To the Editor: Contagious caprine pleuropneumonia is a severe respiratory disease of goats caused by Mycoplasma capricolum subsp. capripneumoniae (Mccp), a member of the M. mycoides cluster (1). Mccp infection is associated with a 60% mortality rate and 90% illness rate, and the disease can cause substantial losses of livestock (1,2). We report a 2012 outbreak of contagious caprine pleuropneumonia in endangered Tibetan antelope (Pantholops hodgsonii) in China.
In 2000, the International Union of Conservation of Nature first listed the Tibetan antelope as an endangered species (3), and in 2004, the number of these antelope was estimated at 150,000 (4). Most Tibetan antelope live on China’s Qinghai–Tibet Plateau at an altitude of 3,700–5,500 m (3).
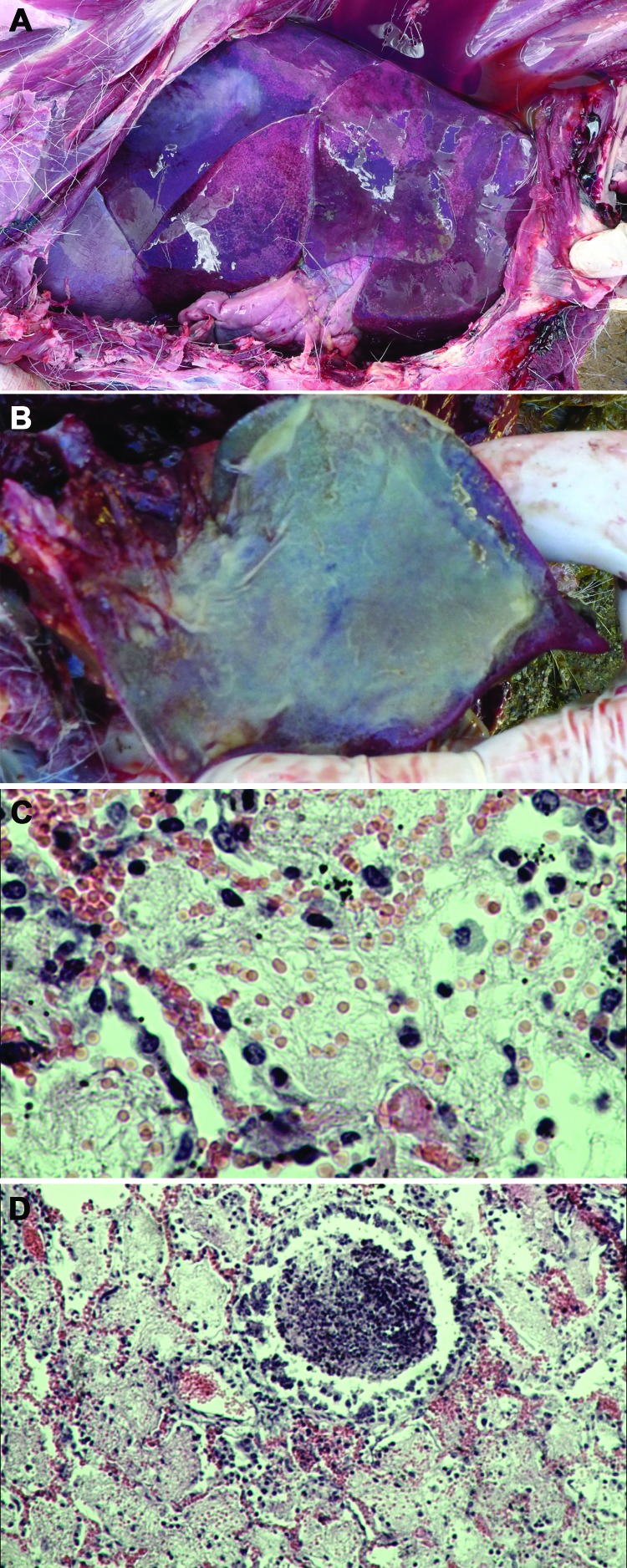
During September–December 2012, ≈2,400 endangered Tibetan antelope were found dead in the Naqu area of Tibet; the dead animals represented 16% of the 15,000 Tibetan antelope thought to live in the area. Necropsy was performed on 13 of the antelope at sites within the Shenzha, Shuanghu, and Nima localities of the Naqu area (Technical Appendix Table 1). Gross pathologic lesions were localized exclusively to the lung, where severe pleuropneumonia with partial hepatization was observed (Figure, panel A). The lungs of some affected antelope displayed a thickening of the interlobular septa, pleuritis, and an accumulation of straw-colored pleural fluid. The pleural exudate solidified to form a gelatinous covering on the lung (Figure, panel B).
Figure.
Pneumonia caused by Mycoplasma capricolum subsp. capripneumoniae in Tibetan antelope (Pantholops hodgsonii), Tibet, 2012. A) Lung of a caprine pleuropneumonia–infected Tibetan antelope (sample SZM2) showing lung hepatization. B) Lung of a caprine pleuropneumonia–infected Tibetan antelope (sample SH3) showing fibrin deposition. C and D) Fibrinous pneumonia with serofibrinous fluid and an inflammatory cell infiltrate, consisting of mainly lymphocytes, in the alveoli (panel C, sample SZM2, hematoxylin and eosin stain; original magnification ×400) and bronchioles (panel D, sample SH3, hematoxylin and eosin stain; original magnification ×100). Refer to Technical Appendix Table 1 for details of the lung samples used to generate images for this figure.
Samples of lung tissue from 5 of the antelope were selected for histologic examination. Four of the samples showed fibrinous pneumonia with serofibrinous fluid and an inflammatory cell infiltrate consisting mainly of lymphocytes in the alveoli (Figure, panel C) and bronchioles (Figure, panel D). One sample showed pulmonary edema with a protein-rich fluid effusion in alveoli.
Lung tissue from each of the 13 antelope was minced and inoculated into modified Hayflick broth, which has been used extensively to isolate Mycoplasma spp. from animals. Cultures were incubated at 37°C in a humidified atmosphere of 5% CO2 (5). The medium was examined daily by comparing inoculated broth with an uninoculated control broth. Moderate turbidity, a color change from pink to yellow, and an appreciable swirl of the culture when rotated were used as indicators of mycoplasma growth. After 2–3 passages in culture, 11 of 13 samples showed growth of mycoplasma. The presence of mycoplasma-like particles in the 11 growth-positive cultures was confirmed by electron microscopy (Technical Appendix Figure 1). Collectively, these observations implicated mycoplasma as the cause of disease in the affected antelope.
We next screened lung samples from each of the 13 Tibetan antelope by PCR for evidence of M. mycoides cluster and M. bovis. Eleven samples were positive for Mccp, but no other types of mycoplasma were detected (Technical Appendix Tables 1, 2). We conducted PCR as described (6) on the arcD gene of Mccp. In brief, we conducted 35 cycles of 30 s at 94°C, 15 s at 47°C, and 15 s at 72°C. Of note, lung sample SH7, which showed pulmonary edema, was negative for mycoplasma by PCR and culture. Lung samples from the 13 dead Tibetan antelope were also tested for an additional 16 potential pathogens (Technical Appendix Tables 1, 2) by PCR or reverse transcription PCR. No pathogens other than Mccp were detected.
To assess the relationship of the Mccp strain isolated from infected Tibetan antelope with previously isolated Mccp strains and the closely related M. capricolum subsp. capricolum (Mcc), we analyzed a 562-bp segment of the H2 gene of Mccp, which was used to distinguish the Mccp and Mcc as reported by Lorenzon et al. (7), isolated from an infected Tibetan antelope in Shuanghu county (sample SH3). The partial H2 sequence (GenBank accession no. KC441725) had higher sequence identity with Mccp isolates (99.3%–99.7%) than with Mcc isolates (90.2%–91.2% (Technical Appendix Figure 2). This phylogenetic analysis demonstrated that the Mccp isolated from infected Tibetan antelope belongs to the same clade as Mccp strains previously isolated in Africa and Asia.
The changing habitat of endangered Tibetan antelope may lead to increased exposure to Mccp, which can cause devastating outbreaks, such as the one reported here. Goats and sheep are herded on grasslands at an altitude of 4,300–5,000 m, the same area where Tibetan antelope reside. Goats are a reservoir for Mccp, and Mccp has been isolated from sheep in mixed herds with goats (8). Rail lines traverse the rangelands in this region, limiting the normal migration patterns of the Tibetan antelope population. Interaction among goats, sheep, and Tibetan antelope in this region, combined with the effect of human infringement on their rangeland, may increase the risk for disease emergence and transmission.
Our results show that contagious caprine pleuropneumonia may pose a substantial threat to the survival of endangered Tibetan antelope. Surveillance for Mccp infection among Tibetan antelope populations and domestic and wild goat and sheep populations that have close contact with the Tibetan antelope should be considered.
Histopathologic findings and results of pathogen testing, primer pairs used for pathogen testing, lung tissue findings, and phylogenetic tree.
Acknowledgments
We thank Peter Wilker for editing the manuscript and Jun Liu, Hongyang Su, and, Xiaohuan Zou for their help in sample processing and histologic observation.
This work was supported by the National Key Technologies R&D Program (grant no. 2013BAD12B04 and 2010BAD04B01) and by the Department of Wildlife Conservation and Nature Reserve Management of the State Forestry Administration, China.
Suggested citation for this article: Yu Z, Wang T, Sun H, Xia Z, Zhang K, Chu D, et al. Contagious caprine pleuropneumonia in endangered Tibetan antelope, China [letter]. Emerg Infect Dis. 2013 Dec [date cited]. http://dx.doi.org/10.3201/eid1912.130067
These authors contributed equally to this article.
These authors contributed equally to this article.
References
- 1.Nicholas R, Churchward C. Contagious caprine pleuropneumonia: new aspects of an old disease. Transbound Emerg Dis. 2012;59:189–96 . 10.1111/j.1865-1682.2011.01262.x [DOI] [PubMed] [Google Scholar]
- 2.Fischer A, Shapiro B, Muriuki C, Heller M, Schnee C, Bongcam-Rudloff E, et al. The origin of the 'Mycoplasma mycoides cluster' coincides with domestication of ruminants. PLoS ONE. 2012;7:e36150 . 10.1371/journal.pone.0036150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Union for Conservation of Nature and Natural Resources. Pantholops hodgsonii. In: IUCN red list of threatened species [cited 2013 Oct15]. http://www.iucnredlist.org/details/15967/0.
- 4.Zhinong X, Lei W. Tracking down Tibetan antelopes. Beijing: Foreign Languages Press; 2004. p. 28. [Google Scholar]
- 5.Eshetu L, Yigezu L, Asfaw Y. A study on contagious caprine pleuropneumonia (CCPP) in goats at an export oriented abattoir, Debrezeit, Ethiopia. Trop Anim Health Prod. 2007;39:427–32. 10.1007/s11250-007-9041-1 [DOI] [PubMed] [Google Scholar]
- 6.Woubit S, Lorenzon S, Peyraud A, Manso-Silvan L, Thiaucourt F. A specific PCR for the identification of Mycoplasma capricolum subsp. capripneumoniae, the causative agent of contagious caprine pleuropneumonia (CCPP). Vet Microbiol. 2004;104:125–32. 10.1016/j.vetmic.2004.08.006 [DOI] [PubMed] [Google Scholar]
- 7.Lorenzon S, Wesonga H, Ygesu L, Tekleghiorgis T, Maikano Y, Angaya M, et al. Genetic evolution of Mycoplasma capricolum subsp. capripneumoniae strains and molecular epidemiology of contagious caprine pleuropneumonia by sequencing of locus H2. Vet Microbiol. 2002;85:111–23. 10.1016/S0378-1135(01)00509-0 [DOI] [PubMed] [Google Scholar]
- 8.Bölske G, Mattsson JG, Bascuñana CR, Bergström K, Wesonga H, Johansson KE. Diagnosis of contagious caprine pleuropneumonia by detection and identification of Mycoplasma capricolum subsp. capripneumoniae by PCR and restriction enzyme analysis. J Clin Microbiol. 1996;34:785–91 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Histopathologic findings and results of pathogen testing, primer pairs used for pathogen testing, lung tissue findings, and phylogenetic tree.