Abstract
The secretion of fluid, electrolytes, and protein by exocrine gland acinar cells is a vectorial process that requires the coordinated regulation of multiple channel and transporter proteins, signaling components, as well as mechanisms involved in vesicular fusion and water transport. Most critical in this is the regulation of cytosolic free [Ca2+] ([Ca2+]i) in response to neurotransmitter stimulation. Control of [Ca2+]i increase in specific regions of the cell is the main determinant of fluid and electrolyte secretion in salivary gland acinar cells as it regulates several major ion flux mechanisms as well as the water channel that are required for this process. Polarized [Ca2+]i signals are also essential for protein secretion in pancreatic acinar cells. Thus, the mechanisms that generate and modulate these compartmentalized [Ca2+]i signals are central to the regulation of exocrine secretion. These mechanisms include membrane receptors for neurotransmitters, intracellular Ca2+ release channels, Ca2+ entry channels, as well Ca2+ as pumps and mitochondria. The spatial arrangement of proteins involved in Ca2+ signaling is of primary significance in the generation of specific compartmentalized [Ca2+]i signals. Within these domains, both local and global [Ca2+]i changes are tightly controlled. Control of secretion is also dependent on the targeting of ion channels and transporters to specific domains in the cell where their regulation by [Ca2+]i signals is facilitated. Together, the polarized localization of Ca2+ signaling and secretory components drive vectorial secretion of fluid, electrolytes, and proteins in the exocrine salivary glands and pancreas. This review will discuss recent findings which have led to resolution of the molecular components underlying the spatio-temporal control of [Ca2+]i signals in exocrine gland cells and their role in secretion.
Keywords: Calcium homeostasis, calcium influx, ion channels, fluid secretion, salivary gland cells, physiology, knockout mouse models
OVERVIEW
Ca2+ is critical for the physiological function of both excitable and non-excitable cells [1, 2]. It is the primary intracellular factor in the regulation of fluid secretion in salivary and lacrimal glands and for protein secretion in pancreas [3–5]. The basic Ca2+ signaling mechanisms that regulate secretory function are remarkably similar in these exocrine glands. This review will highlight recent studies and our current understanding of the regulation of Ca2+ signaling in salivary gland acinar cells and the role of Ca2+ in fluid secretion. Further, we will discuss Ca2+ signaling mechanisms in pancreatic acinar cells to compare exocrine pancreas and salivary glands. Salivary glands have been widely used to determine the main pathways involved in the regulation of Ca2+ signaling in non-excitable cells. These early studies demonstrated that under normal physiological conditions, salivary glands maintain a continuous low level of saliva flow, often referred to as "resting" or "basal" secretion, which is dramatically increased upon demand. The actual up-regulation of secretion in salivary and other exocrine glands is achieved when glands are stimulated by sympathetic and parasympathetic stimuli and mediated by a coordinated cascade of signal transduction and intracellular signaling events [3, 4].
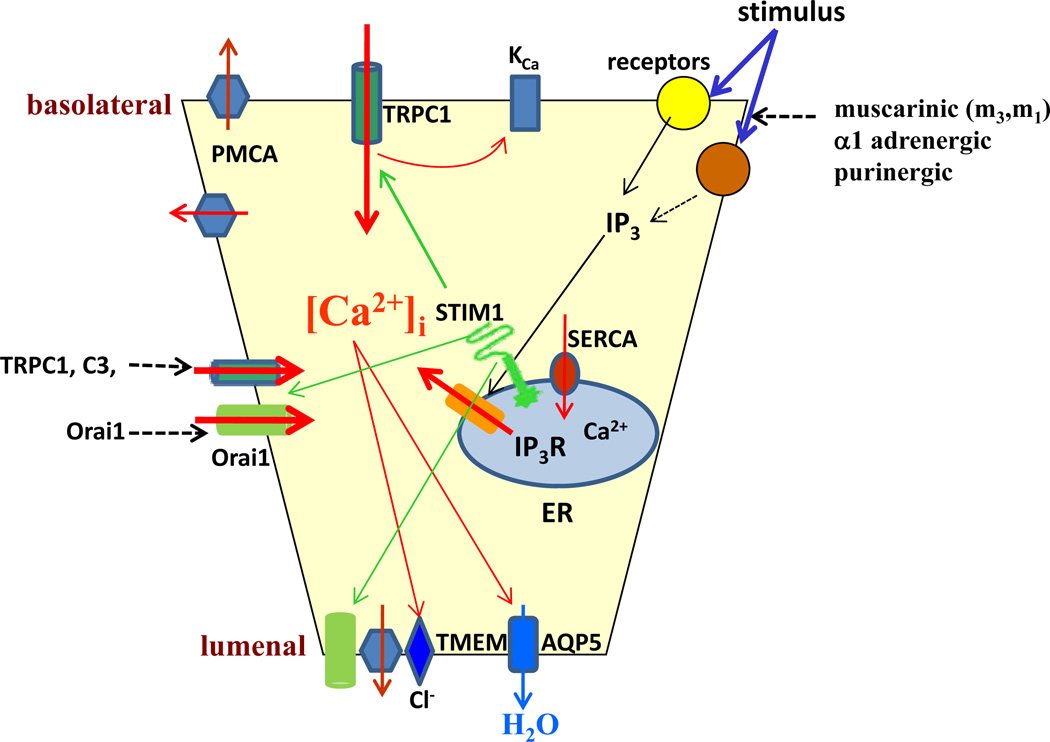
Salivary gland cells have a variety of neurotransmitter receptors, many of which are localized in the basolateral plasma membrane region of the cell; such as muscarinic, adrenergic, and purinergic receptors Fig. (1) which when stimulated trigger intracellular signaling events that culminate in an increase in fluid and protein secretion. Typically, stimulation of Ca2+ mobilizing receptors, e.g. muscarinic, leads to G-protein-mediated activation of phosphatidylinositol 4,5, bisphosphate (PIP2)-specific phospholipase C (PLC), hydrolysis of PIP2, and generation of inositol 1,4,5, trisphosphate (IP3) and diacylglycerol. IP3 is the central mediator in cellular Ca2+ signaling events. It diffuses into the cytosol and binds to a receptor (IP3 receptor, IP3R) localized in the endoplasmic reticulum (ER) membrane. The IP3R serves as a Ca2+ release channel and mediates rapid release of Ca2+ from the ER-Ca2+ store(s) resulting in an increase in [Ca2+]i. Three subtypes of the IP3 receptors have identified, (IP3R1, IP3R2 and IP3R3) and of these, IP3R2 and 3 are the major subtypes found in exocrine gland cells. Importantly, these are concentrated in the apical pole of the cells and have been associated with the generation of spatially distinct [Ca2+]i signals in that region [4–9]. In pancreatic acinar cells, the initial internal Ca2+ release is detected in the apical region of the cell and at relatively low levels of agonist stimulation, the [Ca2+]i increase remains restricted within this region [6, 9, 10]. At higher levels of agonist, although the signal again starts at the apical pole of the cells, it propagates to the basolateral regions. In salivary gland acinar cells, agonist-induced cytosolic Ca2+ signals are also first detected in in the apical region, but unlike the response in pancreatic cells, [Ca2+]i increase is propagated in wave-like pattern to the basal region at both low and high levels of stimuli [11]. The net result of IP3-induced release of Ca2+from the ER in either cell type is a spatially and temporally coordinated increase in the [Ca2+]i which determines sustained fluid secretion in salivary gland acini and triggers granule fusion and protein secretion in pancreatic acini [4, 5].
Fig. (1). Ca2+ signaling mechanisms regulating salivary gland fluid secretion.
The figure shows Ca2+ mobilizing events in acinar cells that are initiated by a stimulus and lead to fluid secretion. Red arrows indicate mechanisms regulated by [Ca2+]i increase. The coordinated regulation (spatial and temporal) of Ca2+ signaling as well as ion channel activation achieves the secretion of fluid from acinar cells.
There are a large number of studies to demonstrate that while intracellular Ca2+ release is sufficient to activate mechanisms involved in fluid secretion, sustained secretion is dependent on extracellular Ca2+ influx. It has now been shown that Ca2+ entry into acinar cells is activated in response to agonist-stimulated PIP2 hydrolysis. Two possible Ca2+ entry mechanisms can be activated under these conditions. The first, referred to as store-operated calcium entry (SOCE), is activated as a consequence of depletion of Ca2+ in the ER lumen via IP3-induced Ca2+ release [12–16] and is regulated by the [Ca2+]i status in the ER. SOCE and the resulting regulation of cell function have been well established in almost all cell types. The other type of Ca2+ entry pathway is referred to as store-independent, or receptor-dependent, Ca2+ entry. Although there is not much information available regarding the mechanism of this influx, it is believed that this entry is not regulated by the [Ca2+] of the ER. Rather, direct effects at the plasma membrane level, due to changes in membrane lipids or other components, can trigger Ca2+ entry. The physiological relevance of this type of entry mechanism has not yet been described in exocrine gland cells. The coordinated activation and regulation of intracellular Ca2+ release and extracellular Ca2+ entry generates the optimal temporal and spatial [Ca2+]i signals that drive secretion and are also likely to be critical for the regulation of other cellular functions, such as protein synthesis and gene expression.
Exocrine glands are typically composed of two major cell types: acinar and duct cells. Acinar cells represent a classical model of polarized epithelial cells with extensive rough ER at the basal pole and smooth ER at the apical pole. The nucleus is situated close to the apical end of the cell, while the protein-containing secretory granules in both salivary and pancreatic acini and are localized at the luminal pole [3, 4]. In pancreatic acini, protein secretion is activated by increase in [Ca2+]i while in salivary gland acini is is controlled by intracellular generation of cAMP. These cells are organized to facilitate vectorial secretory processes and it is interesting that the cellular architecture can be correlated with polarized cellular functions, such as ion transport and Ca2+ signaling. For example, Ca2+ signaling proteins are organized as complexes which are compartmentalized at strategic locations in the cell with most of the receptors being localized in the basolateral region where stimuli are detected. Specificity in the cellular location of these proteins allows for the generation of localized Ca2+ signals and the propagation of Ca2+ waves [4, 6–9]. The local and global Ca2+ signals are then sensed and transduced for the regulation of critical ion channels that drive fluid secretion.
THE ROLE OF [Ca2+]i IN THE REGULATION OF FLUID SECRETION
The first studies, reported almost three decades ago, provided strong evidence for the involvement of Ca2+ in the regulation of fluid secretion in the salivary gland and pancreas [16, 17]. Key experiments done with perfused salivary gland preparations demonstrated that sustained fluid secretion is achieved only when Ca2+ is present in the external medium; while in the absence of external Ca2+, fluid secretion is stimulated transiently. This suggested that Ca2+ entry likely contributes to regulation of secretion. The activation of Ca2+ entry in acini was more directly shown by 45Ca2+ flux experiments that revealed an uptake of Ca2+ when cells were stimulated by agonist that also triggered fluid secretion [17]. These studies also provided conclusive evidence that Ca2+ release from, as well as uptake into, cells is initiated in response to agonist stimulation and that these processes occur concurrently with fluid secretion. Thus it was proposed that stimulation of cells lead to changes in intracellular Ca2+ homeostasis and plasma membrane Ca2+ permeability. Furthermore, the release of Ca2+ from the cell was temporally correlated with the efflux of K+ and an increase in fluid secretion, while Ca2+ uptake was associated with prolonged fluid secretion. In aggregate, these data provided strong support for the suggestion that Ca2+ was sequestered in an internal store from where it was released following stimulation. The uptake mechanism into the store was also established by the identification of an ATP-dependent Ca2+ uptake mechanism (i.e. Ca2+ pump) in the ER membrane that mediates active uptake of Ca2+ from the cytosol into the ER-Ca2+ store of exocrine gland cells.
A landmark study in the field of Ca2+signaling was the demonstration that IP3, a diffusible second messenger synthesized in response to agonist stimulation of cells, induced the release of Ca2+ from ER [18]. This finding demonstrated that inositol lipid turnover following receptor stimulation is functionally linked to intracellular Ca2+ mobilization and enzyme secretion in pancreatic acinar cells. These conclusions were further corroborated when fluorescent probes for measuring [Ca2+]i became available. This technique revolutionized the field of Ca2+ signaling and rapidly advanced studies in cell populations and tissue sections to measurements and real-time imaging of [Ca2+]i in single cells. Such studies conclusively established that agonist stimulation of cells induced Ca2+mobilization events that followed a specific temporal and spatial pattern. Stimulation of cells, including exocrine gland cells, induces a biphasic increase in [Ca2+]i, with an initial rapid increase that is not affected by the removal of external Ca2+ and a subsequent lower sustained [Ca2+]i increase that is completely dependent on the presence of external Ca2+ [3, 4, 6, 7, 9, 13]. This sustained [Ca2+]i increase is inhibited by external addition of La3+ or Ca2+ chelators, confirming that it is determined by Ca2+ influx into the cell. Subsequent studies showed that the sustained increase in [Ca2+]i determines salivary gland fluid secretion since it is required for the activation of key ion flux systems, e.g. KCa channels, the Na+/K+/2Cl− cotransporter, and TMEM16A channel, that together drive water secretion in acini. In addition, membrane trafficking of the water channel, AQP5, the primary water channel involved in fluid secretion, is also dependent on sustained [Ca2+]i elevation [4, 12].
MECHANISMS THAT CONTRIBUTE TO CYTOSOLIC [Ca2+]i INCREASE
Major mechanisms that contribute to the regulation of [Ca2+]i are plasma membrane receptors, Ca2+ channels in the ER and plasma membrane, as well as Ca2+ pumps and mitochondria [3, 4, 8, 19, 20]. Studies using knockout mice established that M(1) and M(3) subtypes are the main muscarinic receptors in salivary glands involved in fluid secretion. Mice lacking these receptors display severe diminution of pilocarpine-stimulated saliva flow. Further, acinar cells isolated from these mice show loss of carbachol-stimulated Ca2+ release as well as Ca2+ entry [21, 22]. The two major events required for maintaining a sustained [Ca2+]i elevation are internal Ca2+ release and Ca2+ entry. One of the best characterized and most important Ca2+channels in cells is the IP3R which mediated rapid release of Ca2+ from the ER-Ca2+ store into the cytosol [6, 7, 18, 19]. In salivary gland cells, IP3R2 and IP3R3 are the major isoforms that have been established to have a physiological role in the regulation of fluid secretion [5–7]. Both these receptors are concentrated in the apical region of both pancreatic and salivary acini. Release of Ca2+ via these receptors leads to the initial increase in [Ca2+]i that is detected at the apical pole Fig. (2). Interestingly, IP3R is regulated both by IP3 as well as Ca2+. Ca2+-dependent regulation of IP3R exhibits a biphasic pattern with an increase in IP3-mediated Ca2+ release at relatively low [Ca2+]i and inhibition at higher [Ca2+]i (> 300nM). The ability of Ca2+ to increase IP3R function is completely dependent on [IP3], with the sensitivity to [Ca2+] increasing at higher [IP3]. This feed-forward and feedback regulation of IP3R by Ca2+ ensures that the channel is activated when [Ca2+]i is low but restricted when [Ca2+]i is high. The range of [Ca2+]i changes in each cell type is tightly regulated to achieve the necessary physiological response and also to protect the cell. For example, if ER-Ca2+ stores are excessively depleted, cells can undergo ER stress. Regulation of the IP3R function ensures against such depletion. When IP3Rs are inactivated at high [Ca2+]i, activity of the Ca2+ pump mediates refilling of the ER-Ca2+ stores so that it is primed for the next release event [19].
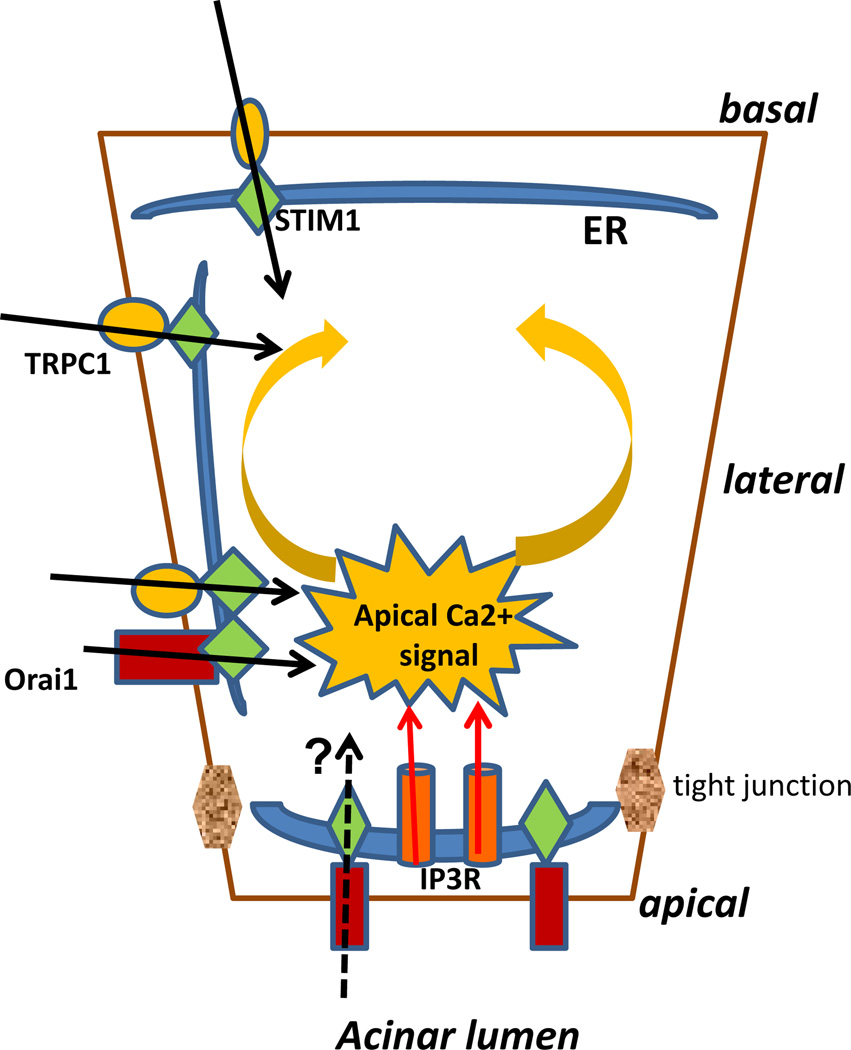
Fig. (2). Initiation and propagation of cytosolic calcium increases in acinar cells.
Release of calcium via apically localized IP3Rs triggers the initial apical rise in cytosolic [Ca2+] following cell stimulation. The calcium signal is then propagated towards the basal region of the cell. It is suggested that apically localized channels are activated first followed by those in the lateral and basal regions of the cell. Activation of calcium entry channels allows a sustained rise in cytosolic [Ca2+] which is required to drive fluid secretion.
The physiological role of IP3Rs in exocrine gland cells was established by studies with knockout mice models. While animals with individual knockout of either the type-2 or type-3 IP3R have no significant effects on muscarinic-receptor stimulated secretion or increases in [Ca2+]i, those lacking both subtypes display a much more striking phenotype. Firstly, although animals are born normally, they failed to survive past weaning. Furthermore, there is complete elimination of agonist-induced Ca2+ signaling, even when supramaximal concentrations of agonist is used [23]. This study not only provided the first physiological evidence that IP3Rs are the main intracellular release channels, but also established the critical role of IP3R2 and IP3R3 in exocrine gland function. The findings also revealed that intracellular Ca2+ release is key to the regulation of fluid secretion. Ca2+ entry mechanism(s) that is not dependent on internal Ca2+ release should remain active and not be affected by deletion of IP3Rs. However, this type of Ca2+ entry appears to have minimal role in stimulating fluid secretion.
MECHANISMS OF Ca2+ INFLUX
As noted above, the requirement of extracellular Ca2+ influx in salivary fluid secretion was established more than three decades ago. However, identification of the molecular components involved in mediating and regulating Ca2+ entry in salivary gland and other non-excitable cells continued to remain a challenging problem for investigators in this field. As noted above, Ca2+ entry into exocrine acinar cells is activated soon after cells are stimulated by an agonist. Since Ca2+ influx is apparently terminated when the agonist is removed or when an antagonist is added to cells, it was suggested that Ca2+influx was activated either directly as a result of agonist binding to the receptor or by an intracellular second messenger generated in response to receptor stimulation [20]. The exact stimulus for Ca2+ entry was established following the identification of thapsigargin as a specific ER-Ca2+ pump inhibitor. Addition of thapsigargin to cells induces a slow depletion of Ca2+ in the ER-Ca2+ store, likely as a result of Ca2+ release via an ER-Ca2+ leak that is normally compensated by the Ca2+ pump activity. While this ER-Ca2+ leak pathway remains to be identified, this important study demonstrated that activation of Ca2+ entry is not dependent on receptor-coupled signaling mechanisms per se but rather on the [Ca2+] status of the ER store. Additional studies showed that refilling of the internal Ca2+ store that occurred following removal of agonist from the external medium, induced inactivation of Ca2+ entry. Thus, the terms capacitative Ca2+ entry (CCE) or store-operated Ca2+ entry (SOCE) were coined to describe this entry mechanism [14].
Several major studies have demonstrated that SOCE is the primary mode of Ca2+ influx in salivary gland and pancreatic acinar cells following receptor stimulation and that critical regulation of fluid and protein secretion, respectively, in the two glands are dependent on this process [4, 5, 12]. The mechanism coupling ER-Ca2+ store depletion to the activation of plasma membrane Ca2+ entry remained an elusive problem that initiated extensive studies. The first model proposed to describe the mechanism of SOCE was that the ER is physically linked to the plasma membrane such that Ca2+ entering the cell directly enters the ER-Ca2+ store from where it is released via the IP3R into the cytosol [13]. When the level of Ca2+ in the ER is high, as in resting cells, Ca2+ entry will be inhibited via a direct effect of the Ca2+ on the entry mechanism. Conversely, when the Ca2+ level decreases following IP3-induced Ca2+ release, the inhibition is depressed and Ca2+ influx is activated. This concept of direct Ca2+ entry into the ER was ruled out based on studies using Mn2+, as a Ca2+ surrogate ion, which enters cells via plasma membrane Ca2+ entry pathways but is not a substrate for the ER-Ca2+ pump. Thus, demonstration that addition of Mn2+ to the external medium of stimulated cells induced quenching of Fura2 in the cytosol provided evidence that Ca2+ enters the cytosol and is subsequently accumulated into the ER [14, 15].
Subsequent studies from many laboratories using a wide variety of cell types showed that SOCE is a ubiquitous pathway that is required for many different physiological functions, such as exocrine secretion, platelet aggregation, endothelial cell permeability and migration, cell proliferation, T-lymphocyte activation, and mast cell degranulation. Identifying the mechanisms and components of this pathway was a major focus of interest in the field of Ca2+ signaling for more than twenty years. The major problems that needed to be addressed in order to fully explain SOCE were: (i) identification of the molecular components of the SOCE channel and (ii) resolution of the mechanism by which the ER-Ca2+ status is transmitted to the Ca2+ channel in the plasma membrane. Initial studies lead to the suggestion that a physical interaction between IP3R in the ER and the plasma membrane channel and regulates SOCE. According to this the IP3R undergoes a conformational change when [Ca2+] in the ER is lowered that is transmitted to the plasma membrane channel resulting in activation of SOCE. However, a direct role for IP3R in gating the SOCE channel was excluded based on studies in DT40 (a chicken B cell line) which had normal SOCE despite deletion of all three IP3R subtypes (further discussed below). The second suggestion was that a diffusible metabolite is released from the ER together with Ca2+ which upon binding to the putative plasma membrane channel induces its activation. This model remains contentious due to lack of data to conclusively support it. Another possible mechanism is the recruitment of channels to the plasma membrane by a regulated trafficking mechanism. Despite the lack of knowledge regarding the exact mechanism of SOCE, it was evident that there must be close physical proximity between the ER and plasma membrane during activation of SOCE as it is a relatively rapid process. Furthermore, Ca2+ entering the cells during store refilling is rapidly taken up into the ER with minimal changes in global [Ca2+]i.
MOLECULAR COMPONENTS OF SOCE
Major breakthroughs in our understanding of the mechanism of SOCE were made over the past ten years. Both the molecular components of the channels as well as the mechanism involved in sensing and transmitting the ER-[Ca2+] status to the plasma membrane have now been identified. Members of the transient receptor potential canonical (TRPC) channels were the first calcium permeable channels to be proposed as candidates for the channel mediating SOCE [8, 24–27]. All TRPC channel members are activated following stimulation of PIP2 hydrolysis by PLC, but only few are also activated in response to thapsigargin stimulation of cells. Thus, some TRPC channels (e.g. TRPC1, TRPC3, and TRPC4) fulfilled the criteria as mediators of SOCE. Although data from heterologous expression studies have not been very consistent, consequences of knockdown of endogenous proteins have provided strong evidence for the involvement of these channels in SOCE. Among these, the strongest data are available for a role for TRPC1 in SOCE. A large number of studies have been carried out in exocrine gland cells. Studies in the human submandibular gland cell line, HSG, first demonstrated that TRPC1 contributes to SOCE [4, 26–30]. Further, generation of mice lacking TRPC1 provided conclusive evidence for the function of this channel in salivary and pancreatic acinar cells. These mice displayed a severe decrease in fluid secretion, almost to the level seen in AQP5−/− mice, which was correlated with a loss of thapsigargin- and carbachol-stimulated Ca2+ entry. Ca2+-dependent potassium (KCa) channel function, which depends on Ca2+ entry, was also significantly depressed. This study demonstrated that SOCE is the primary Ca2+ entry pathway in these cells that drives fluid secretion (consistent with the lack of Ca2+ influx in acini from IP3R2+IP3R3−/− mice as discussed above). Together, these data finally established TRPC1 as a critical channel component of SOCE in salivary gland acinar cells that is essential for neurotransmitter-mediated regulation of fluid secretion. TRPC1 also contributes to SOCE in pancreatic acinar cells where it has a role in modulation of the Ca2+-activated Cl− current, another key ion channel required for secretion. In addition, TRPC3 contributes to SOCE and mediates a significant portion of the receptor-stimulated Ca2+ influx in exocrine pancreatic acinar cells. TRPC3-mediated Ca2+ influx in these cells affected the frequency of Ca2+ oscillations [31].
Although several studies demonstrated that TRPC channels contributed to SOCE, they were associated with the activation of relatively non-selective cation channels in cells, referred to as SOC, store-operated calcium, channels. Thus TRPC proteins were ruled out as components of the Calcium Release-Activated Calcium (CRAC) channel, the first SOCE-associated current identified in mast cells and lymphocytes. Search for the mechanism regulating SOCE and CRAC channel components led to the exciting identification of two protein families, STIM and Orai [14, 32]. While the STIM family consists of STIM1 and STIM2, a large number of studies have focused on STIM1 and presently there is not much information regarding STIM2. STIM1 is an ER protein with a single membrane spanning region, an N-terminal EF-hand domain residing within the ER lumen and a cytosolic domain. The Ca2+-binding domain serves as a sensor for Ca2+ within the ER. When ER-Ca2+ stores are full in resting cells, STIM1 is diffusely localized in the ER. IP3-mediated release of Ca2+ from the ER induces a drop in ER-[Ca2+] and dissociation of Ca2+ from the EF-hand domain. This is the initial trigger for the aggregation, clustering and translocation of STIM1 to the periphery of the cells, where it accumulates in specific ER-plasma membrane junctional domains. Accumulation of STIM1 in this region can be detected and these aggregates have been referred to as punctae. It has been suggested that STIM1 interacts with and activates the channels involved in SOCE within these domains. The other critical SOCE component that was identified was the Orai family of calcium channels which includes three members, Orai1, Orai2, and Orai3. Among these, Orai1 has been most widely studied and established as the poreforming component of CRAC channels. Orai1 is a four transmembrane plasma membrane protein that is assembled in tetramers. There is convincing evidence to show that co-expression of Orai1 and STIM1 is sufficient for generating CRAC channel function [33–35]. As predicted by the contribution of TRPC1 to SOCE, it was reported that STIM1 interacts with and activates TRPC1 and TRPC3 [36–38]. When the TRPC channels are co-expressed with STIM1, there is an increase in Ca2+ entry, measured by Fura2 fluorescence assays. Further, SOC channel activity is generated under these conditions, consistent with previous studies. More importantly, it has been shown that STIM1 gates Orai1 and TRPC1 via distinct C-terminal domains [34, 39, 40]. The STIM1-SOAR domain in the C-terminus of STIM1 (also referred to as the CAD domain) interacts with Orai1 and gates the CRAC channel. In contrast, the C-terminal polybasic (KK) motif of STIM1 activates TRPC1 by an electrostatic gating mechanism that results in SOC channel activation [40, 41]. Together, these data finally validated the “conformational coupling” model for the regulation of SOCE, although the protein in the ER was established as STIM1 and not IP3R.
A very important but somewhat contentious finding was that in addition to STIM1, TRPC1 function was also dependent on functional Orai1 channels. [26, 38, 41, 42]. These studies showed that when endogenous Orai1 expression is suppressed or when functionally deficient Orai1 mutants are expressed, the increase in TRPC1-STIM1 function in response to store depletion is eliminated. Further, TRPC1 is recruited into a complex with Orai1 and STIM1 following stimulation of cells which is dependent on STIM1. Two possible explanations were suggested to explain the critical requirement of Orai1 in TRPC1 function. The first proposed that TRPC1 and Orai1 contribute to a heteromeric channel, where loss of either component leads to deficient channel function. This is inconsistent with the generation of CRAC channel activity in the absence of TRPC1. According to the second, Orai1 function leads to activation of TRPC1 via a secondary mechanism, as demonstrated in a recent report. This important study revealed that Orai1 and TRPC1 constitute distinct Ca2+ channels and do not contribute to a single channel. Suppression of TRPC1 activation or expression of the STIM1-SOAR domain leads to activation of CRAC channel current in cells that normally display SOC currents, which suggests that TRPC1 function masks the underlying Orai1-CRAC channel activity. This study further demonstrated that Orai1-mediated Ca2+ entry triggers recruitment of TRPC1 to the plasma membrane where the channel is gated by STIM1 [43]. Based on these findings, it was proposed that Orai1 is the first channel to be activated in response to store depletion and it provides a trigger pool of calcium that regulates plasma membrane recruitment of TRPC1, a critical step in TRPC activation.
Despite the close proximity of these channels to each other, TRPC1 and Orai1 contribute to the regulation of distinct downstream Ca2+-dependent cell function. TRPC1 is not required for NFAT activation, which is almost exclusively regulated by Ca2+ entering via Orai1. On the other hand TRPC1 contributes to NFκB and KCa channel activation. Thus, the physiological function of each channel appears to be quite distinct even though they both are are assembled within a protein complex, are active at the same time, and contribute to [Ca2+]i elevation induced in response to agonist-stimulation of cells and intracellular Ca2+ store depletion. Together these findings suggest the possibility that Ca2+ entry via these two channels lead to generation of distinct Ca2+ signaling microdomains. The nature of Ca2+ signals generated by them and the identities of Ca2+ sensors and mediators in the vicinity of these channels, which determine the specificity of functional regulation, remains to be elucidated. Furthermore, the physiological regulation of TRPC1 by Orai1 has also not been demonstrated as yet within the intact gland. Studies with TRPC1−/− mice suggest that TRPC1 is not redundant in salivary gland fluid secretion. Further, it is also clear that the residual Orai1 in the TRPC1−/− glands cannot sustain the activation of the ion channel mechanisms required for salivary secretion; e.g. KCa channel [12]. While the exact physiological role of Orai1 and STIM1 in exocrine gland function needs to be confirmed, a recent study showed that knockdown of TRPC1, STIM1 or Orai1 expression in isolated exocrine cell preparations led to reduced SOCE and frequency of Ca2+ oscillations [44]. Thus, these data suggest that all three proteins are required for agonist-induced Ca2+ signaling in exocrine acinar cells.
COMPARTMENTALIZATION OF Ca2+ SIGNALING IN EXOCRINE GLAND CELLS
Exocrine gland cells have a polarized architecture with distinct basal and apical regions. Apart from the morphological distinctions, these cellular regions are also functionally distinct. The primary reason for this is to achieve and facilitate a vectorial secretory process. Polarization of the structure and function necessitates targeting and compartmentalization of functionally relevant proteins to specific locations in the cells. This is primarily achieved by the assembly of functionally related proteins into complexes that are localized in specific cellular regions. Ca2+ signaling mechanisms are also similarly compartmentalized. As discussed above, the [Ca2+]i signals generated inside the cell in response to stimulation are spatially and temporally specific. This is accomplished by localizing the relevant Ca2+ signaling proteins within the cellular region where the [Ca2+]i signal is required for the regulation of cell function. Such polarized localization has been shown for almost all Ca2+ signaling proteins in exocrine acinar cells; e.g. the three IP3Rs, SERCA and PMCA pumps, GPCRs, TRPC channels, Orai channels, and STIM1 [6–9, 44–46]. Compartmentalization of these proteins launches the Ca2+ signal in the apical region of the cell and initiates the vectorial secretion, exocytosis of secretory granules or fluid and electrolyte secretion [4, 6, 7, 9]. Previous studies have demonstrated that agonist-stimulated Ca2+ signalling in exocrine gland cells is highly polarized, initiating at the apical pole and propagating to the basal pole. The Ca2+ signal evoked by physiological agonist concentrations is in the form of Ca2+ oscillations, where the Ca2+ signal is periodically repeated. Frequency and amplitude of these oscillations are determined by the intensity of receptor stimulation. Although the fundamental aspects of Ca2+ signaling are similar, the details of Ca2+ signals are specific to pancreatic and salivary cells in terms of sensitivity to agonist stimulation, frequency of the oscillations, and speed of the Ca2+ waves. In salivary glands, the Ca2+ oscillations always start at the apical pole and propagate to the basal pole in the form of Ca2+ waves. In pancreatic acinar cells, the oscillations can remain confined to the apical pole, especially following stimulation with lower levels of agonist. Interestingly, the different spatial and temporal aspects of the Ca2+ oscillations in pancreatic and parotid acinar cells [6, 10] reflect the specific Ca2+ requirements for the regulation of secretion in the two types of glands. The Ca2+ signaling pathway mediates exocytotic enzyme secretion in pancreatic acini and fluid secretion in salivary glands.
Measurements of ion flux mechanisms localized in different regions of the cell have provided insight as to why local [Ca2+]i signals might be extremely important in these cells. However, little is known about the mechanism(s) that is involved in the targeting, assembly and retention of Ca2+ signaling complexes. Examples from several other cell types, and a few from exocrine gland cells, suggest that scaffolding proteins are likely to mediate the retention of proteins within specific cellular domains. Nonetheless, it is possible that the localization of cellular organelles and their dynamic regulation, such as rearrangement or trafficking, also contribute to the pattern of Ca2+ signals in the cells. For example in pancreatic acinar cells, mitochondria cause a fire-wall effect and restrict [Ca2+]i elevations to the apical region [9, 11]. Similarly recruitment of ion channels such as TRPC1 into the plasma membrane could lead to increases in [Ca2+]i in the vicinity of ion flux components. Finally, clustering of the channels could also provides a way of increasing the amount of [Ca2+] entering a particular location in the cell. The initiation and propagation pattern of Ca2+ waves evoked by the same agonist originate from the exact same site and are propagated along the same path. Such specificity in the generation and migration of cellular Ca2+ suggests that stably localized Ca2+ signaling proteins are involved in defining the pattern of Ca2+ signals. Consistent with this suggestion, immunolocalization studies revealed that Ca2+ signaling proteins (e.g. IP3Rs) are concentrated at the apical pole of polarized cells. What is really interesting, although not completely proven as yet by experimental evidence, is that the Ca2+ signaling complex at the apical pole appears to be much more sensitive to agonist stimulation than the complexes at the basal pole since Ca2+ responses are detected in this region at very low levels of agonists. Whether this is due to differences in the components of IP3Rs per se or a consequence of spatial aspects of their assembly remains to be established.
In addition to protein microdomains, [Ca2+]i signals are also compartmentalized. A particularly interesting Ca2+ microdomain was described in a recent study in which [Ca2+]i in the sub-plasma membrane region was measured using TIRF microscopy [47]. The findings from this study revealed relatively high and sustained [Ca2+]i elevation underneath the plasma membrane when SOCE is activated. It has been suggested that the [Ca2+] near the channel pore is likely to be relatively high compared to areas of the cell that are further away from the cell. Fast diffusion in the cytosol as well as other buffering mechanisms allow the quick dissipation of the initial [Ca2+]i signal. The functional relevance of this localized high [Ca2+] signal is not yet known, although it is reasonable to suggest that it is likely utilized for regulation of specific functions such as activation of ion channels and Ca2+-dependent signaling components (e.g. CaM, calcineurin, NFAT) that are localized within the vicinity of the channel. The pattern of [Ca2+]i change is determined by the type of cell function that is activated and the Ca2+ sensor that transduces the signal to the effector protein. An example of this is evident in HSG cells, where Orai1-specific Ca2+ influx but not global Ca2+ increase accounts for the activation of NFAT Fig. (3).
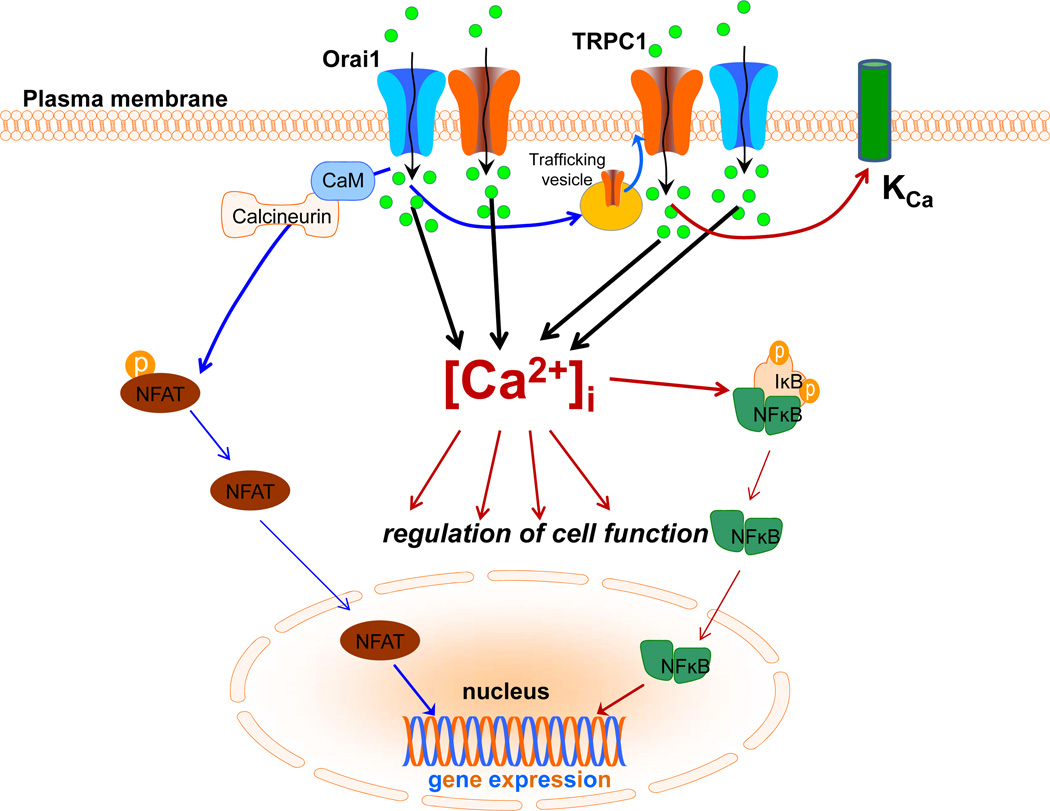
Fig. (3). Ca2+-dependent regulation of cell function.
Agonist-stimulated cytosolic calcium changes result from intracellular Ca2+ release as well as Ca2+ entry. Sustained calcium entry drives several key cellular functions including Ca2+-dependent gene expression as well as regulation of ion channel activities. In salivary gland cells, cell lines and acinar cells from mice, TRPC1-mediated Ca2+-entry is the primary determinant of sustained KCa channel activation. On the other hand, studies in cell lines show that Orai1-mediated Ca2+ entry drives NFAT activation, without any contribution of TRPC1. In contrast, NFκB activation requires contributions from both Orai1 and TRPC1.
TRPC1 and Orai1 also display spatially distinct localizations in acinar cells. TRPC1 is primarily localized in the basal and lateral regions of acinar cells while TRPC3 is detected both in the apical as well as basolateral region of the cell [12, 31, 46]. Orai1 is localized in the lateral membrane towards the apical region, although low levels of the channel could be present in the basolateral membrane region. Orai1 and TRPC1 overlap in the luminal end of the lateral membrane [43, 44]. Under resting conditions, STIM1 appears to be localized in a diffused pattern in the cell but following stimulation, it translocates to the lateral and basal regions where it co-localizes with Orai1 and TRPC channels. The localization of these key components of SOCE suggests that they can contribute to the apical and basal [Ca2+]i signals. Based on the data obtained with TRPC1−/− mice, it can be hypothesized that TRPC1 is a non-redundant component in salivary gland cells and the primary contributor of the sustained Ca2+ influx that drives fluid secretion. While Orai1 is likely to regulate TRPC1 function, a direct contribution to secretion has not yet been established. Furthermore, neither Orai1 nor any other channel appears to compensate for the loss of TRPC1 function in these cells.
Ion flux components involved in the regulation of fluid secretion are also localized within specifc cellular regions in acinar cells which are defined by their specific role in the process of fluid secretion. For example, AQP5 mediates water flow out of the cell (the final step in fluid secretion) and is located in the apical membrane. On the other hand, NKCC1 mediates influx of Cl− into the cell for its transcellular movement and is localized in the basolateral region of acinar cells, while the chloride channel involved in Cl− secretion from the cell, TMEM16A, is localized in the apical region. KCa channels are found at both locations and have a role in maintaining membrane potential. Importantly, all these ion flux pathways are regulated by agonist-dependent [Ca2+]i increases [3, 4, 7]. It can be predicted that both apical and basolateral Ca2+ signals contribute to the regulation of these ion fluxes. These include local changes in [Ca2+]i due to Ca2+ entry via calcium channels localized within the specific domains or global [Ca2+]i changes that facilitate the spread of Ca2+ from the site of Ca2+ channels to the ion flux systems. The exact local and global [Ca2+]i increases contributed by Orai1 channels that are localized in the lateral and luminal region of the cells, as well as TRPC1 channels localized in the basolateral regions, in response to stimulation of cells by agonists need to be determined. Future studies should be directed towards addressing the mechanisms which control the temporal and spatial specificity of [Ca2+]i signals and how a particular [Ca2+]i signal is decoded by the cell for regulation of specific downstream effectors.
CONCLUSIONS
In the past four decades since the identification of Ca2+ as a critical factor in salivary gland function, several key components (e.g. receptors, G proteins, phospholipase C) that are involved in generating and regulating cellular [Ca2+] signals in exocrine glands have now been identified. IP3R2 and IP3R3 have been established as the primary intracellular Ca2+ release channels involved in agonist-mediated Ca2+ mobilization. Interestingly, localization of these receptors in the apical region of the cell has been linked to the initiation of receptor-mediated intracellular Ca2+ release in this domain. The key molecular components and mechanism(s) that underlie regulation of SOCE have also been elucidated. TRPC1 is a critical contributor to SOCE and secretion in both salivary and pancreatic acinar cells. Further, Orai1 and STIM1 have been identified as central components of SOCE and are required for TRPC1 function. While STIM1 gates TRPC1, Orai1 controls its function by regulating the trafficking of TRPC1 to the plasma membrane. Important insights into the assembly of the TRPC1/STIM1/Orai1 complex, which leads to the activation of TRPC1 and Orai1 channels, have also been described. Emerging studies highlight the functional specificity of the different calcium channels within the same cell. These distinct properties are determined by the local Ca2+ signaling initiated by each channel and mediated by the compartmentalization of specific Ca2+ sensors and signaling proteins within the channel microdomains. Although the physiological relevance of Orai1 and STIM1 in the exocrine glands have yet to be described, occurrence of these two proteins in areas where TRPC1 is located suggest close functional interactions between these proteins. Together, these studies provide significant insights into the assembly and compartmentalization of these crucial Ca2+ signaling components in the exocrine gland cells and their roles in the control of polarized secretion in each gland.
ACKNOWLEDGEMENT
Declared none.
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Berridge MJ. Calcium microdomains: organization and function. Cell Calcium. 2006;40:405–412. doi: 10.1016/j.ceca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 3.Ambudkar IS. Regulation of calcium in salivary gland secretion. Crit Rev Oral Biol Med. 2000;11:4–25. doi: 10.1177/10454411000110010301. [DOI] [PubMed] [Google Scholar]
- 4.Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol. 2005;67:445–469. doi: 10.1146/annurev.physiol.67.041703.084745. [DOI] [PubMed] [Google Scholar]
- 5.Mikoshiba K, Hisatsune C, Futatsugi A, Mizutani A, Nakamura T, Miyachi K. The role of Ca2+ signaling in cell function with special reference to exocrine secretion. Cornea. 2008;27(Suppl 1):S3–S8. doi: 10.1097/ICO.0b013e31817f246e. [DOI] [PubMed] [Google Scholar]
- 6.Yule DI. Subtype-specific regulation of inositol1,4,5-trisphosphate receptors: controlling calcium signals in time and space. J Gen Physiol. 2001;117:431–434. doi: 10.1085/jgp.117.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee MG, Xu X, Zeng W, Diaz J, Wojcikiewicz RJ, Kuo TH, Wuytack F, Racymaekers L, Muallem S. Polarized expression of Ca2+ channels in pancreatic and salivary gland cells. Correlation with initiation and propagation of [Ca2+]i waves. J Biol Chem. 1997;272:15765–15770. doi: 10.1074/jbc.272.25.15765. [DOI] [PubMed] [Google Scholar]
- 8.Kiselyov K, Wang X, Shin DM, Zang W, Muallem S. Calcium signaling complexes in microdomains of polarized secretory cells. Cell Calcium. 2006;40:451–459. doi: 10.1016/j.ceca.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Petersen OH, Tepikin AV. Polarized calcium signaling in exocrine gland cells. Annu Rev Physiol. 2008;70:273–299. doi: 10.1146/annurev.physiol.70.113006.100618. [DOI] [PubMed] [Google Scholar]
- 10.Sneyd J, Tsaneva-Atanasova K, Bruce JI, Straub SV, Giovannucci DR, Yule DI. A model of calcium waves in pancreatic and parotid acinar cells. Biophys J. 2003;85:1392–1405. doi: 10.1016/S0006-3495(03)74572-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruce JI, Giovannucci DR, Blinder G, Shuttleworth TJ, Yule DI. Modulation of [Ca2+]i signaling dynamics and metabolism by perinuclear mitochondria in mouse parotid acinar cells. J Biol Chem. 2004;279:12909–12917. doi: 10.1074/jbc.M309070200. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Cheng KT, Bandyopadhyay BC, Pani B, Dietrich A, Paria BC, Swaim WD, Beech D, Yildrim E, Singh BB, Birnbaumer L, Ambudkar IS. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1(−/−) mice. Proc Natl Acad Sci U S A. 2007;104:17542–17547. doi: 10.1073/pnas.0701254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Putney JW., Jr Capacitative calcium entry revisited. Cell Calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- 14.Putney JW. Capacitative calcium entry: from concept to molecules. Immunol Rev. 2009;231:10–22. doi: 10.1111/j.1600-065X.2009.00810.x. [DOI] [PubMed] [Google Scholar]
- 15.Putney JW, Jr, Broad LM, Braun FJ, Lievremont JP, Bird GS. Mechanisms of capacitative calcium entry. J Cell Sci. 2001;114:2223–2229. doi: 10.1242/jcs.114.12.2223. [DOI] [PubMed] [Google Scholar]
- 16.Putney JW., Jr Identification of cellular activation mechanisms associated with salivary secretion. Annu Rev Physiol. 1986;48:75–88. doi: 10.1146/annurev.ph.48.030186.000451. [DOI] [PubMed] [Google Scholar]
- 17.Putney JW, Jr, Weiss SJ. Relationship between receptors, calcium channels, and responses in exocrine gland cells. Methods Cell Biol. 1981;23:503–511. doi: 10.1016/s0091-679x(08)61516-2. [DOI] [PubMed] [Google Scholar]
- 18.Streb H, Heslop JP, Irvine RF, Schulz I, Berridge MJ. Relationship between secretagogue-induced Ca2+ release and inositol polyphosphate production in permeabilized pancreatic acinar cells. J Biol Chem. 1985;260:7309–7315. [PubMed] [Google Scholar]
- 19.Mikoshiba K. The IP3 receptor/Ca2+ channel and its cellular function. Biochem Soc Symp. 2007;74:9–22. doi: 10.1042/BSS0740009. [DOI] [PubMed] [Google Scholar]
- 20.Li Q, Luo X, Muallem S. Functional mapping of Ca2+ signaling complexes in plasma membrane microdomains of polarized cells. J Biol Chem. 2004;279:27837–27840. doi: 10.1074/jbc.C400184200. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura T, Matsui M, Uchida K, Futatsugi A, Kusakawa S, Matsumoto N, Nakamura K, Manabe T, Taketo MM, Mikoshiba K. M(3) muscarinic acetylcholine receptor plays a critical role in parasympathetic control of salivation in mice. J Physiol. 2004;558:561–575. doi: 10.1113/jphysiol.2004.064626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gautam D, Heard TS, Cui Y, Miller G, Bloodworth L, Wess J. Cholinergic stimulation of salivary secretion studied with M1 and M3 muscarinic receptor single- and double-knockout mice. Mol Pharmacol. 2004;66:260–267. doi: 10.1124/mol.66.2.260. [DOI] [PubMed] [Google Scholar]
- 23.Futatsugi A, Nakamura T, Yamada MK, Ebisui E, Nakamura K, Uchida K, Kitaguchi T, Takahashi-Iwanaga H, Noda T, Aruga J, Mikoshiba K. IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science. 2005;309:2232–2234. doi: 10.1126/science.1114110. [DOI] [PubMed] [Google Scholar]
- 24.Birnbaumer L, Zhu X, Jiang M, Boulay G, Peyton M, Vannier B, Brown D, Platano D, Sadeghi H, Stefani E, Birnbaumer M. On the molecular basis and regulation of cellular capacitative calcium entry: roles for Trp proteins. Proc Natl Acad Sci U S A. 1996;93:15195–151202. doi: 10.1073/pnas.93.26.15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montell C. The TRP superfamily of cation channels. Sci STKE. 2005;(272):re3. doi: 10.1126/stke.2722005re3. (2005) [DOI] [PubMed] [Google Scholar]
- 26.Birnbaumer L. The TRPC class of ion channels: a critical review of their roles in slow, sustained increases in intracellular Ca(2+) concentrations. Annu Rev Pharmacol Toxicol. 2009;49:395–426. doi: 10.1146/annurev.pharmtox.48.113006.094928. [DOI] [PubMed] [Google Scholar]
- 27.Liu X, Wang W, Singh BB, Lockwich T, Jadlowiec J, O'Connell B, Wellner R, Zhu MX, Ambudkar IS. Trp1, a candidate protein for the store-operated Ca(2+) influx mechanism in salivary gland cells. J Biol Chem. 2000;275:3403–3411. doi: 10.1074/jbc.275.5.3403. [DOI] [PubMed] [Google Scholar]
- 28.Ambudkar IS. Ca2+ signaling microdomains:platforms for the assembly and regulation of TRPC channels. Trends Pharmacol Sci. 2006;27:25–32. doi: 10.1016/j.tips.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Ambudkar IS. TRPC1: a core component of store-operated calcium channels. Biochem Soc Trans. 2007;35:96–100. doi: 10.1042/BST0350096. [DOI] [PubMed] [Google Scholar]
- 30.Ambudkar IS, Ong HL, Liu X, Bandyopadhyay BC, Cheng KT. TRPC1: the link between functionally distinct store-operated calcium channels. Cell Calcium. 2007;42:213–223. doi: 10.1016/j.ceca.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Kim MS, Hong JH, Li Q, Shin DM, Abramowitz J, Birnbaumer L, Muallem S. Deletion of TRPC3 in mice reduces store-operated Ca2+ influx and the severity of acute pancreatitis. Gastroenterology. 2009;137:1509–1517. doi: 10.1053/j.gastro.2009.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 34.Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–890. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 36.Worley PF, Zeng W, Huang GN, Yuan JP, Kim JY, Lee MG, Muallem S. TRPC channels as STIM1-regulated store-operated channels. Cell Calcium. 2007;42:205–211. doi: 10.1016/j.ceca.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, Gill DL, Ambudkar IS. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx: evidence for similarities in store-operated and calcium release-activated calcium channel components. J Biol Chem. 2007;282:9105–9116. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as storeoperated channels. Nat Cell Biol. 2007;9:636–645. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeng W, Yuan JP, Kim MS, Choi YJ, Huang GN, Worley PF, Muallem S. STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol Cell. 2008;32:439–448. doi: 10.1016/j.molcel.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009;11:337–343. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee KP, Yuan JP, Hong JH, So I, Worley PF, Muallem S. An endoplasmic reticulum/plasma membrane junction: STIM1/Orai1/TRPCs. FEBS Lett. 2010;584:2022–2027. doi: 10.1016/j.febslet.2009.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng KT, Liu X, Ong HL, Ambudkar IS. Functional requirement for Orai1 in store operated TRPC1-STIM1 channels. J Biol Chem. 2008;283:12935–12940. doi: 10.1074/jbc.C800008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng KT, Liu X, Ong HL, Swaim W, Ambudkar IS. Local Ca2+ entry via Orai1 regulates plasma membrane recruitment of TRPC1 and controls cytosolic Ca2+ signals required for specific cell functions. PLoS Biol. 2011;9:e1001025. doi: 10.1371/journal.pbio.1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong JH, Li Q, Kim MS, Shin DM, Feske S, Birnbaumer L, Cheng KT, Ambudkar IS, Muallem S. Polarized but Differential Localization and Recruitment of STIM1, Orai1 and TRPC Channels in Secretory Cells. 2011;12:232–245. doi: 10.1111/j.1600-0854.2010.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lur G, Haynes LP, Prior IA, Gerasimenko OV, Feske S, Petersen OH, Burgoyne RD, Tepikin AV. Ribosome-free terminals of rough ER allow formation of STIM1 puncta and segregation of STIM1 from IP(3) receptors. Curr Biol. 2009;19:1648–1653. doi: 10.1016/j.cub.2009.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bandyopadhyay BC, Swaim WD, Liu X, Redman RS, Patterson RL, Ambudkar IS. Apical localization of a functional TRPC3/TRPC6-Ca2+-signaling complex in polarized epithelial cells. Role in apical Ca2+ influx. J Biol Chem. 2005;280:12908–12916. doi: 10.1074/jbc.M410013200. [DOI] [PubMed] [Google Scholar]
- 47.Won JH, Yule DI. Measurement of Ca2+ signaling dynamics in exocrine cells with total internal reflection microscopy. Am J Physiol Gastrointest Liver Physiol. 2006;291:G146–G155. doi: 10.1152/ajpgi.00003.2006. [DOI] [PubMed] [Google Scholar]