Abstract
Extracapillary-proliferative glomerulonephritis is a rare complication of multiple myeloma. Partial remission of kidney involvement with cyclophosphamide therapy has previously been described. We report the case of a 60-year-old male patient diagnosed with rapidly progressive glomerulonephritis associated with IgG kappa monoclonal gammopathy. His kidney biopsy revealed pauci-immune extracapillary-proliferative glomerulonephritis without cryoglobulinaemia. Treatment with the proteasome inhibitor bortezomib induced rapid clinical and histological remission of his kidney disease. The patient's renal function remained stable on bortezomib maintenance therapy. Our findings suggest that bortezomib is a promising therapeutic approach to ameliorate severe kidney damage in monoclonal gammopathy- and myeloma-associated pauci-immune extracapillary-proliferative glomerulonephritis.
Keywords: bortezomib, glomerulonephritis, MGRS, MGUS, multiple myeloma
Background
Renal manifestations of multiple myeloma are clinically and histologically diverse. The most common form of renal involvement, accounting for 33 to >60% of cases, is cast nephropathy characterized by an overabundance of toxic light chains in the tubular system. Light-chain deposition disease, primary amyloidosis, often complicated by nephrotic syndrome, proximal and distal tubular dysfunction, renal vein thrombosis due to hyperviscosity or type 1 cryoglobulinaemia are less commonly seen. Rapidly progressive glomerulonephritis is an unusual complication of multiple myeloma that has rarely been reported in the literature [1–5]. It is characterized by severe glomerular damage often involving >50% of the glomeruli in a renal biopsy [6]. Due to the associated rapid decline of renal function, often within weeks, prompt initiation of therapy is crucial to prevent additional damage. In general, the most common cause is pauci-immune glomerulonephritis with a mean age at presentation of 60 years [6–8]. This form of extracapillary-proliferative glomerulonephritis is closely correlated with circulating pathogenic anti-neutrophil cytoplasmic antibodies (ANCAs) in 80–90% of patients [8]. Immune complex-mediated glomerulonephritis and anti-glomerular basement membrane nephritis are less frequently diagnosed in this setting. Standard treatment of extracapillary-proliferative glomerulonephritis includes induction therapy with cyclophosphamide and steroids. In severe kidney and pulmonary disease, plasmapheresis to remove circulating antibodies may be beneficial. Mycophenolate mofetil or azathioprine is usually employed for maintenance immunosuppression. Rituximab as a B-cell-depleting therapy has also been successfully used [9, 10].
In multiple myeloma, bortezomib therapy in combination with dexamethasone recently became a first-line therapy for patients with myeloma-induced renal insufficiency [11]. Several studies documented a significant improvement in kidney function, usually within the initial two to three cycles of treatment [12].
We report a case of monoclonal gammopathy-associated pauci-immune extracapillary-proliferative glomerulonephritis successfully treated with the proteasome inhibitor bortezomib.
Case report
A 60-year-old male was referred to our department in April 2011 by his nephrologist due to an increase in his serum creatinine to 140.8 µmol/L (1.6 mg/dL), haematuria of 290 erythrocytes/µL and spot urine protein–creatinine ratio (UPCR) of 1.9 g/g. He had also developed hypertension during the previous months. His medical history was uneventful, and physical examination did not show any pathologies. He subsequently underwent renal biopsy showing pauci-immune extracapillary-proliferative glomerulonephritis (4 of 14 crescents) with mild tubular atrophy and interstitial fibrosis (20%), as well as lymphohistiocytic infiltration (Figure 1A). Polymorphonuclear leukocytes were present in the glomerulus and in the interstitium. Immunofluorescence revealed minimal mesangial deposits of C3 and complement complex C5b-9 as well as IgM. Immunofluorescence for IgA, IgG and fibrinogen was negative. No amyloid deposits or myeloma casts were identified. Serum C3 and C4 complement levels were normal. Serum ANCAs and cryoglobulines showed negative results. No anti-glomerular basement membrane antibodies were present. The patient received four monthly cycles of cyclophosphamide i.v. along with steroids. Informed consent was obtained from the patient prior to submission of this manuscript. During therapy, serum creatinine stabilized to 132.0 µmol/L (1.5 mg/dL), proteinuria (UPCR) decreased to 0.5 g/g and haematuria improved to 32 erythrocytes/µL, respectively. Maintenance treatment with azathioprine was started and the patient returned to the care of his nephrologist.
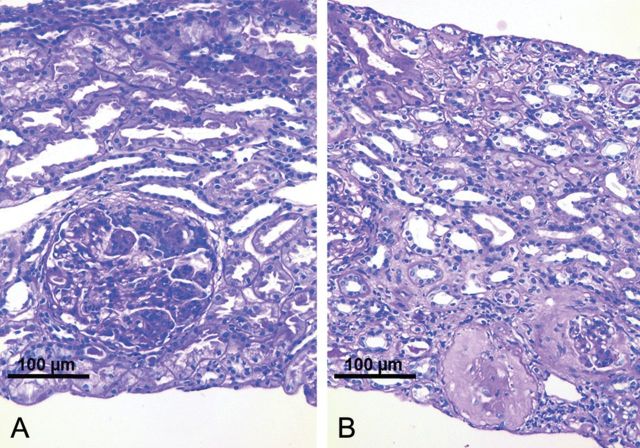
Fig. 1.
Renal biopsy findings in a 60-year-old patient with pauci-immune extracapillary-proliferative glomerulonephritis due to IgG kappa multiple myeloma. (A) Biopsy before bortezomib treatment (200×, PAS) showing a proliferating glomerular crescent. The tubulo-interstitial space appears to be normal. Polymorphonuclear leucocytes within the glomerular tuft. No infiltrates. (B) Biopsy after bortezomib treatment (200×, PAS) showing sclerosing glomeruli and sclerosing crescents, interstitial fibrosis and tubular atrophy with only scarce inflammatory infiltrates. The majority of glomeruli showed normal appearance.
After 10 months, the patient was referred to our service again with newly diagnosed macrohaematuria, increased UCPR of 5 g/g and elevated serum creatinine of 169.8 µmol/L (1.93 mg/dL). A thorough investigation revealed an increased serum β2-microglobulin of 3.5 mg/L and a pathological serum IgG kappa/lambda free light-chain ratio of 5.2 (normal value: 0.26–1.65) due to elevated serum IgG kappa light chains of 74.0 mg/L (normal range: 3.3–19.4 mg/L). A bone marrow biopsy showed an increase of monoclonal kappa-positive plasma cells to 10%. Re-evaluating the initial renal biopsy, no evidence of light-chain deposition disease, fibrillary glomerulonephritis or amyloidosis was present.
A new renal biopsy was performed, again showing pauci-immune extracapillary-proliferating glomerulonephritis (4 of 10 crescents) with mild interstitial fibrosis. In this biopsy, there was also no evidence of classical myeloma-associated kidney disease. Due to the relapse of rapidly progressive glomerulonephritis after cyclophosphamide therapy and leucopenia during azathioprine treatment, we decided to administer two doses of 1 g of rituximab i.v. within 4 weeks and maintained the patient on a reduced dose of azathioprine in combination with cyclosporine A [1, 10]. Unfortunately, the patient showed rapid deterioration of his renal function within the following 8 weeks to a serum creatinine level of 303.6 µmol/L (3.45 mg/dL), an increase of proteinuria to 9 g/g and an increase of haematuria. The serum kappa/lambda free light-chain ratio also increased to 9.1. Because of the underlying plasma cell dyscrasia and rapidly worsening kidney function, we decided to start the patient on the proteasome inhibitor bortezomib (1.3 mg/m2 body surface i.v. on Days 1, 8, 15, 22) in combination with dexamethasone based on the treatment recommendations for multiple myeloma. After the first cycle of bortezomib/dexamethasone, serum creatinine decreased to 140.8 µmol/L (1.6 mg/dL), and urinalysis showed reduced proteinuria of 2 g/g. A control biopsy was performed revealing residual sclerosed crescents, completely sclerosed glomeruli, mild interstitial fibrosis and tubular atrophy (Figure 1B). No signs of active extracapillary proliferations were detected. After the second cycle of bortezomib/dexamethasone, the patient showed clinical remission with serum creatinine levels of 103.8 µmol/L (1.18 mg/dL), minimal proteinuria of 0.48 g/g, no haematuria and well-controlled hypertension. Maintenance therapy of monthly bortezomib was initiated, and the patient showed stable serum creatinine values as well as stable proteinuria with 8 months of follow-up.
Discussion
Glomerulonephritis with crescents, although rare, is a well-documented complication of multiple myeloma. This association was first described by Kaplan and Kaplan in 1970, presenting a 49-year-old patient with renal failure due to extracapillary-proliferative glomerulonephritis, nephrotic syndrome and an IgG paraprotein [13]. Meyrier et al. [2] described three cases of extracapillary-proliferative glomerulonephritis in which plasma cell dyscrasia was identified in two patients and Waldenstrom's macroglobulinaemia in one patient as the underlying cause of renal disease. Renal function was stabilized by melphalan and steroids in the first patient and by steroids in combination with plasmapheresis in the third patient. Rapidly progressive glomerulonephritis has also been reported in patients with primary and secondary amyloidosis [1, 4, 14, 15].
The presence of paraproteins without characteristics of multiple myeloma (hypercalcaemia, anaemia, bone disease) is referred to as ‘monoclonal gammopathy of undetermined significance’ (MGUS). Recently, Leung et al. [16] suggested to introduce the term ‘monoclonal gammopathy of renal significance’ (MGRS) if renal damage is present in these patients.
In this report, we describe a patient with pauci-immune extracapillary-proliferative glomerulonephritis due to IgG kappa monoclonal gammopathy, in which bortezomib and dexamethasone treatment significantly improved his renal function. Therefore, this patient suffers from MGRS. Histologically, after bortezomib therapy, crescents were sclerosed, a mild interstitial fibrosis and tubular atrophy developed and proliferative glomerulonephritis was stopped. The improvement of renal function after treatment with bortezomib was very rapid, suggesting a direct effect of bortezomib on the proliferative and inflammatory glomerular lesions. Bortezomib is a highly selective inhibitor of the 26S proteasome. This drug is known to inhibit protein degradation especially in high-turnover tumour cells, interfering with cell-cycle regulation and cell proliferation [17]. Due to the rapid improvement of the patient's kidney function, we hypothesize that bortezomib exerted its beneficial effects not only through a control of plasma cell proliferation and paraprotein secretion, but also through direct inhibition of cell proliferation in the kidney. In a mouse model of ANCA-associated necrotizing crescentic glomerulonephritis, bortezomib was able to prevent renal disease [18].
Interestingly, no additional hallmarks of myeloma-associated kidney involvement were present in this patient's renal biopsy. Histologically, no deposition of light chains or protein casts was detected. There was also no evidence for amyloidosis. Crosthwaite et al. [4] recently described a patient with primary AL amyloidosis and IgG kappa multiple myeloma that developed rapidly progressive glomerulonephritis in the setting of renal amyloidosis. In this patient, bortezomib and dexamethasone treatment led to a decrease of paraproteinaemia, but one month after initiation of therapy his serum creatinine started to rise and he became dialysis-dependent, suggesting that bortezomib treatment might be less effective if renal amyloidosis is present.
In our patient, the diagnosis of his underlying disease was not made until the second episode of rapid deterioration of kidney function when a thorough workup revealed elevated serum IgG kappa light chains. The fact that monoclonal gammopathy was not discovered earlier indicates that this disease was not primarily taken into account as a cause for his progressive glomerulonephritis. Therefore, we suggest that monoclonal gammopathy and multiple myeloma should be ruled out, especially in patients with ANCA-negative rapidly progressive glomerulonephritis.
In summary, this case demonstrates that in pauci-immune extracapillary-proliferative glomerulonephritis, the presence of monoclonal gammopathy and multiple myeloma, although a rare differential diagnosis, should be considered. Bortezomib therapy is a promising therapeutic approach to reverse severe kidney damage in myeloma-associated pauci-immune extracapillary-proliferative glomerulonephritis.
Conflict of interest statement
None declared.
Footnotes
A version has been published to make this article Open Access.
References
- 1.Schafernak KT, Chugh SS, Kanwar YS. Co-existent crescentic glomerulonephritis and renal amyloidosis: a case report and literature review. J Nephrol. 2005;18:616–622. [PubMed] [Google Scholar]
- 2.Meyrier A, Simon P, Mignon F, et al. Rapidly progressive ('crescentic’) glomerulonephritis and monoclonal gammapathies. Nephron. 1984;38:156–162. doi: 10.1159/000183299. [DOI] [PubMed] [Google Scholar]
- 3.Vedder AC, Weening JJ, Krediet RT. Intracapillary proliferative glomerulonephritis due to heavy chain deposition disease. Nephrol Dial Transplant. 2004;19:1302–1304. doi: 10.1093/ndt/gfg575. [DOI] [PubMed] [Google Scholar]
- 4.Crosthwaite A, Skene A, Mount P. Rapidly progressive glomerulonephritis complicating primary AL amyloidosis and multiple myeloma. Nephrol Dial Transplant. 2010;25:2786–2789. doi: 10.1093/ndt/gfp715. [DOI] [PubMed] [Google Scholar]
- 5.Moscoso-Solorzano GT, Madureira-Silva MV, Balda C, et al. Crescentic glomerulonephritis in IgA multiple myeloma: a case report. Nephron Extra. 2011;1:69–72. doi: 10.1159/000331217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jennette JC. Rapidly progressive crescentic glomerulonephritis. Kidney Int. 2003;63:1164–1177. doi: 10.1046/j.1523-1755.2003.00843.x. [DOI] [PubMed] [Google Scholar]
- 7.Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med. 1997;337:1512–1523. doi: 10.1056/NEJM199711203372106. [DOI] [PubMed] [Google Scholar]
- 8.Kambham N. Crescentic Glomerulonephritis: an update on pauci-immune and anti-GBM diseases. Adv Anat Pathol. 2012;19:111–124. doi: 10.1097/PAP.0b013e318248b7a1. [DOI] [PubMed] [Google Scholar]
- 9.Niles J. Rituximab in induction therapy for anti-neutrophil cytoplasmic antibody (ANCA) vasculitis. Clin Exp Immunol. 2011;164:27–30. doi: 10.1111/j.1365-2249.2011.04363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221–232. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kastritis E, Dimopoulos MA, Bladé J. Evolving chemotherapy options for the treatment of myeloma kidney: a 40-year perspective. Adv Chronic Kidney Dis. 2012;19:312–323. doi: 10.1053/j.ackd.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Dimopoulos MA, Richardson PG, Schlag R, et al. VMP (bortezomib, melphalan, and prednisone) is active and well tolerated in newly diagnosed patients with multiple myeloma with moderately impaired renal function, and results in reversal of renal impairment: cohort analysis of the phase III VISTA study. J Clin Oncol. 2009;27:6086–6093. doi: 10.1200/JCO.2009.22.2232. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan NG, Kaplan KC. Monoclonal gammopathy, glomerulonephritis and the nephrotic syndrome. Arch Intern Med. 1970;125:696–700. [PubMed] [Google Scholar]
- 14.Andreu FJ, Almirall J, Jurado I, et al. Renal failure in a patient with two renal diseases: renal amyloidosis and rapidly progressive glomerulonephritis. Nephrol Dial Transplant. 1997;12:341–343. doi: 10.1093/ndt/12.2.341. [DOI] [PubMed] [Google Scholar]
- 15.Bernheim J, Bernheim J. The patient with two renal diseases: crescentic glomerulonephritis and renal AA amyloid. Nephrol Dial Transplant. 1999;14:1315–1316. doi: 10.1093/ndt/14.5.1315. [DOI] [PubMed] [Google Scholar]
- 16.Leung N, Bridoux F, Hutchison CA, et al. Monoclonal gammopathy of renal significance: when MGUS is no longer undetermined or insignificant. Blood. 2012;120:4292–4295. doi: 10.1182/blood-2012-07-445304. [DOI] [PubMed] [Google Scholar]
- 17.Bianchi G, Ghobrial IM. Molecular mechanisms of effectiveness of novel therapies in multiple myeloma. Leuk Lymphoma. 2013;54:229–241. doi: 10.3109/10428194.2012.706287. [DOI] [PubMed] [Google Scholar]
- 18.Bontscho J, Schreiber A, Manz RA, et al. Myeloperoxidase-specific plasma cell depletion by bortezomib protects from anti-neutrophil cytoplasmic autoantibodies-induced glomerulonephritis. J Am Soc Nephrol. 2011;22:336–348. doi: 10.1681/ASN.2010010034. [DOI] [PMC free article] [PubMed] [Google Scholar]