Abstract
We isolated a novel influenza virus A(H1N2) strain from a pig on January 13, 2012, in Gunma Prefecture, Japan. Phylogenetic analysis showed that the strain was a novel type of double-reassortant virus derived from the swine influenza virus strains H1N1pdm09 and H1N2, which were prevalent in Gunma at that time.
Keywords: Swine, influenza A (H1N2), virus, reassortant viruses, H1N1 subtype
Influenza A viruses can be transmitted between humans, swine, and birds; virus subtypes have the potential to reassort and generate new viruses by cross-breeding in the various hosts (1). For example, influenza A subtype H1N1 viruses reassorted in swine, and the resulting swine influenza viruses (SIVs) were transmitted to humans. The reassorted combinations have resulted in pandemic viruses as well as low-pathogenicity viruses with low transmissibility among humans. Similarly, seasonal human subtypes of influenza are transmissible to swine (2). In 2009, a novel strain of the H1N1 SIV subtype emerged and was associated with a pandemic (3,4). The virus, later termed influenza A(H1N1)pdm09, hereafter referred to as pH1N1, was confirmed as a reassortant virus resulting from cross-breeding of a European avian subtype H1N1 virus and a North American triple reassortant virus (5). Subsequently, other strains reassorted from the pH1N1 virus (6–8). We report on an isolated new reassortant H1N2 SIV derived from the pH1N1 virus and SIVs originating in Japan.
The Study
We collected 109 nasal swab samples from pigs for swine influenza surveillance during November 2011–February 2012. Nasal swab samples were collected from healthy pigs, 6 months of age, at an abattoir in Gunma Prefecture, Japan. All samples were inoculated onto MDCK cells (9). All cell culture supernatants were tested by using a hemagglutination assay of a 0.7% solution of guinea pig erythrocytes (9). To determine the subtype of the isolate, a hemagglutination inhibition assay was performed by using ferret antiserum for A/California/07/2009 [A(H1N1)pdm09], A/Victoria/210/2009 [A(H3N2)], B/Bangladesh/3333/2007 [B/Yamagata-lineage], and B/Brisbane/60/2008 [B/Victoria-lineage] (9). One strain of influenza A virus, designated A/swine/Gunma/1/2012, was isolated from the samples.
For full genome sequencing of the influenza A/swine/Gunma/1/2012 strain, we conducted reverse transcription PCR (10). Segment-specific primers used for amplification and sequencing are shown in Technical Appendix Figure, panel A. Phylogenetic analysis of the nucleotide sequences was conducted by using MEGA version 5 software (www.megasoftware.net) and Tree Explorer version 2.12 (http://en.bio-soft.net/tree/TreeExplorer.html) (11). Evolutionary distances were estimated according to the Kimura 2-parameter method (12). The phylogenetic trees of hemagglutinin (HA) and neuraminidase (NA) genes were constructed by using the neighbor-joining method (13). In addition, phylogenetic trees based on the matrix protein, nucleoprotein genes, nonstructural protein, polymerase acid, polymerase basic 1, and polymerase basic 2 were constructed by using the neighbor-joining method. The reliability of the trees was estimated with 1,000 bootstrap replications. GenBank accession numbers assigned to the gene sequences of the analyzed strain are the following: polymerase basic 2 (AB731582), polymerase basic 1 (AB731583), polymerase acid (AB731584), HA (AB731585), nucleoprotein (AB731586), NA (AB731587), matrix protein (AB731588), and nonstructural protein (AB731589).
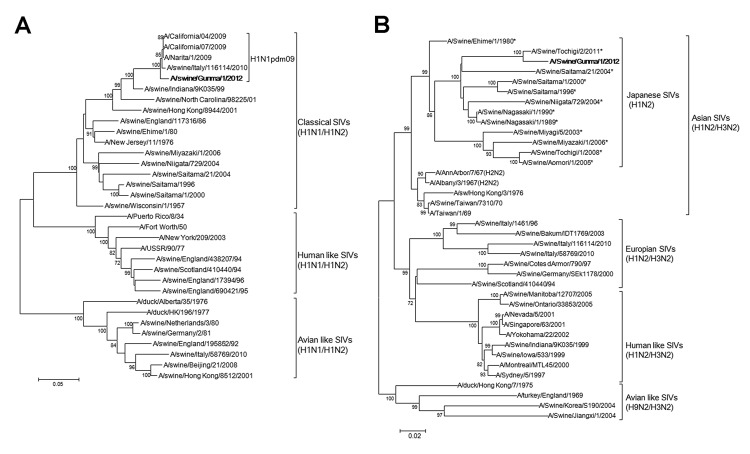
Phylogenetic trees based on HA and NA gene sequences are shown in the Figure, panels A and B. The identities of the nucleotide sequences of each gene are shown in the Table. The A/swine/Gunma/1/2012 strain was confirmed as a strain of pH1N1 virus Figure, panel A). NA gene sequences showed that the virus was located within clusters of swine-type viruses documented in Japan as the representative strains, such as A/swine/Ehime/1/1980 (Figure, panel B). The sequence identity of the NA gene between the A/swine/Gunma/1/2012 strain and other Japanese H1N2 SIV strains ranged from 85.0 to 97.5%. The identities of other genes between the A/swine/Gunma/1/2012 strain and pH1N1 virus vaccine strain (A/California/07/2009) were highly homologous (>90%; Table). These results suggest that the A/swine/Gunma/1/2012 strain was a new reassortant of the H1N2 SIV subtype derived from the pH1N1 virus.
Figure.
Phylogenetic tree based on the nucleotide sequences of hemagglutinin (A) and neuraminidase (B) genes of A/swine/Gunma/1/2012, a novel H1N2 swine influenza virus (SIV) strain. Distance was calculated according to the Kimura 2-parameter method; the trees were constructed by using the neighbor-joining method with labeling of the branches showing at least 70% bootstrap support. Boldface text indicates the novel strain reassorted from strains of the SIV H1N2 subtype. Asterisks indicate reference strains compared with A/Swine/Gunma/1/2012 used to calculate the identity of neuraminidase gene. Scale bars indicate nucleotide substitutions per site.
Table. Sequence identity of each gene of influenza strain A/swine/Gunma/1/2012, reassorted from influenza A(H1N1)pdm09 and A/California/07/2009*.
| Gene | Identity (%) |
|---|---|
| PB2 | 98.9 |
| PB1 | 98.7 |
| rPA | 98.7 |
| HA | 98.4 |
| NP | 98.7 |
| MP | 99.3 |
| NS | 99.3 |
*PB, polymerase basic; PA, polymerase acid; HA, hemagglutinin; NP, nucleoprotein; MP, matrix protein; NS, nonstructural protein.
We isolated 1 strain in this study. The samples (109 nasal swabs) were collected from different pig farms ≈60 km apart. The epidemiologic association may be low among the samples, because the quarantine inspection system is well established in Japan. All samples were collected from pigs 6 months of age; therefore, the potential for infection with the virus could have been low. Additional and larger studies investigating the emergence of the parent virus of the strain may be needed.
Conclusions
Vijaykrisna et al. found a new reassortant virus among avian-type, swine-type, and pH1N1 viruses (6). In addition, Monero et al. reported a new reassortant virus between SIV, identified in Italy, and pH1N1 viruses (7). Thus, pH1N1 virus and other types of influenza viruses can be reassorted. However, to our knowledge, reassortant H1N2 SIV strains derived from pH1N1 virus in Japan have not been identified before this report. Although the transmission of SIVs to humans has been reported sporadically, the infectious nature of this reassortant H1N2 strain among humans is unknown. The emergence of a novel H1N2 SIV strain raises further concerns about whether the virus will generate further genetic reassortments and gain virulence. Systematic influenza virus surveillance in pigs and humans should be considered.
Genome Amplification, Sequencing, and Phylogeny of Novel Reassortant Influenza A(H1N2) Virus
Acknowledgments
This study was supported by National Epidemiological Surveillance of Vaccine-Preventable Diseases by the Ministry of Health, Labor and Welfare, Japan.
Biography
Ms Kobayashi is a research worker in the Gunma Prefectural Institute of Public Health and Environmental Sciences, Gunma, Japan. Her research interests are the epidemiology and molecular biology of respiratory viruses.
Footnotes
Suggested citation for this article: Kobayashi M, Takayama I, Kageyama T, Tsukagoshi H, Saitoh M, Ishioka T, et al. Novel reassortant influenza A(H1N2) virus derived from A(H1N1)pdm09 virus isolated from swine, Japan. Emerg Infect Dis. 2013 Dec [date cited]. http://dx.doi.org/10.3201/eid1912.120944
References
- 1.Brown IH. The epidemiology and evolution of influenza viruses in pigs. Vet Microbiol. 2000;74:29–46 . 10.1016/S0378-1135(00)00164-4 [DOI] [PubMed] [Google Scholar]
- 2.Katsuda K, Sato S, Shirahata T, Lindstrom S, Nerome R, Ishida M, et al. Antigenic and genetic characteristics of H1N1 human influenza virus isolated from pigs in Japan. J Gen Virol. 1995;76:1247–9. 10.1099/0022-1317-76-5-1247 [DOI] [PubMed] [Google Scholar]
- 3.Peiris JS, Poon LL, Guan Y. Emergence of a novel swine-origin influenza A virus (S-OIV) H1N1 virus in humans. J Clin Virol. 2009;45:169–73. 10.1016/j.jcv.2009.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Novel Swine-Origin Influenza A. (H1N1) Virus Investigation Team, Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–15.http:// [DOI] [PubMed]
- 5.Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009;325:197–201. 10.1126/science.1176225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vijaykrishna D, Poon LL, Zhu HC, Ma SK, Li OT, Cheung CL, et al. Reassortment of pandemic H1N1/2009 influenza A virus in swine. Science. 2010;328:1529. 10.1126/science.1189132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moreno A, Di Trani L, Faccini S, Vaccari G, Nigrelli D, Boniotti MB, et al. Novel H1N2 swine influenza reassortant strain in pigs derived from the pandemic H1N1/2009 virus. Vet Microbiol. 2011;149:472–7. 10.1016/j.vetmic.2010.12.011 [DOI] [PubMed] [Google Scholar]
- 8.Howard WA, Essen SC, Strugnell BW, Russell C, Barass L, Reid SM, et al. Reassortant pandemic (H1N1) 2009 virus in pigs, United Kingdom. Emerg Infect Dis. 2011;17:1049–52 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO Global Influenza Surveillance Network. Manual for the laboratory diagnosis and virological surveillance of influenza. Geneva: World Health Organization; 2011. p. 35–77. [Google Scholar]
- 10.Hoffmann E, Stech J, Guan Y, Webster RG, Perez DR. Universal primer set for the full-length amplification of all influenza A viruses. Arch Virol. 2001;146:2275–89 . 10.1007/s007050170002 [DOI] [PubMed] [Google Scholar]
- 11.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–9. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–20. 10.1007/BF01731581 [DOI] [PubMed] [Google Scholar]
- 13.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–25 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Genome Amplification, Sequencing, and Phylogeny of Novel Reassortant Influenza A(H1N2) Virus