Abstract
Objectives: Bioengineered skin grafts, including acellular dermal matrices, may be effective in treating lower extremity and trunk wounds that are not responsive to traditional wound management. Acellular dermal wound matrix is derived from human acellular dermal wound matrix (HADWM) tissue and provides a scaffold that supports cellular repopulation and revascularization. The major structural components of the dermis are retained during processing, and a single application has been shown to help achieve wound closure. Methods: This patient case series examined the use of HADWM on lower extremity and trunk wounds in 11 patients (6 male and 5 female) with a mean age of 55 years (range: 31–83 years). Wounds were debrided 1 to 2 times, followed by placement of HADWM (range: 4–330 cm2) on wounds that varied from the dorsal surface of the foot, lower abdomen, and lower extremity to the Achilles flap. A nonadherent layer in conjunction with bacitracin was placed over HADWM. Negative pressure wound therapy (NPWT) was placed over the HADWM and initiated continuously at −125 mm Hg for 1 to 2 weeks. After the application of NPWT, HADWM was covered with various gauze dressings using mineral oil. Results: All patients completed their treatment successfully, and follow-up ranged from 1 week to 6 months. One patient experienced an infection, which resulted in partial graft loss that required replacement with HADWM and NPWT. No additional complications occurred in the other patients. Conclusions: This patient case series demonstrated successful use of HADWM and NPWT, which further supports published studies documenting HADWM success in chronic wounds.
Keywords: chronic wound, human acellular dermal matrix, lower extremity, matrix, NPWT
Wounds of the lower extremity currently affect more than 6 million people in the United States.1 Chronic full-thickness wounds, such as lower extremity wounds, can be hard to heal and can become a problem to treat.2 Nonhealing or slow healing wounds create a major health problem that contributes to substantial disability, morbidity, and costs. Currently, there is a need to develop treatment modalities to reduce the risks of severe extremity wounds that can lead to amputations.3
Bioengineered skin grafts, including acellular dermal matrices, may be effective in treating lower extremity wounds that are not responsive to traditional wound management.4 In order for skin substitutes to be effective, the wound bed must be well vascularized and debrided to healthy tissue.5 Tissue engineering research has shown benefits to using allografts to reach faster wound healing as compared with conservative care.6 Also, several studies have shown the successful use of human acellular dermal regenerative matrices to heal chronic, full-thickness wounds of the lower extremity.2,6
When using the appropriate acellular matrix, wounds may heal faster than standard treatment in chronic hard-to-heal wounds.4 Acellular dermal matrices may be used to replace damaged extracellular matrix, fill defects, and optimize the wound environment for healing lower extremity wounds.7 By combining an acellular matrix with other advanced treatment modalities, wounds may continue the progression of healing.7 For example, the use of negative pressure wound therapy (NPWT, V.A.C. Therapy, KCI USA, Inc, San Antonio, Texas) may help to control excessive exudate and, in addition, hold the matrix in place to maximize contact with the wound bed.8
Graftjacket regenerative tissue matrix (Wright Medical Technology, Inc, licensed by KCI USA, Inc, San Antonio, Texas) is a human acellular dermal wound matrix (HADWM) that is derived from human tissue and processed from screened donated human skin.4 The HADWM is processed to remove the living cells while preserving dermal structure4 and also serves as a scaffold to support cellular repopulation and revascularization.4 Wound bed preparation is crucial and requires debridement of necrotic tissue and control of infection and edema.9 This article reports clinical experience using HADWM in conjunction with NPWT on patients with lower extremity and trunk wounds.
METHODS
Each patient underwent a complete medical history, a physical examination, and full assessment of the wound. Informed consent was obtained for each patient before treatment.
All chronic wounds were debrided 1 to 2 times to remove necrotic tissue. After sharp debridement, a bleeding wound base was created, followed by placement of HADWM (range: 4–330 cm2).
If needed, multiple HADWMs were combined to completely cover the surface area of larger wounds. The reticular layer (shiny side) of the HADWM was placed against the wound bed, which aligned this layer to the vascular supply of adjacent tissue and the basement membrane. HADWM was secured with staples to the wound margins to ensure coverage of the whole wound bed. A nonadherent layer (Adaptic, Systagenix Wound Management, Gatwick, UK) in conjunction with bacitracin was placed over the HADWM.
Negative pressure wound therapy was used as a bolster over the HADWM, and pressure was initiated continuously at −125 mm Hg for 1 to 2 weeks. After NPWT application, HADWM was covered with various gauze dressings moistened with mineral oil. Dressings were changed and reapplied every 1 to 2 days.
RESULTS
All patients completed their treatment successfully, and follow-up ranged from 1 week to 6 months. Patient demographics are summarized in Table 1. A total of 11 patients (6 male and 5 female) received HADWM to treat lower extremity and trunk wounds followed by NPWT. The mean patient age was 55 years, with patients ranging in age from 31 to 83 years. Patient comorbidities are listed in Table 2. Two patients experienced an infection; one resulted in partial graft loss that required replacement of HADWM and NPWT. Both patients’ wounds healed completely. All wounds reached complete healing with 10 of 11 patient wounds receiving a single application of HADWM. No complications specifically associated with the use of the HADWM were noted.
Table 1.
Patient demographics
| Patient no. | Sex | Age | Comorbidities | Wound etiology | Wound location | Prior treatments | Human Acellular Dermal Matrix size | Bolster |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 83 | Diabetes mellitus, hypertension, osteoarthritis, heart disease, vascular insufficiency | Postsurgical, vascular insufficiency to the foot | Right dorsal foot | Debridement by orthopedic surgeon, attempted closure | 4 cm2 | NPWT |
| 2 | M | 53 | Venous insufficiency, morbid obesity | Venous stasis ulcer, lymphedema skin breakdown | Lower extremity | Compression therapy, non-adherent dressing changes daily | 40 cm2 | NPWT |
| 3 | F | 52 | Uterine cancer, smoking, thyroid cancer | Failed full-thickness skin graft, exposed tendon | Right dorsal foot | Negative pressure wound therapy | 4 cm2 | NPWT |
| 4 | M | 72 | Venous insufficiency | Multiple debridements of venous stasis ulcers | Right lower extremity | Compression therapy, Apligraf® | 330 cm2 | NPWT |
| 5 | M | 39 | None | Postsurgical wound after Achilles repair | Right Achilles adipofascial flap | Wet to dry dressings | 32 cm2 | NPWT |
| 6 | F | 31 | Diabetes mellitus, kidney disease, liver failure | Venous stasis and posttraumatic wound | Right lower extremity | Wet to dry dressings | 120 cm2 | NPWT |
| 7 | M | 64 | Diabetes mellitus, Fournier gangrene | Debridements of necrotic tissue | Lower left abdomen | Negative pressure wound therapy | 32 cm2 | NPWT |
| 8 | M | 54 | Diabetes mellitus | Debridement of pressure ulcers | Sacrum ulcer, Diabetic ulcer on left foot, distal forefoot ulcer on right foot, exposed toe bone on dorsum of foot, and large ulcer on midfoot | Wet to dry dressings | 64 cm2 | NPWT |
| 9 | M | 65 | Diabetes mellitus, hypertension, Fournier gangrene, coronary heart disease, renal disease | Debridement of pressure ulcers | Bilateral calcaneal ulcers | Wet to dry dressings | 64 cm2 | NPWT |
| 10 | F | 53 | Uterine cancer, smoking, thyroid cancer | Postsurgical | Right dorsal foot | Wet to dry dressings | 4 cm2 | NPWT |
| 11 | F | 34 | Smoking | Posttraumatic | Left | Negative pressure wound therapy followed by wet to dry dressings | 150 cm2 | NPWT |
CASE STUDIES
Three cases are highlighted in Figures 1 to 3 and demonstrate the successful use of HADWM in conjunction with NPWT.
Figure 1.
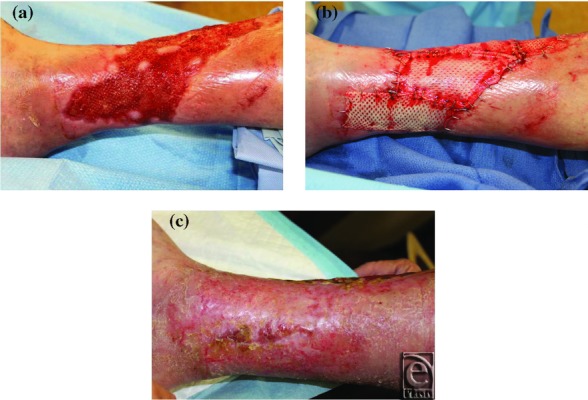
(a) Wound at initial presentation. (b) Wound after HADWM placement. (c) 30 days after placement of HADWM.
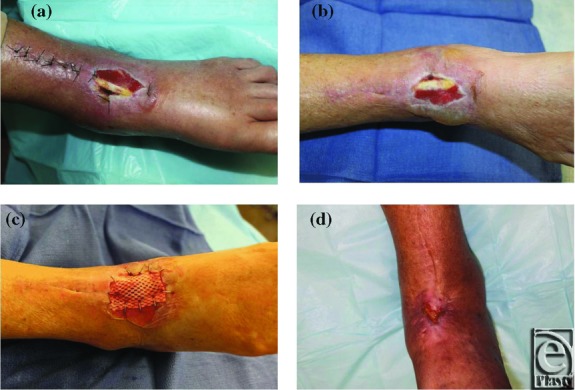
Figure 3.
(a) Wound at initial presentation. (b) Wound after HADWM placement. (c) 8 days after HADWM placement. (d) 45 days after HADWM placement.
Patient 1
A 72-year-old man with history of venous insufficiency ulcers in both legs with a large, chronic, lower extremity wound (Figures 1a-c).
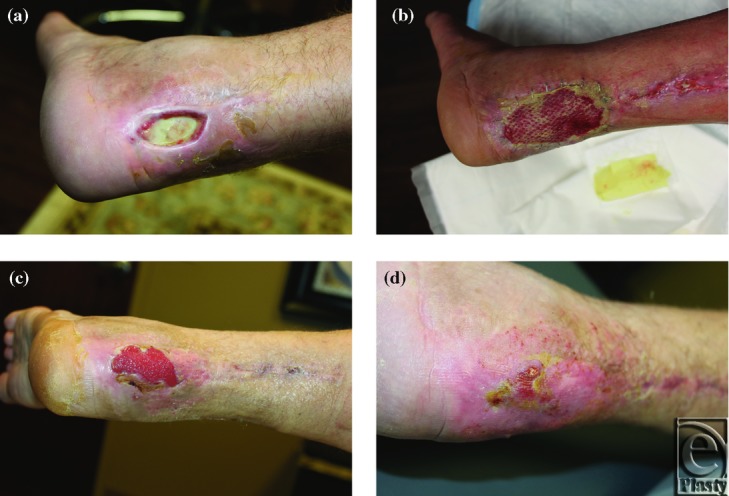
Patient 2
An 83-year-old woman with a postsurgical right dorsal foot wound after a total ankle arthroplasty (Figures 2a-d).
Figure 2.
(a) Wound at initial presentation. (b) Sutures removed. (c) Wound after HADWM placement. (d) Approximately 6 months after HADWM placement.
Patient 3
A 39-year-old man with chronic postsurgical Achilles wound with exposed tendon and scar tissue (Figures 3a-d).
DISCUSSION
The use of HADWM in conjunction with NPWT resulted in complete healing in all of the lower extremity and trunk wounds in this series of patients. These results further support previous findings of the successful use of acellular matrices used and in combination with NPWT.2,3,8,10,11
Negative pressure wound therapy has been shown to increase perfusion, contraction, and rate of granulation in acute and chronic wounds.5 By using NPWT in conjunction with HADWM, the wound is kept moist, thus enhancing its integration. Negative pressure wound therapy also acts as a bolster and further secures the HADWM to the wound bed. The use of NPWT with HADWM may enhance the adherence and survival of matrices. Several clinical studies have shown the successful use of NPWT in managing matrices.12-16
Human acellular dermal wound matrix has several advantages over other bioengineered skin grafts.4 For instance, HADWM is derived from human cadaver tissue and composed of collagen and extracellular protein matrices. These proteins promote nutritional diffusion and cellular proliferation at the graft site, leading to rapid revascularization and cellular repopulation of the matrix. The scaffold then transitions into essential dermal tissue, which further reproduces its own tissues around it. The patients in this series have regenerated tissue that is more functionally stable than scar tissue. Another benefit of HADWM is the avoidance of any donor site, which may be problematic in a patient who is immunocompromised or has other comorbidities that may lead to a healing delay.
A single application of HADWM is often sufficient for complete healing and may potentially translate into a further cost saving when compared with other bioengineered grafts that require multiple treatments. Other studies have shown that multiple applications of dermal matrices may be required to heal chronic wounds.17,18 In addition, there is a cost to the patient in terms of lost days of work and longer time to healing. Benefits of combination therapy, such as HADWM and NPWT, are that the procedure can be performed in a clinical setting, further avoiding cost of surgery and preoperative testing as well as eliminating the need for daily dressing changes. Our initial experience further supports evidence that skin grafts, such as HADWM used in conjunction with NPWT, are effective treatment options for lower extremity wounds. While our experience was limited to 11 patients, larger studies with defined endpoints (eg, time to wound closure) are needed to confirm our results of this combination therapy.
Acknowledgements
The author thanks Kristine Villarreal, MS (KCI USA, Inc) for assisting with preparation of the manuscript.
REFERENCES
- 1.American Society of Plastic Surgeons. Evidence-Based Clinical Practice Guideline: Chronic Wounds of the Lower Extremity. Arlington Heights, IL: American Society of Plastic Surgeons; 2012. [Google Scholar]
- 2.Brigido SA, Boc SF, Lopez RC. Effective management of major lower extremity wounds using an acellular regenerative tissue matrix: a pilot study. Orthopedics. 2004;27(1) suppl:s145–9. doi: 10.3928/0147-7447-20040102-14. [DOI] [PubMed] [Google Scholar]
- 3.Brigido SA. The use of an acellular dermal regenerative tissue matrix in the treatment of lower extremity wounds: a prospective 16-week pilot study. Int Wound J. 2006;3(3):181–7. doi: 10.1111/j.1742-481X.2006.00209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reyzelman A, Crews RT, Moore JC, et al. Clinical effectiveness of an acellular dermal regenerative tissue matrix compared to standard wound management in healing diabetic foot ulcers: a prospective, randomised, multicentre study. Int Wound J. 2009;6(3):196–208. doi: 10.1111/j.1742-481X.2009.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hopf HW, Humphrey LM, Puzziferri N, West JM, Attinger CE, Hunt TK. Adjuncts to preparing wounds for closure: hyperbaric oxygen, growth factors, skin substitutes, negative pressure wound therapy (vacuum-assisted closure) Foot Ankle Clin. 2001;6(4):661–82. doi: 10.1016/s1083-7515(02)00008-6. [DOI] [PubMed] [Google Scholar]
- 6.Martin BR, Sangalang M, Wu S, Armstrong DG. Outcomes of allogenic acellular matrix therapy in treatment of diabetic foot wounds: an initial experience. Int Wound J. 2005;2(2):161–5. doi: 10.1111/j.1742-4801.2005.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harding K, Kirsner R, Lee D, Mulder G, Serena T. An expert working group review. London: Wounds International; 2010. International consensus: acellular matrices for the treatment of wounds. [Google Scholar]
- 8.Randall KL, Booth BA, Miller AJ, Russell CB, Laughlin RT. Use of an acellular regenerative tissue matrix in combination with vacuum-assisted closure therapy for treatment of a diabetic foot wound. J Foot Ankle Surg. 2008;47(5):430–3. doi: 10.1053/j.jfas.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Ehrenreich M, Ruszczak Z. Update on tissue-engineered biological dressings. Tissue Eng. 2006;12(9):2407–24. doi: 10.1089/ten.2006.12.2407. [DOI] [PubMed] [Google Scholar]
- 10.Winters CL, Brigido SA, Liden BA, Simmons M, Hartman JF, Wright ML. A multicenter study involving the use of a human acellular dermal regenerative tissue matrix for the treatment of diabetic lower extremity wounds. Adv Skin Wound Care. 2008;21(8):375–81. doi: 10.1097/01.ASW.0000323532.98003.26. [DOI] [PubMed] [Google Scholar]
- 11.Menn ZK, Lee E, Klebuc MJ. Acellular dermal matrix and negative pressure wound therapy: a tissue-engineered alternative to free tissue transfer in the compromised host. J Reconstr Microsurg. 2012;28(2):139–44. doi: 10.1055/s-0031-1289167. [DOI] [PubMed] [Google Scholar]
- 12.Blume PA, Key JJ, Thakor P, Thakor S, Sumpio B. Retrospective evaluation of clinical outcomes in subjects with split-thickness skin graft: comparing V.A.C.? Therapy and conventional therapy in foot and ankle reconstructive surgeries. Int Wound J. 2010;7(6):480–7. doi: 10.1111/j.1742-481X.2010.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blackburn JH, Boemi L, Hall WW, et al. Negative-pressure dressings as a bolster for skin grafts. Ann Plast Surg. 1998;40(5):453–7. doi: 10.1097/00000637-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Stone P, Prigozen J, Hofeldt M, Hass S, DeLuca J, Flaherty S. Bolster versus negative pressure wound therapy for securing split-thickness skin grafts in trauma patients. Wounds. 2004;16(7):219–23. [Google Scholar]
- 15.Scherer LA, Shiver S, Chang M, Meredith JW, Owings JT. The vacuum assisted closure device: a method of securing skin grafts and improving graft survival. Arch Surg. 2002;137(8):930–4. doi: 10.1001/archsurg.137.8.930. [DOI] [PubMed] [Google Scholar]
- 16.Carson SN, Overall K, Lee-Jahshan S, Travis E. Vacuum-assisted closure used for healing chronic wounds and skin grafts in the lower extremities. Ostomy Wound Manage. 2004;50(3):52–8. [PubMed] [Google Scholar]
- 17.Marston WA, Hanft J, Norwood P, Pollak R. Dermagraft Diabetic Foot Ulcer Study Group. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care. 2003;26(6):1701–5. doi: 10.2337/diacare.26.6.1701. [DOI] [PubMed] [Google Scholar]
- 18.Veves A, Falanga V, Armstrong DG, Sabolinski ML. Apligraf Diabetic Foot Ulcer Study. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care. 2001;24(2):290–5. doi: 10.2337/diacare.24.2.290. [DOI] [PubMed] [Google Scholar]


