | 0.7359 | |
| F2 | r 2 | 0.9748 | 0.9001 | 0.9443 | 0.3574 |
| k | 5.6750 | −0.2143 | 1.4521 | 0.2611 | |
| n | – | – | – | 0.8708 | |
| F3 | r 2 | 0.9953 | 0.8742 | 0.9814 | 0.9927 |
| k | 7.8763 | −0.2876 | 2.8976 | 0.2535 | |
| n | – | – | – | 0.8441 | |
| F4 | r 2 | 0.9949 | 0.9019 | 0.7217 | 0.9934 |
| k | 10.2134 | −0.1238 | −0.3425 | 0.2773 | |
| n | – | – | – | 0.9551 | |
| F5 | r 2 | 0.9003 | 0.7715 | 0.9537 | 0.9518 |
| k | −0.1238 | −0.3287 | 3.8764 | 0.2669 | |
| n | – | – | – | 0.9312 | |
| F6 | r 2 | 0.9750 | 0.9015 | 0.9446 | 0.9800 |
| k | 1.3706 | −0.2960 | 30.2320 | 0.2322 | |
| n | – | – | – | 0.7086 | |
| F7 | r 2 | 0.8877 | 0.7429 | 0.9519 | 0.9944 |
| k | 8.3679 | −0.3018 | 4.3322 | 0.1522 | |
| n | – | – | – | 0.6954 | |
| F8 | r 2 | 0.9517 | 0.8264 | 0.9733 | 0.9760 |
| k | 2.5501 | −0.3267 | 7.8760 | 0.2188 | |
| n | – | – | – | 0.7976 | |
| F9 | r 2 | 0.9844 | 0.8445 | 0.9687 | 0.9830 |
| k | 5.8971 | 0.9832 | −0.1398 | 0.2613 | |
| n | – | – | – | 0.8214 |
Optimization of Formulation
The data generated by evaluation of the formulations were subjected to statistical analysis using 32 full factorial design, with the help of Design Expert Software version 8.0.5 (Table V). Polynomial equations were generated, representing the relationship between the level of independent variable and observed response. After omitting the nonsignificant figures by application of analysis of variance (ANOVA), the final reduced (transformed) equations were obtained for the observed responses, i.e., mucoadhesion time (Y1), mucoadhesive force (Y2), and %CDR at 8 h (Y3). The transformed equations were as follows:
 |
4 |
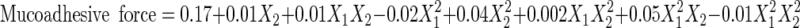 |
5 |
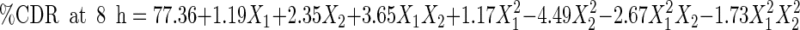 |
6 |
Table V.
One-Way ANOVA for Responses of Factorial Model: Mucoadhesion Time, Mucoadhesion Force, and %CDR at 8 h
| Source | Sum of squares | Degree of freedom | Mean square | F value | p value | Probability > F |
|---|---|---|---|---|---|---|
| Mucoadhesion time | ||||||
| Model | 3,065.22 | 4 | 383.15 | <0.0001 | Significant | |
| X 1—Ethylcellulose | 1,120.67 | 2 | 123.86 | 0.559 | <0.0001 | |
| X 2—HPMC K4M | 104.17 | 2 | 966.36 | 4.360 | <0.0005 | |
| X 1 X 2 | 4.00 | 4 | 221.29 | |||
| Pure error | 0.00 | 0 | ||||
| Cor error | 3,065.22 | 8 | ||||
| Mean | 521.06 | |||||
| Mucoadhesive force | ||||||
| Model | 31.96 | 4 | 39.96 | <0.0039 | Significant | |
| X 1—Ethylcellulose | 4.35 | 2 | 21.76 | 0.589 | <0.0006 | |
| X 2—HPMC K4M | 12.85 | 2 | 64.26 | 1.74 | <0.0129 | |
| X 1 X 2 | 0.534 | 4 | 36.90 | |||
| Pure error | 0.00 | 0 | ||||
| Cor error | 31.96 | 8 | ||||
| Mean | 0.179 | |||||
| %CDR at 8 h | ||||||
| Model | 346.41 | 4 | 43.30 | <0.0016 | Significant | |
| X 1—Ethylcellulose | 131.41 | 2 | 65.70 | 1.410 | <0.0043 | |
| X 2—HPMC K4M | 28.94 | 2 | 14.47 | 0.311 | <0.0116 | |
| X 1 X 2 | 186.06 | 4 | 46.51 | |||
| Pure error | 0.00 | 0 | ||||
| Cor error | 17.57 | 8 | ||||
| Mean | 77.36 | |||||
In these equations, coefficients with more than one factor represent the interaction between factors, while coefficients with second-order terms indicate the quantitative effect of independent variables (X1 and X2) upon the responses (Y1, Y2, and Y3). From these polynomial equations, response surface graphs of the respective responses were generated, which were used to predict the responses of dependent variables at the intermediate levels of independent variables (29).
The three-dimensional response surface graph for mucoadhesion time and mucoadhesive force showed an increase in mucoadhesion time and mucoadhesive force with a corresponding increase in the amount of HPMC K4M (Fig. 4a, b). This may be due to the ability of HPMC K4M to form hydrogen bonds with the glycoprotein-rich mucous membrane (30). An increase in the amount of HPMC K4M in the experimental formulations led to an increase in the possibility of formation of hydrogen bonds between the polymer and mucous, thereby leading to an increase in the mucoadhesion time and mucoadhesive force. The %CDR was found to be governed by the amount of both hydrophilic and hydrophobic polymers (Fig. 4c). The hydrophilicity of HPMC K4M was found to be modified in the presence of the hydrophobic character of EC which led to retardation of drug release from the swollen matrix. An increase in the amount of HPMC K4M in the formulations resulted in higher water uptake, thereby leading to considerable swelling of the polymer matrix, and thus, allowing the drug to diffuse out at a faster rate from the films. Formulation F4 was optimized on the basis of satisfactory mucoadhesion time, mucoadhesive force, and highest %CDR after 8 h. The film was later on developed as nonporous film of glibenclamide (NF4), and various parameters were compared.
Fig. 4.

Three-dimensional response surface plots for ex vivo mucoadhesion time (a), ex vivo mucoadhesive force (b), and %CDR at 8 h (c)
Validation of Experimental Design
In order to establish the validity of generated equations in the optimization procedure, a new formulation of porous film having the amount of polymers that was not included in the experimental design (extra-design checkpoint) was prepared. Comparative analysis of the predicted values and experimental values of responses using paired t test indicated no significant (p < 0.05) difference between the two values, thereby establishing the validity of the generated model to precisely predict the values of the responses (Table VI).
Table VI.
Predicted and Experimental Values of Responses for Extra-Design Checkpoint (F10) Formulation
| Response | Predicted value | Experimental value | Paired t test |
|---|---|---|---|
| Mucoadhesion time (min) | 515.67 | 514.33 ± 2.51 | p < 0.05 |
| Mucoadhesive force (N) | 0.17 | 0.18 ± 0.03 | p < 0.05 |
| %CDR at 8th h | 77.34 | 75.62 ± 0.99 | p < 0.05 |
Ex Vivo Drug Permeation
The optimized porous film (F4) was subjected to the evaluation of ex vivo drug permeation and showed 68.33 ± 0.67% drug permeation in 8 h through the chicken buccal mucosa (Fig. 5a). Flux was calculated and was found to be 0.0860 μmol/cm2/h. The value of flux was found to be higher than the target flux of glibenclamide, i.e., 0.0393 μmol/cm2/h (31). Hence, it can be anticipated that formulation F4 would be able to achieve the required permeation rate across the buccal mucosa. The correlation between in vitro drug release and ex vivo drug permeation across the chicken buccal mucosa was found to be positive with a correlation coefficient of 0.9931 (Fig. 5b). This indicates that the amount of drug permeated is directly proportional to the amount of drug released.
Fig. 5.

a Comparative in vitro permeation profiles and correlation plots of b F4 and c NF4
Stability in Simulated Saliva
The optimized formulation F4 was evaluated for any change in their drug content in simulated saliva. The film did not exhibit any change, indicating the stability of the developed mucoadhesive system in simulated saliva (data not shown). The thickness of the film was found to increase slightly (p > 0.05), owing to swelling of the system in simulated saliva.
Evaluation of Mucoadhesive Nonporous Film
The composition of the optimized porous film (F4) was selected for the preparation of nonporous film (NF4) and evaluated for various parameters. Thickness, surface pH, folding endurance, and drug content (in percent) of NF4 was found to be 0.24 ± 0.01 mm, 6.97 ± 0.09, 236 ± 3.0, and 92.17 ± 0.25%, respectively. The swelling index of the nonporous film was found to be 35.71 ± 0.7 in 120 min. The nonporous film showed a mucoadhesion time of 510 ± 2.00 min and a mucoadhesive force of 0.20 ± 0.55 N using fresh chicken buccal mucosa. In vitro drug release from nonporous film was found to be 62.66 ± 0.87% (Fig. 3) over a period of 8 h and was found to follow a mixed-order kinetics. The value of n was found to be between 0.5 and 1.0, and the r2 value of 0.9953 indicated non-Fickian diffusion as the possible release mechanism involved. The nonporous film showed 53.47 ± 0.78% ex vivo drug permeation in 8 h (Fig. 5a) through the chicken buccal mucosa. Flux was found to be 0.0668 μmol/cm2/h. Correlation between in vitro drug release and ex vivo drug permeation of F4 across the chicken buccal mucosa was found to be positive, with a correlation coefficient of 0.9846 (Fig. 5b). Ex vivo drug permeation through the chicken buccal mucosa indicated higher permeation of the drug from porous film in comparison to nonporous film. This higher drug permeation might be due to better drug release from the porous film, leading to the development of a higher concentration gradient of drug at the mucosal side in comparison to nonporous film. As a result of higher concentration gradient, higher flux was observed in porous film (0.0860 μmol/cm2/h) than nonporous film (0.0668 μmol/cm2/h). Better positive correlation was observed between in vitro drug release and ex vivo drug permeation for porous film with a correlation coefficient of 0.9931 in comparison to the nonporous film with a correlation coefficient of 0.9846 (Fig. 5c).
Comparison of Optimized Mucoadhesive Porous Film with Nonporous Film
Mucoadhesive buccal delivery systems of glibenclamide in the forms of porous and nonporous films were found to be satisfactory when evaluated for mean weight, thickness, folding endurance, and drug content. The surface pH of porous and nonporous film was found to be 6.90 ± 0.13 and 6.97 ± 0.03, respectively, i.e., close to neutral pH. Hence, these buccal devices are not likely to cause any irritation to the buccal mucosa. The swelling index of porous film was found to be higher as compared to nonporous film. This variation may be due to the presence of pores in the porous films which allowed easy penetration of the release medium to facilitate more swelling.
Ex vivo mucoadhesion time and mucoadhesive force were observed to be higher in the case of porous film than nonporous film (Table VII). This behavior of the porous film may be due to its porous nature, which allows rapid swelling to facilitate better mucoadhesion. As a result of rapid swelling of the polymer, immediate initiation of diffusion of the drug occurs, which leads to the formation of adhesive bonds resulting in faster initiation of bioadhesion (32).
Table VII.
Comparison of Optimized Porous (F4) and Nonporous (NF4) Film of Glibenclamide
| Parameters | Porous film (F4) | Nonporous film (NF4) |
|---|---|---|
| Swelling index after 120 min | 51.26 ± 0.70 | 35.71 ± 0.70 |
| Mucoadhesion time (min) | 539.11 ± 3.05 | 510.00 ± 2.00 |
| Mucoadhesive force (N) | 0.24 ± 0.04 | 0.20 ± 0.05 |
| % Cumulative drug release at 8 h | 84.47 ± 0.94 | 62.66 ± 0.87 |
| % Cumulative drug permeated at 8 h | 68.33 ± 0.67 | 53.47 ± 0.78 |
| Release kinetics | Zero order | Peppas |
| Correlation between in vitro drug release and ex vivo drug permeation (r 2) | 0.9931 | 0.9846 |
In vitro drug release study showed that both porous and nonporous films had an initially slow release, but after 2 h, the porous film showed higher release rate in a controlled manner as compared to nonporous film (Fig. 3). This might be due to the porous polymeric network, which allowed better entry of the release medium, than that of nonporous film, to facilitate increased diffusion of the drug out of the matrix. Increased drug release is advantageous as it is likely to promote increased permeation of the drug across the buccal mucosa by providing higher concentration at the mucosal side. The release data of NF4 best fitted the Korsmeyer–Peppas equation and elucidated non-Fickian diffusion as the release mechanism of glibenclamide molecules (r2 = 0.9846; n = 0.67). On the contrary, F4 (porous film) demonstrated zero-order release with higher r2 of 0.993. The controlled release from F4 can be visualized as multipocket simultaneous release of the drug from porous channels (microsized cylinders) that was not slowed down due to increase in diffusional path length, which probably was the limiting factor in drug release from nonporous film, NF4. The microsized cylinders facilitated free movement of the release medium across the film and this also affected the swelling dynamics of the film as visualized by SEM.
SEM (Fig. 6) revealed the presence of numerous pores on the surface of the porous film prior to drug release (Fig. 6a), while the nonporous film exhibited a smooth surface devoid of pores (Fig. 6c). After a drug release of 8 h, both films demonstrated swelling of the hydrophilic polymer which might be contributing toward the controlled drug release in the case of both films. Some pores were observed in the case of nonporous film after drug release (Fig. 6d), while in the case of porous film, the pores got fused to create larger pores (Fig. 6b). The increased drug release observed in the case of porous films may be attributed to the fusion of existing pores to form bigger pores during the course of drug release.
Fig. 6.
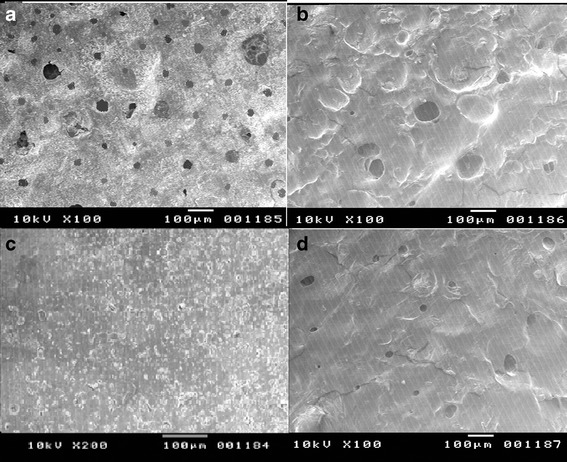
SEM image of a porous film before release, b porous film after release, c nonporous film before release, and d nonporous film after release in simulated saliva, pH 6.8
Histological Analysis
The microscopical representation of normal mucosal histology in the controls (Fig. 7a) indicated no damage on treatment with saline phosphate buffer, pH 6.8, while the positive control (Fig. 7c) indicated extensive damage to the mucosal cells, indicating sensitivity of the experimental model to the test conditions. The histology of mucosa treated with porous (Fig. 7b) and nonporous (Fig. 7d) films did not reveal any kind of cellular damage to the integrity of the buccal mucosa. In both microscopic views, apparently intact flattened surface cell layers were visible after treatment.
Fig. 7.
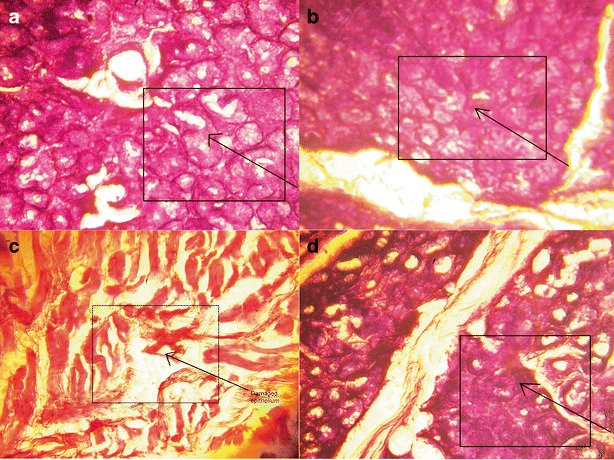
Histological images of chicken buccal mucosa treated with a saline phosphate buffer, pH 6.8, b F4, c isopropyl alcohol, and d NF4. The inset boxes highlight the intact flattened mucosal cells in a, c, and d
CONCLUSION
The porous mucoadhesive buccal film of glibenclamide was successfully developed using 32 full factorial design. EC as the rate-controlling polymer was able to delay the drug release for more than 8 h. However, a higher amount of EC tends to decrease the mucoadhesive characteristics. Porous film provided higher in vitro drug release with better control than nonporous film. Ex vivo permeation study revealed that the porous film showed higher drug permeation through the buccal mucosa with higher flux rate than the nonporous film. The porous film showed better correlation between in vitro drug release and ex vivo drug permeation.
ACKNOWLEDGMENTS
The authors are thankful to Sun Pharmaceutical Industries Ltd. (Sikkim, India) for providing the gift sample of glibenclamide.
REFERENCES
- 1.Bhanja S, Ellaiah P, Choudhury R, Murthy KVR, Panigrahi B, Padhy SK. Formulation, development and evaluation of mucoadhesive buccal patches of methotrexate. J Adv Pharm Res. 2010;1:17–25. [Google Scholar]
- 2.Rossi S, Sandri G, Caramella CM. Buccal drug delivery: a challenge already won? Drug Discov Today Technol. 2005;2:59–65. doi: 10.1016/j.ddtec.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 3.Swamy PV, Kumar AT, Shirsand SB, Patil AN, Farhana L. Design and evaluation of buccal patches of granisetron hydrochloride. Ind J Pharm Edu Res. 2010;44:95–101. [Google Scholar]
- 4.Chaudhary R, Qureshi MS, Patel J, Panigrahi UP, Giri IC. Formulation, development and in vitro evaluation of mucoadhesive buccal patches of methotrexate. Int J Pharm Sci Res. 2010;1:357–65. [Google Scholar]
- 5.McElnay JC, Hughes CM. Drug delivery—buccal route. In: Swarbrick J, Boylan JC, editors. Encyclopedia of pharmaceutical technology. New York: Dekker; 2002. pp. 800–9. [Google Scholar]
- 6.Wong CF, Yuen KH, Peh KK. Formulation and evaluation of controlled release Eudragit buccal patches. Int J Pharm. 1999;178:11–22. doi: 10.1016/S0378-5173(98)00342-1. [DOI] [PubMed] [Google Scholar]
- 7.Patel VM, Prajapati BG, Patel MM. Formulation, evaluation and comparison of bilayered and multilayered mucoadhesive buccal devices of propranolol hydrochloride. AAPS PharmSciTech. 2007;8:E1–8. doi: 10.1208/pt0804089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nafee NA, Ismail FA, Boraie NA, Mortada LM. Mucoadhesive buccal patches of miconazole nitrate: in vitro/in vivo performance and effect of ageing. Int J Pharm. 2003;264:1–14. doi: 10.1016/S0378-5173(03)00371-5. [DOI] [PubMed] [Google Scholar]
- 9.Satishbabu BK, Srinivasan Formulation and evaluation of buccoadhesive films of atenolol. Ind J Pharm Sci. 2008;70:175–9. doi: 10.4103/0250-474X.41451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park CR, Munday DL. Evaluation of selected polysaccharide excipient in buccoadhesive tablets for sustained release of nicotine. Drug Dev Ind Pharm. 2004;30:609–17. doi: 10.1081/DDC-120037492. [DOI] [PubMed] [Google Scholar]
- 11.Alur HH, Johnston TP, Mitra AK. Peptides and proteins: buccal absorption. In: Swarbrick J, Boylan JC, editors. Encyclopedia of pharmaceutical technology. New York: Dekker; 2001. p. 206. [Google Scholar]
- 12.Philip AK, Srivastava M, Pathak K. Buccoadhesive gels of glibenclamide: a means for achieving enhanced bioavailability. Drug Deliv. 2009;16:405–15. doi: 10.1080/10717540903126314. [DOI] [PubMed] [Google Scholar]
- 13.Gaudanavar PS, Bagali RS, Patil SM, Chandashkhara S. Formulation and in vitro evaluation of mucoadhesive buccal films of glibenclamide. Der Pharmacia Lettre. 2010;2:382–7. [Google Scholar]
- 14.Marikanti R, Kumar AK, Nagaraju I, Sowjanya TL, Srikanth B, Venkateswarlu G. Design and in vitro evaluation of drug release and bioadhesive properties from buccoadhesive tablets of glibenclamide for systemic delivery. J Chem Pharm Res. 2010;2:291–303. [Google Scholar]
- 15.Anders R, Merkle HP. Evaluation of laminated mucoadhesive patches for buccal drug delivery. Int J Pharm. 1989;49:231–40. doi: 10.1016/0378-5173(89)90347-5. [DOI] [Google Scholar]
- 16.Ilango R, Kavimani S, Mullaicharam AR, Jayakar B. In vitro studies on buccal strips of glibenclamide using chitosan. Ind J Pharm Sci. 1997;59:232–5. [Google Scholar]
- 17.Muzib YI, Kumari KS. Mucoadhesive buccal films of glibenclamide: development and evaluation. Int J Pharm Investig. 2011;1:42–7. doi: 10.4103/2230-973X.76728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Attama AA, Akpa PA, Onugwu LE, Igwilo G. Novel buccoadhesive delivery system of hydrochlorothiazide formulated with ethyl cellulose–hydroxypropyl methylcellulose interpolymer complex. Sci Res Essays. 2008;3:343–7. [Google Scholar]
- 19.Semalty M, Semalty A, Kumar G, Juyal V. Development of mucoadhesive buccal films of glipizide. Int J Pharm Sci Nanotechnol. 2008;1:184–90. doi: 10.4103/0250-474X.40330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peppas NA. Analysis of Fickian and non-Fickian drug release from polymers. Pharm Acta Helv. 1985;60:110–1. [PubMed] [Google Scholar]
- 21.Park H, Robinson JR. Physico-chemical properties of water insoluble polymers important to mucin epithelial adhesion. J Control Rel. 1985;2:47–57.
- 22.Junginger HE, Hoogstaate JA, Verhoef JC. Recent advances in buccal drug delivery and absorption—in vitro and in vivo studies. J Control Release. 1999;62:149–59. doi: 10.1016/S0168-3659(99)00032-2. [DOI] [PubMed] [Google Scholar]
- 23.Chen JL, Cyr GN. Compositions producing adhesion through hydration. In: Manly RS, editor. Adhesion in biological systems. London: Academic Press; 1970. pp. 163–81. [Google Scholar]
- 24.Koland M, Charyulu RN, Prabhu P. Mucoadhesive films of losartan potassium for buccal delivery: design and characterization. Ind J Pharm Educ Res. 2010;44:315–23. [Google Scholar]
- 25.Majithiya RJ, Raval AJ, Umrethia ML, Ghosh PK, Murthy RSR. Enhancement of mucoadhesion by blending anionic, cationic and nonionic polymers. Drug Deliv Technol. 2008;8:40–5. [Google Scholar]
- 26.Morales JO, McConville JT. Manufacture and characterization of mucoadhesive buccal films. Eur J Pharm Biopharm. 2011;77:187–99. doi: 10.1016/j.ejpb.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 27.Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanism of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15:25–35. doi: 10.1016/0378-5173(83)90064-9. [DOI] [PubMed] [Google Scholar]
- 28.Pathak K. Mucoadhesion—a prerequisite or a constraint in nasal drug delivery? Int J Pharm Investig. 2011;2:62–3. doi: 10.4103/2230-973X.82383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao MRP, Borate SG, Thanki KC, Ranpise AA, Parikh GN. Development and in vitro evaluation of floating rosiglitazone maleate microspheres. Drug Dev Ind Pharm. 2009;35:834–42. doi: 10.1080/03639040802627421. [DOI] [PubMed] [Google Scholar]
- 30.Desai KGH, Kumar TMP. Preparation and evaluation of a novel buccal adhesive system. AAPS PharmSciTech. 2004;5:1–9. doi: 10.1208/pt050335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain A, Ghosh B, Nayak S, Soni V. A study of transdermal delivery of glibenclamide using iontophoresis. Int J Health Res. 2009;2:83–91. [Google Scholar]
- 32.Anlar S, Capan Y, Hincal A. Physico-chemical and bioadhesive properties of polyacrylic acid polymers. Pharmazie. 1993;48:285–7. [PubMed] [Google Scholar]


