Abstract
The aim of this study was the optimization of a lidocaine-based film formulation for the prevention of pain from needle prick during the injection of local anesthetic in dentistry. Film performances were evaluated in vitro by studying lidocaine permeation across pig esophageal epithelium as model for nonkeratinized buccal mucosa. The results obtained showed that the molecular weight of the film-forming polymer had no effect on lidocaine transport. The introduction of the adhesive Plastoid® into the film determined a significant increase of drug permeation rate, which was further improved by the addition of Azone®. On the contrary, the effect of sodium taurocholate was negligible.
KEY WORDS: buccal delivery, esophageal epithelium, lidocaine, permeation enhancers, polymeric films
INTRODUCTION
Pain from needlestick during local anesthetic injection is described as the major deterrent to dental care for many patients, especially in pediatric age (1). The application of topical anesthetics to the oral mucosa to reduce the discomfort before the injection is a very common practice in dentistry (2). Lidocaine hydrochloride is the most important amide local anesthetic, characterized by a rapid onset of action (15–30 min) and, together with benzocaine, is the most commonly topically used agent. Different studies demonstrated its efficacy in reducing the discomfort of intraoral local anesthetic injections compared to placebo (3–6). The efficacy in reducing pain was also demonstrated in comparison to other anesthetic agents, in particular benzocaine (4).
In most cases, the formulations used for topical anesthesia are solutions or gels, whose main drawback is the lack of bioadhesiveness. This means reduction of contact time and efficacy, and dilution by the saliva, that leads to an unpleasant taste and discomfort for the patient. These problems may be reduced by using bioadhesive films that, with their small size and thickness, may improve patients’ compliance.
The aim of this work was the optimization of a lidocaine-based film formulation for the prevention of pain from needle prick during the injection of local anesthetics in dentistry. The effect of film-forming polymer molecular weight of the adhesive Plastoid® E35H and of permeation enhancers was studied. An additional objective was to evaluate the effect of occlusion on lidocaine transport. Lidocaine permeation was studied across pig esophageal epithelium as model for nonkeratinized buccal mucosa.
EXPERIMENTAL
Materials
Lidocaine hydrochloride (molecular weight (m.w.) 270.33) was a gift of Lisapharma S.p.A. (Erba, Italy). Polyvinyl alcohol (PVA), m.w. 83,400 and 115,000 and degree of hydrolysis 87%, was obtained from Nippon Gohsei (Osaka, Japan). Azone® was from Netqem (Durham, NC, USA) and Na taurocholate from Sigma (St Louis, MO, USA). Plastoid® E 35 H was prepared according to the protocol of Rofarma: Eudragit® E 100 (15.9%, w/w, Rohm, Darmstadt, Germany), lauric acid (9.2%, w/w, Fluka Chemika, Buchs, Switzerland), and adipic acid (1.8%, w/w, Fluka Chemika, Buchs, Switzerland) were added to hot water (72.1%, w/w, temperature ∼80°C). The mixture was stirred, maintaining the temperature at ∼80°C, until a clear solution was formed. The solution was then cooled to 60°C, and glycerol (1.0%, w/w, Merck, Darmstadt, Germany) was added. The mixture was then gradually cooled to room temperature while stirring. All others chemicals were of analytical grade.
Film Preparation
Films containing lidocaine HCl were prepared as previously described (7). The composition of the mixtures to be laminated is reported in Table I. Lidocaine HCl was dissolved in a mixture of water and plasticizer (sorbitol). Permeation enhancers (Azone® or sodium taurocholate) were incorporated, and the solution/suspension was then added to the PVA 20% (w/w) water solution and to the adhesive Plastoid® E35H and mixed overnight. The mixtures were spread on siliconized paper using a film casting knife (BYK Gardner, Silverspring, USA; gap 450 μm) and oven-dried at 80°C for 30 min. The films were covered with a second siliconized paper and sealed in aluminum pouches.
Table I.
Composition of the Mixtures Used for Film Preparation (in percent, w/w) and Lidocaine Content on Finished Products (Mean Values ± SD)
| Component | A | B | C | D | E | F |
|---|---|---|---|---|---|---|
| PVA 83 Ka | 84.0 | – | 56.0 | – | 55.8 | 55.1 |
| PVA 115 Ka | – | 84.0 | – | 56.0 | – | – |
| Plastoid E35H | – | – | 27.0 | 27.0 | 26.9 | 26.6 |
| Sorbitolb | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 3.9 |
| Lidocaine HCl | 7.2 | 7.2 | 8.0 | 8.0 | 8.0 | 7.9 |
| Sodium taurocholate | – | – | – | – | 0.3 | – |
| Azone | – | – | – | – | – | 1.6 |
| Water | 4.8 | 4.8 | 5.0 | 5.0 | 5.0 | 4.9 |
| Drug contentc | ||||||
| mg/cm2 | 1.70 ± 0.07 | 1.89 ± 0.22 | 1.83 ± 0.21 | 2.06 ± 0.44 | 2.09 ± 0.44 | 2.05 ± 0.15 |
| % (w/w) | 19.30 ± 0.63 | 21.61 ± 2.38 | 19.93 ± 2.50 | 23.89 ± 3.92 | 20.29 ± 2.40 | 20.99 ± 2.50 |
PVA polyvinyl alcohol
aAs 20% (w/w) water solution
bAs 70% (w/w) water solution
cAs lidocaine base
In Vitro Permeation Test
Esophageal epithelium was prepared according to Diaz del Consuelo et al. (8). The esophageal mucosa was separated from the outer muscle layer with a scalpel, and the epithelium was peeled off from the connective tissue after immersion in distilled water at 60°C for 120 s. Samples obtained were frozen until use.
Permeation experiments were performed using Franz type diffusion cells with an available diffusion area of 0.6 cm2. Esophageal isolated epithelium, spread over a 0.45-μm regenerate cellulose filter, was mounted between the two compartments of the cell with the connective side facing the membrane. Phosphate buffer solution pH 7.4 (5.98 g Na2HPO4∙12H2O, 0.19 g KH2PO4, and 8.80 g NaCl per liter of distilled water adjusted with phosphoric acid to pH 7.4) was used as the receptor medium, maintained at 37 ± 1°C, and magnetically stirred at 300 rpm. After application of the test formulations on the donor side, 300-μl aliquots were collected from the receptor at designed time intervals and replaced by the same volume of fresh buffer to maintain a constant volume. As donor, 0.7 ml of lidocaine hydrochloride water solution (0.5% w/v expressed as base, pH 6.8) or films were used. Before the application of film formulations, the epithelial side of the tissue was dried, and 30 μl of phosphate buffer (2.38 g Na2HPO4, 0.19 g KH2PO4, and 8.00 g NaCl per liter of distilled water adjusted with phosphoric acid to pH 6.75) was spread on the surface to simulate the salivary film (9). The amount of lidocaine transported was determined by HPLC. Diffusion experiments were conducted using tissue from at least four animals.
In the case of solution, permeation data were analyzed using a first-order model equation (Eq. 1) (10):
 |
1 |
where Mt/M∞ is the fraction of drug present in the donor chamber at time t (in seconds), A is the permeation area (in square centimeters), V is the donor volume (in milliliters), and P is the permeation coefficient (in centimeters per second). P was calculated from the slope of the linear regression analysis of the data.
For polymeric films, permeation data were analyzed using the Higuchi model, and permeation rate was calculated using Eq. 2
 |
2 |
where Q is the amount of lidocaine permeated per unit area (in micrograms per square centimeter), t is time (in hours), and K is the permeation rate (in micrograms per square centimeter per square root of hour).
Chromatographic Conditions
Lidocaine was quantified using an HPLC system consisting of a PerkinElmer liquid chromatograph (PerkinElmer, Norwalk, CT, USA) equipped with a Varian ProStar 410 autosampler (Palo Alto, CA, USA). Analytes were separated by Waters Nova-Pak® C8 (4 μm, 3.9 × 150 mm) (Milford, MA, USA) column maintained at 30°C. The mobile phase consisted of a mixture of methanol and ammonium dihydrogen phosphate buffer (0.277 mM) adjusted to pH 7. The flow rate was 0.9 ml/min. The eluted substance was monitored at 220 nm. In these conditions, the retention time of lidocaine was 7.6 min. The analytical method was validated according to USP 34 (11).
Statistical Analysis
Results are expressed as the means of at least eight experiments ± SD. Statistical analysis was performed using ANOVA followed by Dunnet or Bonferroni post test (GraphPad Prism 5, GraphPad Software Inc., San Diego, CA, USA). The chosen level of significance was p < 0.05.
RESULTS AND DISCUSSION
Permeation from Aqueous Solution
The first part of this work concerned the evaluation of the suitability of pig esophageal epithelium as a barrier for the study of lidocaine permeation. Pig buccal mucosa is often used in in vitro permeation studies as a model for human buccal mucosa. Both tissues are nonkeratinized and showed to be very similar in terms of structure and permeability (12). However, mucosal tissue from pig cheeks is often damaged by mastication and is strongly attached to the muscular tissue; thus, the excision is difficult and time consuming. Diaz del Consuelo and collaborators proposed pig esophageal mucosa as a valid permeability barrier model for buccal tissue (8). In their studies, they found that esophageal and buccal epithelium have a comparable structure and that in both cases, the permeability barrier is represented by intercellular lipids, whose composition resulted to be very similar both qualitatively and quantitatively (13). Esophageal epithelium is less damaged and has a more uniform thickness, if compared to buccal epithelium, and its separation from muscular tissue is very easy. The authors have also shown that the in vitro permeability of fentanyl across the two tissues is very similar (14, 15). Similar results have been reported for carbamazepine, but not for triamcinolone (16), to point out that the model is promising but that the physicochemical properties of permeants could have a relevant effect. Therefore, a preliminary evaluation of lidocaine permeation across esophageal epithelium was necessary.
Lidocaine permeation was studied from aqueous solution (0.5%, w/v, pH 6.8) in the same conditions used by Kokate et al. (17) across fresh porcine buccal tissue. The permeation data obtained through esophageal fresh and frozen tissue are reported in Fig. 1a. The permeation profiles were not linear as a function of time but showed a reduction of the permeation rate due to the depletion of the donor, since the amount of lidocaine permeated after 8 h exceeded 50% of the applied dose. For this reason, a first-order equation (Eq. 1) was used to process the data (Fig. 1b) and calculate the permeability coefficient (P) that resulted in 7.78 ± 0.62 × 10−6 and 7.25 ± 0.75 × 10−6 cm/s, respectively, for fresh and frozen esophageal tissue. Both values are approximately half of that reported by Kokate (17.0 ± 1.8 × 10−6 cm/s) for buccal mucosa. No difference was found between fresh and frozen tissue, indicating that freezing does not affect the permeation barrier properties of esophageal epithelium and that fresh tissue can be substituted by frozen tissue in permeation studies of lidocaine.
Fig. 1.
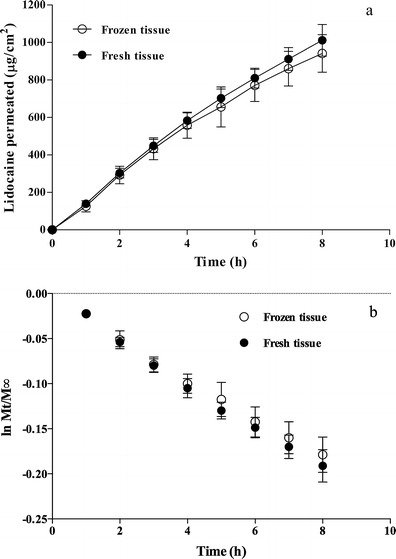
Lidocaine permeation across fresh and frozen esophageal tissue from aqueous solution (0.5% w/v, pH 6.8) (mean values ± SD). a Permeation profiles. b Data processed using Eq. 1
Permeation from Polymeric Films
The second part of the work was devoted to the preparation of buccal films containing polyvinyl alcohol. The film composition is shown in Table I. To evaluate the film performance, we measured the permeation of lidocaine across the esophageal epithelium and not drug retention in the epithelium. In fact, in a recent work on liposomal benzocaine gel formulation (18), a correlation between in vitro permeation parameters and in vivo topical anesthesia was studied. Results obtained showed that drug flux across pig esophageal epithelium was strongly correlated with in vivo anesthetic efficacy, while mucosal drug retention was not correlated with any of the in vivo parameters.
Polymeric films are relatively recently developed dosage forms for buccal delivery. They are more comfortable and flexible than adhesive tablets and exhibit higher residence time on the mucosa, compared to gels and ointments that can be easily washed away by saliva.
The list of polymers used for the formulation of buccal mucoadhesive films includes, among others, chitosan, hydroxypropylcellulose, polycarbophil, and Eudragit S-100 (19). For this work, we decided to use PVA because it is a well-known polymer, widely used in the pharmaceutical field with good hydrogen bonding capacity, an important characteristic for bioadhesion.
The first formulation prepared was a film of PVA containing 20% (w/w) of lidocaine. Two different films were prepared using PVA of different molecular weights, 83 and 115K (Table I, formulations A and B), in order to evaluate the effect of this parameter on lidocaine permeation across pig esophageal epithelium. Figure 2 reports the results obtained. Permeation rate, calculated using Eq. 2, resulted to be 92.58 ± 28.75 and 114.00 ± 51.95 μg cm−2 h−0.5, respectively, for formulations A and B. No significant difference was observed (p > 0.05); it means that PVA molecular weight has no effect on lidocaine permeation.
Fig. 2.
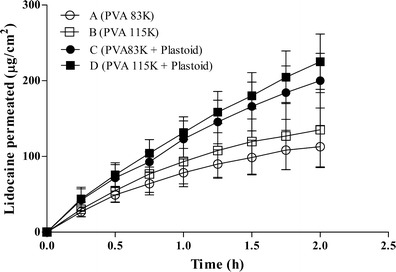
Lidocaine permeation profiles across esophageal tissue from polymeric films (mean values ± SD)
The composition of polymeric films was then modified by introducing Plastoid® E 35 H, an adhesive hydrophilic mixture. The presence of Plastoid® increased lidocaine permeation (Fig. 2) both in the case of PVA 83K (formulation A vs C) and of PVA 115 K (formulation B vs D). However, it did not modify the permeation kinetics; in fact, the Higuchi model (Eq. 2) can still be used to calculate the permeation rate (Table II). The difference between formulations C and D was not statistically significant.
Table II.
Lidocaine Permeation Parameters Across Esophageal Mucosa from Polymeric Films (Mean Values ± SD)
| Formulation | Permeation rate (μg cm−2 h−0.5) |
|---|---|
| A | 92.58 ± 28.75 |
| B | 114.00 ± 51.95 |
| C | 187.16 ± 39.18 |
| C occluded | 172.59 ± 34.64 |
| D | 213.06 ± 35.48 |
| E | 227.87 ± 34.97 |
| F | 334.05 ± 89.88 |
The effect of Plastoid® on lidocaine permeation was already observed across the skin (20). Plastoid® is a mixture of acrylic polymer (Eudragit® E 100) with lauric and adipic acid; lauric acid is able to form ion pairs with lidocaine, and this results in an increased transdermal flux because of higher lipophilicity (21). The same mechanism of action was proposed for sumatriptan transport across buccal mucosa (22). To verify this hypothesis, the composition of film A was modified by introducing lauric acid. The amount added corresponded to that present in film C. The permeation profile obtained was superimposed to that obtained from film A (data not shown). Thus, the increase in permeation observed in the presence of Plastoid® is not ascribable to the formation of ion pairs, but is probably due to the higher adhesion and more intimate contact of the film to the tissue because of the formation of additional hydrogen bonding with the acrylic polymer (19). On the basis of the results obtained, the best permeation performances were obtained with films containing Plastoid®. The effect of PVA molecular weight was negligible also in this case, and the study continued only on the film with PVA 83 K (formulation C) because of the higher water solubility of the polymer. Different enhancement strategies were then applied to this film.
Occlusion is a very simple method to enhance the permeation of a drug across the skin (23, 24). In the present work, the use of an occlusive backing was mainly used to avoid the release of lidocaine in the oral cavity, thus reducing systemic absorption. The effect of the presence of an impermeable backing on the permeation of lidocaine across the esophageal epithelium is shown in Fig. 3 (C vs C + occlusion). Occlusion did not modify the permeation profile, as expected with a nonkeratinized tissue, such as the esophageal epithelium (8), characterized by high water content and constantly in contact with saliva.
Fig. 3.
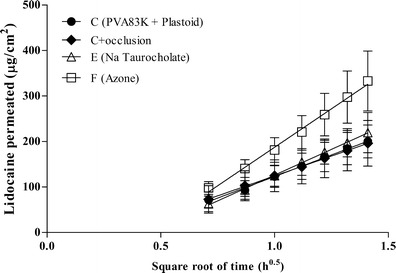
Lidocaine permeation profiles across esophageal tissue from polymeric films against the square root of time (mean values ± SD)
The use of chemical permeation enhancers was then considered. Bile salts were successfully used to promote drug absorption across different epitheliums such as nasal, pulmonary, ocular, and vaginal (25). The buccal penetration enhancement of sodium taurocholate has been reported for peptides and alpha interferon (26) and for insulin (27). The amount of lidocaine permeated did not increase in the presence of sodium taurocholate (Fig. 3, formulation C vs E). Similar results were reported by (28) for thiocolchicoside, stressing that the effect of the chemical enhancer depends on the permeant (25). The other chemical enhancer tested was Azone®. Azone® (laurocapram) is a hydrophobic nonionic molecule extensively used as transdermal penetration enhancer (29) that also has found use in buccal drug delivery (30). In some cases the enhancing effect of Azone® was demonstrated using hamster cheek buccal mucosa (30) that is a keratinized tissue with a structure very similar to the stratum corneum. When tested on nonkeratinized tissue, the effect is controversial since some authors report an increase of the flux (31, 32) while others report no effect or a reduction of the flux (33, 34), pointing out that, also in this case, the effect depends on the permeant. In our study, the presence of Azone® in the film produced a significant increase of both flux and amount permeated (p < 0.0001) when compared to the control (Table II and Fig. 3, formulation C vs F).
The different performance of the two enhancers tested could be ascribed to their different mechanism of action. In fact, it is reported that bile salts in buccal mucosa mainly alter paracellular or polar transport and have limited effect on lipophilic compound (as lidocaine is; logP 2.6). On the contrary, Azone® acts by improving partitioning of lipophilic drug in the buccal mucosa and then can be responsible for an increased concentration gradient and transmucosal flux (30).
CONCLUSIONS
Film-forming polymer molecular weight has no effect on lidocaine permeation across pig esophageal epithelium, since no significant difference on lidocaine permeation rate was observed. The addition of Plastoid® E35H determined a significant increase of the lidocaine permeation rate, probably due to the higher adhesion of the film to the tissue because of the formation of additional hydrogen bonding with the acrylic polymer. Among the permeation enhancers studied, Azone® effectively promoted lidocaine transport compared to the control film while sodium taurocholate did not have any effects.
The results obtained in this paper represent an interesting starting point for the realization of a convenient formulation for local anesthesia. The use of a mucoadhesive film, in fact, could avoid the drawbacks of semisolid formulations such as the low residence time, assuring, at the same time, a suitable drug release to the mucosa.
Additionally, the study performed in the present work using Azone® and sodium taurocholate can contribute to increasing the knowledge on the mechanisms of action of these enhancers on the buccal mucosa, a topic that has not been fully understood and still need additional experimental data.
REFERENCES
- 1.Milgrom P, Coldwell SE, Getz T, Weinstein P, Ramsay DS. Four dimensions of fear of dental injections. J Am Dent Assoc. 1997;128:756–66. doi: 10.14219/jada.archive.1997.0301. [DOI] [PubMed] [Google Scholar]
- 2.Meechan JG. Intraoral topical anesthesia. Periodontology. 2008;46:56–79. doi: 10.1111/j.1600-0757.2008.00231.x. [DOI] [PubMed] [Google Scholar]
- 3.Rosivack RG, Koenigsberg SR, Maxwell KC. An analysis of the effectiveness of two topical anesthetics. Anesth Prog. 1990;37(6):290–2. [PMC free article] [PubMed] [Google Scholar]
- 4.Carr MP, Horton JE. Clinical evaluation and comparison of 2 topical anesthetics for pain caused by needle sticks and scaling and root planing. J Periodontol. 2001;72(4):479–84. doi: 10.1902/jop.2001.72.4.479. [DOI] [PubMed] [Google Scholar]
- 5.Carr MP, Horton JE. Evaluation of a transoral delivery system for topical anesthesia. J Am Dent Assoc. 2001;132(12):1714–9. doi: 10.14219/jada.archive.2001.0127. [DOI] [PubMed] [Google Scholar]
- 6.Yaacob HB, Noor GM, Malek SN. The pharmacological effect of xylocaine topical anaesthetic—a comparison with a placebo. Singapore Dent J. 1981;6(2):55–7. [PubMed] [Google Scholar]
- 7.Padula C, Colombo G, Nicoli S, Catellani P, Massimo G, Santi P. Bioadhesive film for the transdermal delivery of lidocaine: in vitro and in vivo behavior. J Control Release. 2003;88:277–85. doi: 10.1016/S0168-3659(03)00015-4. [DOI] [PubMed] [Google Scholar]
- 8.Diaz del Consuelo I, Pizzolato GP, Falson F, Guy RH, Jacques Y. Evaluation of pig esophageal mucosa as a permeability barrier model for buccal tissue. J Pharm Sci. 2005;94(12):2777–88. doi: 10.1002/jps.20409. [DOI] [PubMed] [Google Scholar]
- 9.Peh KK, Wong CF. Polymeric films as vehicle for buccal delivery: swelling, mechanical, and bioadhesive properties. J Pharm Pharmaceut Sci. 1999;2(2):53–61. [PubMed] [Google Scholar]
- 10.Baker RW, Londsdale HK. Controlled release of biologically active agents. In: Tanquary AC, Lacey RE, editors. Controlled release of biologically active agents. New York: Plenum Press; 1974. [Google Scholar]
- 11.USP. United States Pharmacopeia and National Formulary USP 34-NF 29 Rockville, MD. 2011.
- 12.Shojaei AH. Buccal mucosa as a route for systemic drug delivery: a review. J Pharm Pharmaceut Sci. 1998;1(1):15–30. [PubMed] [Google Scholar]
- 13.Diaz del Consuelo I, Jacques Y, Pizzolato GP, Guy RH, Falson F. Comparison of the lipid composition of porcine buccal and esophageal permeability barriers. Arch Oral Biol. 2005;50(12):981–7. doi: 10.1016/j.archoralbio.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Diaz del Consuelo I, Falson F, Guy RH, Jacques Y. Transport of fentanyl through pig buccal and esophageal epithelia in vitro. Influence of concentration and vehicle pH. Pharm Res. 2005;22(9):1525–9. doi: 10.1007/s11095-005-6020-y. [DOI] [PubMed] [Google Scholar]
- 15.Diaz del Consuelo I, Falson F, Guy RH, Jacques Y. Ex vivo evaluation of bioadhesive films for buccal delivery of fentanyl. J Control Release. 2007;123(2):135–40. doi: 10.1016/j.jconrel.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Caon T, Simoes CM. Effect of freezing and type of mucosa on ex vivo drug permeability parameters. AAPS PharmSciTech. 2011;12(2):587–92. doi: 10.1208/s12249-011-9621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kokate A, Li X, Jasti B. HPLC detection of marker compounds during buccal permeation enhancement studies. J Pharm Biomed Anal. 2008;47(1):190–4. doi: 10.1016/j.jpba.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 18.Franz-Montan M, Cereda CM, Gaspari A, da Silva CM, de Araujo DR, Padula C, et al. Liposomal-benzocaine gel formulation: correlation between in vitro assays and in vivo topical anesthesia in volunteers. J Liposome Res. 2013;23(1):54–60. doi: 10.3109/08982104.2012.742536. [DOI] [PubMed] [Google Scholar]
- 19.Salamat-Miller N, Chittchang M, Johnston TP. The use of mucoadhesive polymers in buccal drug delivery. Adv Drug Deliv Rev. 2005;57(11):1666–91. doi: 10.1016/j.addr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Padula C, Nicoli S, Colombo P, Santi P. Single-layer transdermal film containing lidocaine: modulation of drug release. Eur J Pharm Biopharm. 2007;66:422–8. doi: 10.1016/j.ejpb.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Nash RA, Metha DB, Matias JR, Orentreich N. The possibility of lidocaine ion pair absorption through excised hairless mouse skin. Skin Pharmacol Appl. 1992;5(3):160–70. doi: 10.1159/000211033. [DOI] [PubMed] [Google Scholar]
- 22.van der Bijl P, Penkler L, van Eyk AD. Permeation of sumatriptan through human vaginal and buccal mucosa. Headache. 2000;40:137–41. doi: 10.1046/j.1526-4610.2000.00019.x. [DOI] [PubMed] [Google Scholar]
- 23.Thassu D, Vyas SP. Controlled transdermal occlusive delivery device of primaquine. Drug Dev Ind Pharm. 1993;19:1343–56. doi: 10.3109/03639049309074405. [DOI] [Google Scholar]
- 24.Mak VH, Potts RO, Guy RH. Does hydration affect intercellular lipid organization in the stratum corneum? Pharm Res. 1991;8(8):1064–5. doi: 10.1023/A:1015873511692. [DOI] [PubMed] [Google Scholar]
- 25.Senel S, Hincal AA. Drug permeation enhancement via buccal route: possibilities and limitations. J Control Release. 2001;72(1–3):133–44. doi: 10.1016/S0168-3659(01)00269-3. [DOI] [PubMed] [Google Scholar]
- 26.Steward A, Bayley DL, Howes C. The effect of enhancers on the buccal absorption of hybrid (BDBB) alpha interferon. Int J Pharm. 1994;104:145–9. doi: 10.1016/0378-5173(94)90189-9. [DOI] [Google Scholar]
- 27.Zhang J, Nin S, Ebert C, Stanley TH. An in vivo dog model for studying recovery kinetics of the buccal mucosa permeation barrier after exposure to permeation enhancers: apparent evidence of effective enhancement without tissue damage. Int J Pharm. 1994;101:15–22. doi: 10.1016/0378-5173(94)90071-X. [DOI] [Google Scholar]
- 28.Artusi M, Santi P, Colombo P, Junginger HE. Buccal delivery of thiocolchicoside: in vitro and in vivo permeation studies. Int J Pharm. 2003;250(1):203–13. doi: 10.1016/S0378-5173(02)00545-8. [DOI] [PubMed] [Google Scholar]
- 29.Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliv Rev. 2004;56(5):603–18. doi: 10.1016/j.addr.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 30.Nicolazzo JA, Reed BL, Finnin BC. Buccal penetration enhancers—how do they really work? J Control Release. 2005;105(1–2):1–15. doi: 10.1016/j.jconrel.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 31.Hu L, Damaj BB, Martin R, Michniak-Kohn BB. Enhanced in vitro transbuccal drug delivery of ondansetron HCl. Int J Pharm. 2011;404(1–2):66–74. doi: 10.1016/j.ijpharm.2010.10.052. [DOI] [PubMed] [Google Scholar]
- 32.Nicolazzo JA, Reed BL, Finnin BC. Enhancing the buccal mucosal uptake and retention of triamcinolone acetonide. J Control Release. 2005;105(3):240–8. doi: 10.1016/j.jconrel.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 33.Heemstra LB, Finnin BC, Nicolazzo JA. The buccal mucosa as an alternative route for the systemic delivery of risperidone. J Pharm Sci. 2010;99(11):4584–92. doi: 10.1002/jps.22175. [DOI] [PubMed] [Google Scholar]
- 34.Cid YP, Pedrazzi V, de Sousa VP, Pierre MB. In vitro characterization of chitosan gels for buccal delivery of celecoxib: influence of a penetration enhancer. AAPS PharmSciTech. 2012;13(1):101–11. doi: 10.1208/s12249-011-9725-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


