Abstract
Objectives
Without resistance tests, deciding which patients with virological failure should switch to second-line antiretroviral therapy (ART) is difficult. The factors influencing this decision are poorly understood. We assess predictors of switching regimens following virological failure.
Design
Retrospective cohort study using clinical data from a South African ART programme with 6-monthly viral load (VL) monitoring
Methods
We constructed a dataset of patient visits occurring following first-line virological failure, and used random effects logistic regression (accounting for individual- and clinic-level clustering) to assess predictors of switching at each visit.
Results
1668 patients with virological failure (73% male, mean age 41yrs, median CD4 184 cells/mm3, mean log10VL 4.3) contributed 1922 person-years of viraemia. 12 months following failure, the cumulative incidence of switching regimen, viral re-suppression or death was 16.9%, 13.2% and 4.6% respectively. In adjusted analysis, switching was more likely at the third or subsequent visit following failure; in visits occurring in 2008 vs.2003-2007; and in patients with ART-experience pre-programme, current high VL or low CD4 count. Switching was less likely in patients with no clinic contact for 4 months, or declining VL. Switching rates varied between clinics with clinic-level clustering evident in the final model (p<0.001).
Conclusion
Despite 6-monthly virological monitoring and recommendations to switch following adherence interventions and confirmed viraemia, patients experienced delayed switching. Individual-level covariates influenced switching but did not account for variable switching rates between clinics, suggesting differences in guideline implementation. In certain circumstances delays may be warranted; however understanding barriers to guideline implementation will limit unnecessary delays.
Keywords: Virological failure, switching, second-line antiretroviral therapy, South Africa, predictors
Introduction
Patients with antiretroviral treatment (ART) failure are increasingly encountered in resource-limited settings.1-3 Rates of switching to second-line ART are low and concerns have been raised that patients may be experiencing long periods with virologic failure.4-5
In settings without access to resistance tests more evidence is needed to refine the monitoring strategy for detecting treatment failure and switching regimens.6 Laboratory monitoring (CD4 count +/− viral load [VL]) improves immune recovery7 and reduce long-term morbidity or mortality;8-9 however the optimal use of these tests is unclear.9-10 Virological monitoring may conserve first-line regimens by detecting non-adherence before viral resistance develops,11 limiting resistance accumulation through earlier detection of virological failure,12-13 and reducing transmission of resistant virus.14 While late switching, with complex resistance, appears to have minimal effect on early second-line outcomes, the role of virological monitoring in determining longer-term outcomes is yet to be determined.15 Resistance, however, may not be the primary aetiology in all patients with virological failure and switching early, without adequately excluding non-adherence, could rapidly exhaust available treatment options and drive up programme costs.16 The optimal adherence support, monitoring frequency, acceptable duration of viraemia, and virological threshold at which to switch regimens, whether from a clinico-immunological, resistance or cost-effectiveness perspective, are ill-defined.12, 14, 17-22
The World Health Organization (WHO) provides pragmatic recommendations; in countries without resistance tests but with routine virological monitoring, adherence interventions should be instigated following a first raised VL. Patients considered adherent with confirmed VL >5000 copies/ml should switch regimens.23 Few studies have described programmatic practice of this approach.5, 24-25 In South Africa, the country with the world’s largest antiretroviral programme, six-monthly virological monitoring is the standard of care, routine resistance testing is not available and current national guidelines (2010) advocate switching at a VL threshold of 1000 copies/ml.26 In a multi-site treatment programme we aimed to describe rates of virological failure, subsequent outcomes, and factors associated with a decision to switch to second-line ART during the viraemic episode.
Methods
Study design and setting
This observational retrospective cohort study used prospectively-collected clinical data from the Aurum Institute ART programme, South Africa. The workplace component comprised 56 clinics serving employees of large, predominantly mining companies. The community programme had 81 urban and peri-urban private general practitioner and non-government organization clinics serving patients with limited resources. In both programmes HIV-related treatment was provided free of charge.
Ethics statement
This study was approved by the Research Ethics Committees of the University of KwaZulu Natal, South Africa and the London School of Hygiene and Tropical Medicine, UK. Access to the programme database was granted by Aurum Institute. This database contains clinical data collected as part of routine clinical care for the purposes of monitoring and evaluation. The workplace employers also provide additional data on reasons for leaving the programme through employers’ records and hospital death registers, and dates of death are confirmed through programme links with the National Death Register. The Research Ethics Committees approval of this study waived the need for patient consent as data had been collected as part of routine practice, and all data we received was both retrospective and anonymised.
Clinical management
In the workplace, patients were eligible for first-line ART (efavirenz [EFV] or nevirapine [NVP] with zidovudine [AZT], lamivudine [3TC] until 2008, then tenofovir / emtricitabine thereafter) if CD4 ≤250 cells/mm3, WHO stage IV, or CD4 ≤350 cells/mm3 plus WHO stage III. In the community programme, eligibility criteria for first-line ART (NVP or EFV with stavudine [d4T], 3TC) were WHO stage IV or CD4 ≤200 cells/mm3. Treatment monitoring involved three-monthly visits, with CD4 count and VL monitored at baseline, six weeks and six-monthly intervals thereafter. Programme guidelines recommended intensifying adherence counselling following a first raised VL with healthcare workers trained in identifying appropriate, patient-centred, interventions e.g. treatment partners, medication alarms. If subsequent VL remained high a switch to second-line ART was recommended; the threshold triggering a switch changed from 5000 copies/ml to 1000 copies/ml over the course of the programme. Second-line ART in the workplace programme comprised abacavir (ABC), didanosine (ddI), boosted lopinavir (bLPV), and in the community programme AZT, ddI and bLPV. All community clinics were doctor-led; however some workplace clinics were nurse-led with doctors consulted concerning complications, including virological failure.
Study population
Patients ≥15 years old commencing first-line ART between 1/1/2003 and 31/12/2008, and with at least six months of follow-up were eligible for inclusion. Clinics with <50 patients on first-line ART were excluded.
Data management
At each visit, healthcare workers used standardised data collection forms to record clinical and programme data, e.g. WHO staging diagnoses, adverse events, prescriptions, reported reasons for leaving the programme. Self-reported adherence was recorded as part of routine data collection; however no data was collected on adherence interventions implemented as part of the adherence support package. Data were entered into a central database with laboratory data transferred electronically. Where civil identification numbers were available, deaths were verified through the National death register; and in the workplace, through employment records and hospital death registers.
Outcomes
Virological failure on first-line ART was defined as the second of two consecutive (≤9 months apart) measurements, both >1000 copies/ml and occurring ≥6 months after commencing therapy. Patients were defined as having viral re-suppression if a subsequent VL measurement on first-line ART was <400 copies/ml, and to have switched to second-line ART if a protease inhibitor [PI]-based regimen was prescribed. If the patient died within three months of the last visit, death was determined to be the primary reason for loss to the programme. Patients with no clinic contact for ≥6 months, and with no known reason for leaving the programme, were defined as lost to follow-up three months after their last visit.
Statistical analysis
Descriptive cohorts
Rates of virological failure, overall and by clinic, were described for patients commencing first-line ART. Analysis was restricted to patients with ≥1 VL measurement after six months of first-line ART with follow-up right censored at earliest of first episode of virological failure, death, loss to programme or end of follow-up (31/12/2009, defined as administrative censoring). Analyses of viral re-suppression and switching to second-line ART following first virological failure were restricted to patients with at least one episode of virological failure prior to 30/6/2009, allowing at least six months potential follow-up for events to occur. Patients entered this analysis on date of second consecutive VL measurement >1000 copies/ml with follow-up right-censored at earliest of event of interest, death, loss to programme or end of follow-up (31/12/2009). In determining rates of switching, follow-up was right censored on date of re-suppression (and vice-versa). Taking account of their competing risks, the cumulative incidence at 12 months of switching regimens, and viral re-suppression and death on first-line ART were described.27
Visit dataset
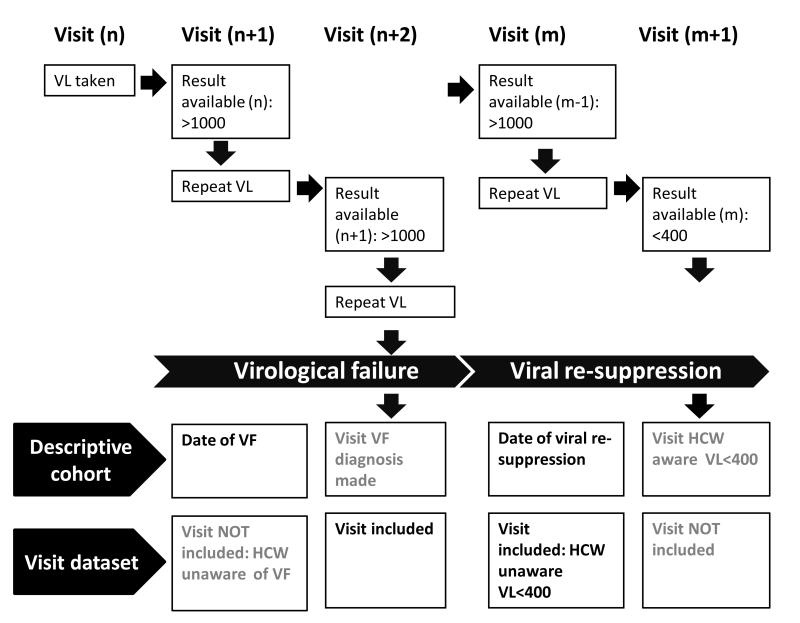
for patients developing virological failure prior to 31/12/2009, factors associated with switching to second-line ART at the next, and subsequent, clinic visits were assessed by constructing a visit dataset. Patients with ≥1 visit following first or subsequent episodes of virological failure were eligible for inclusion. Visits of interest were those at which the healthcare worker believed the patient to be viraemic and made a decision to continue first-line ART or switch to second-line ART (figure 1); thus we included visits occurring between >3 days following date of virological failure to ≤3 days following date of viral re-suppression on first-line ART. Visits after date of switching to second-line ART were excluded. Our rationale was based on discussions with healthcare workers, who reported that a minimum of three days were required for samples to get to the laboratory, be processed and for results to be made available. Therefore in the 3 days following venesection healthcare workers would be unaware that the patient had fulfilled a definition of virological failure or achieved viral re-suppression.
Figure 1. Definitions used in the construction of the descriptive cohort and visit dataset.
Diagram illustrating the relationship between clinic visits and a) definitions of date of virological failure and date of viral re-suppression used in the descriptive cohort and b) eligibility of visits for inclusion in the visit dataset
Abbreviations: VL, viral load; VF, virological failure; HCW, healthcare worker
A switch to second-line ART was defined as the first visit a PI-regimen was prescribed. Covariates were updated using information available to the clinician at the time of the visit. VL and CD4 count were defined as the closest available result from a sample taken 7 months to 3 days before the visit. Change in VL and CD4 count as percentage change in absolute values between the last two available results (within 2-28 weeks of each other, and >3 days before the visit). Duration of viraemia as time between second VL >1000 copies/ml and date of visit. Change in weight between last and current appointment, where appointments were greater than one week apart was measured in kg/month. Patients with no clinic contact in preceding 4 months were defined as having delayed clinic attendance. For each clinic, the clinics’ rate of virological failure, as described above, was used as proxy measure of their experience in managing virological failure.
Logistic regression, using random effects to control for individual- and clinic-level clustering, was used to explore predictors of switching to second-line ART at any given visit. A backward stepwise approach was taken with covariates included and retained in the multivariable model if p<0.2 (likelihood ratio test [LRT]). Co-linearity was assessed by comparing standard errors between successive models. To explore clinic-level clustering we looked to see if clinic factors, although not associated with switching in the univariable analysis, would account for clinic-level clustering in the final model.
Sensitivity analyses were performed with the final model restricted to patients known to be ART-naïve on commencing ART, and to those with virological suppression prior to failure; and also stratified by time between the visit and diagnosis of virological failure (<12 vs. ≥12 months), and programme. Statistical analyses were performed using Stata version 11 (STATA Corporation, College Station, TX)
Results
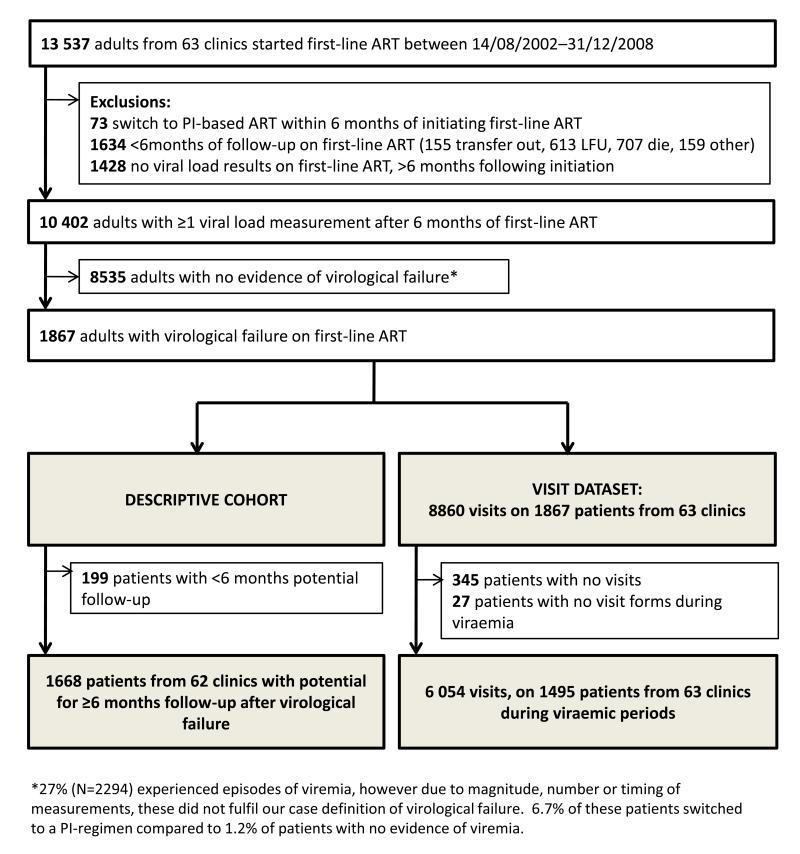
In total, 10,402 out of 13,537 patients starting first-line ART had at least one VL measurement after six months of first-line ART (median 5; inter-quartile range [IQR], 4-7). Of these, 1867 (17.9%) patients (1146 (31.1%) from 12 workplace clinics and 721 (10.7%) from 51 community clinics) experienced virological failure on first-line ART, over a median of 2.6 years (IQR: 1.69-3.51) from initiating ART to leaving programme or administrative censoring (figure 2). The first episode occurred a median of 1.4 years (range: 1.1-2.0) after commencing ART.
Figure 2. Flow chart illustrating the construction of a) descriptive cohort and b) visit dataset.
Abbreviations: ART, antiretroviral therapy; PI, protease inhibitor; LFU, lost to follow-up
Rates of virological failure varied between clinics (median clinic rate 4.8/100py [IQR: 3.3-7.7]), with the median clinic rate in the larger clinics (>500 patients initiated first-line ART, n=7) twice that seen in smaller clinics (<500 patients, n=56); 10.6/100py and 4.6/100py respectively. Accounting for clinic-level clustering by the use of robust standard errors, the overall rate of virological failure in first-line ART was 7.4/100py (95% confidence interval [CI]: 5.8-9.5); 6.8/100py (95% CI: 5.1-9.1) between 6-12 months, 14.1/100py (95% CI: 10.7-18.6) between 12-24 months, and 6.2/100py (95% CI: 4.8-8.1) thereafter. The median time between the first and second raised VL was 4.9 months (IQR: 2.8-6.1).
The characteristics of patients with virological failure and clinics are presented in table 1 and webappendix table 1, respectively. The majority of patients were male (73.1%) with a median age at virological failure of 41 years and median CD4 count 184 cells/mm3. 12 workplace clinics (4 hospital out-patients and 8 occupational health clinics) and 51 community clinics (49 general practitioners, 3 non-government organisation clinics and 1 specialist clinic) were included in the study. 59 clinics started enrolling patients on first-line ART between 2003-2006 and 4 clinics between 2007-2008. On conducting the study 7 clinics had enrolled >500 patients on first-line ART, 7 clinics 250-499 patients and 49 clinics 50-249 patients. 7 clinics had switched >7.5% of patients to second-line ART, 8 clinics 5-7.5%, 16 clinics 2.5-4.9%, 16 clinics <2.5% and in 11 clinics no patients had switched to second-line ART.
Table 1. Characteristics of patients at first episode of virological failure on first-line ART.
| Patients with virological failure on first-line ART, N (%) |
|||||
|---|---|---|---|---|---|
| All patients N=1867 |
Descriptive cohort (>6 months follow-up after VF) N=1668 |
Visit dataset (≥1 visit after VF) N=1495 |
|||
| Programme | Workplace | 1146 (61.4) | 1060 (63.6) | 944 (63.1) | |
| Community | 721 (38.6) | 608 (36.4) | 551 (36.8) | ||
|
| |||||
| Male | 1364 (73.1) | 1238 (74.2) | 1105 (73.9) | ||
|
| |||||
|
Exposure to ART pre-
programme |
Naïve | 1215 (65.1) | 1098 (65.8) | 977 (65.4) | |
| Experienced | 186 (10.0) | 165 (9.9) | 150 (10.0) | ||
| Unknown | 466 (25.0) | 405 (24.3) | 368 (24.6) | ||
|
| |||||
|
Episodes of virological
failure on first-line ART |
1 | 1797 (96.3) | 1598 (95.8) | 1426 (95.4) | |
| 2 | 68 (3.6) | 68 (4.1) | 67 (4.5) | ||
| 3 | 2 (0.1) | 2 (0.1) | 2 (0.1) | ||
|
| |||||
| Months between 1st and 2nd VL, (median; IQR) | 4.86 (2.8-6.1) | 4.83 (2.8-6.1) | 4.83 (2.8-6.1) | ||
|
| |||||
| At time of first virological failure | |||||
| Calendar year | 2003-2007 | 919 (49.2) | 919 (55.1) | 820 (54.9) | |
| 2008 | 531 (28.4) | 531 (31.8) | 430 (28.8) | ||
| 2009 | 417 (22.3) | 218 (13.1) | 245 (16.4) | ||
|
|
|||||
| Age, years (mean; range) | 41.56 (17.2-71.3) | 41.64 (17.2-71.3) | 41.76 (17.2-68.5) | ||
|
|
|||||
| Viral suppression prior to virological failure | 1181 (63.3) | 1046 (62.7) | 959 (64.1) | ||
|
|
|||||
| CD4, cells/mm3 (median; IQR), N=1840/1486 | 184 (108-279) | 183 (106-277) | 187 (112-279) | ||
|
|
|||||
| Log10 VL, (mean, range) | 4.29 (3.0-5.7) | 4.30 (3.0-5.7) | 4.27 (3.0-5.7) | ||
|
| |||||
| First visit following first episode of virological failure | N=1493 1 | ||||
|
Duration of current
viraemic episode (months) |
<6 | 1320 (88.4) | |||
| 6-11.9 | 134 (9.0) | ||||
| 12-18 | 27 (1.8) | ||||
| >18 | 12 (0.8) | ||||
|
|
|||||
| No clinic contact in 4 months preceding visit | 235 (15.7) | ||||
|
|
|||||
| CD4, cells/mm3(median; IQR), N=1421 | 184 (111-277) | ||||
|
|
|||||
|
CD4 count slope preceding visit, N=1222 |
≥50% increase | 251 (20.5) | |||
| No change | 591 (48.4) | ||||
| ≥50% decline | 380 (31.1) | ||||
|
|
|||||
| Log10 VL, (mean, range), N=1425 | 4.28 (2.6-5.7) | ||||
|
|
|||||
|
VL slope preceding visit, N=1235 |
≥50% increase | 375 (30.4) | |||
| No change | 431 (34.9) | ||||
| ≥50% decline | 429 (34.7) | ||||
|
|
|||||
|
Change in weight between last and current visit, N=1268 |
Loss ≥2kg/month | 78 (6.2) | |||
| Weight stable | 1112 (87.7) | ||||
| Gain ≥2kg/month | 78 (6.1) | ||||
|
| |||||
|
Outcome at end of
follow-up |
Dead | 145 (7.8) | 141 (8.5) | 111 (7.4) | |
| Lost to follow-up | 399 (21.4) | 399 (23.9) | 280 (18.7) | ||
| Transferred out | 80 (4.3) | 79 (4.7) | 66 (4.4) | ||
| Other | 94 (5.0) | 92 (5.5) | 68 (4.6) | ||
| Alive in care, 1st-line ART | 858 (46.0)2a | 673 (40.4)2b | 694 (46.4)2c | ||
| Alive in care, 2nd-line ART | 291 (15.6)3a | 284 (17.0)3b | 276 (18.5)3c | ||
Two patients had no visits during first episode of VF, however visits were documented following during subsequent episodes;
20.2% VL<400 copies/ml;
24.5% VL<400 copies/ml;
22.6% VL<400 copies/ml;
3.8% VL<400 copies/ml;
3.9% VL<400 copies/ml;
3.6% VL<400 copies/ml;
Abbreviations: VF, virological failure; IQR, inter-quartile range
Outcomes in patients with first-line virological failure
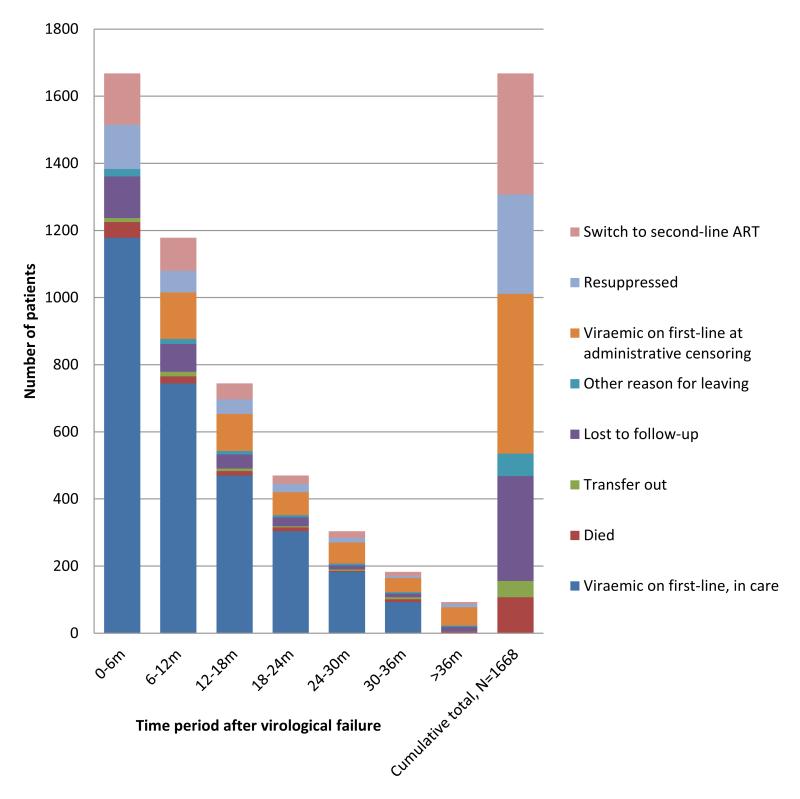
1668 (89.3%) patients had at least six months’ follow-up following a second VL >1000 copies/ml (median 1.6 years [IQR: 0.8-2.9]). Over 1921.8 person-years of first-line virological failure, 296 (17.7%) patients achieved viral re-suppression, 361 (21.6%) switched to second-line ART, 107 (6.4%) died, 312 (18.7%) were lost to follow-up, 49 (2.9%) transferred out, 67 (4.0%) left programme for other reasons, and 476 (28.5%) were administratively censored after ≥6 months of viraemia (figure 3). Taking account of competing risks, at 12 months the cumulative incidence of switching to second-line ART, viral re-suppression and death was 16.9%, 13.2% and 4.6% respectively.
Figure 3. Outcomes in patients following first episode of virological failure (2 consecutive viral loads >1000 copies/ml) in a multi-site workplace and community programme in South Africa.
Following failure the rate of viral re-suppression on first-line ART was 15.4/100py (95%CI: 11.7-20.2); highest in the first year 16.7/100py (95%CI: 12.4-22.4), falling to 14.1/100py (95%CI: 11.0-18.2) in the second year and 11.8/100py (95%CI: 7.4-18.8) thereafter. 60 patients had at least one further episode of first-line virological failure. The rate of switching following failure was 18.8/100py (95%CI: 16.9-20.9); highest in the first year 21.3/100py (95%CI: 13.5-33.4), falling to 14.8/100py (95%CI: 8.1-26.7) in the second year, and 15.0/100py (95%CI: 6.3-35.8) thereafter. Overall rates of re-suppression and rates of switching varied by clinic: 10.62/100py (IQR: 0-25.02) and 16.98/100py (IQR: 2.8-49.73) respectively.
Predictors of switching to second-line ART
345 (18.5%) patients had no recorded visits following virological failure (156 administrative censoring, 117 lost to follow-up, 31 died, 27 other, 14 transferred out). 8860 visits were recorded on the remaining 1522 patients, from 63 clinics. 2806 visits occurred during periods of re-suppression, or following switch to second-line ART, resulting in 6054 visits on 1495 patients from 63 clinics in the visit dataset (figure 1). Visits occurred either during one episode (n=1441 patients), two episodes (n=52 patients), or three episodes (n=2 patients) of virological failure, with a median of 3 visits/episode (IQR: 2-5). Two patients had no visits recorded during the first episode of virological failure; however visits were recorded during subsequent episodes. 88.4% (1320/1493) of patients visited the clinic within six months of date of first virological failure (table 1). During the viremic episodes, the median time-interval between visits was 2.8 months (IQR: 1.4-3.5).
There was strong evidence of within-clinic clustering for switching outcome (LRT, p<0.01), with weak evidence of within-individual clustering (LRT, p=0.08). Random effect analyses which accounted for both clinic- and individual-level clustering did not differ from analyses accounting for clinic-level clustering alone therefore the simpler models are presented. On univariable analysis, switching was associated with ART exposure pre-programme, calendar year of visit, visit number during viremic episode, delayed clinic attendance, current CD4 and VL result, and CD4 and VL trend preceding the visit (table 2).
Table 2. Factors associated with switching to second-line ART at visits following virological failure: univariable and multivariable analysis.
| Visits where decision to switch made/all visits (%) |
OR (95% CI) 6054 visits; 1495 patients; 63 clinics |
P (LRT) | aOR1 (95% CI) 4630 visits; 1335 patients; 60 clinics |
P (LRT) | ||
|---|---|---|---|---|---|---|
| Sex | Male | 233/4704 (4.9) | 1 | 0.82 | ||
| Female | 130/1350 (9.6) | 1.04 (0.76-1.41) | ||||
|
| ||||||
| Age at visit (years) | ≤30 | 36/415 (8.7) | 1 | 0.65 | ||
| 31-40 | 136/1714 (7.9) | 1.06 (0.7-1.59) | ||||
| 41-50 | 131/2333 (5.9) | 1.14 (0.75-1.74) | ||||
| >50 | 60/1692 (3.5) | 0.92 (0.57-1.5) | ||||
|
| ||||||
| Programme | Workplace | 174/4246 (4.1) | 1 | 0.24 | ||
| Community | 189/1808 (10.4) | 1.56 (0.74-3.29) | ||||
|
| ||||||
|
ART exposure pre-
programme |
Naïve | 225/4034 (5.6) | 1 | 0.03 | 1 | <0.01 |
| Experienced | 60/591 (10.2) | 1.45 (1.03-2.04) | 1.65 (1.11-2.46) | |||
| Missing | 78/1429 (5.5) | 0.86 (0.64-1.17) | 0.77 (0.53-1.11) | |||
|
| ||||||
| Year of visit | 2003-7 | 117/2428 (4.8) | 1 | <0.01 | 1 | <0.01 |
| 2008 | 148/1737 (8.5) | 1.55 (1.17-2.04) | 1.69 (1.2-2.38) | |||
| 2009 | 98/1889 (5.2) | 0.96 (0.71-1.3) | 1.11 (0.77-1.61) | |||
|
| ||||||
|
Viral suppression prior to
current episode of VF |
No | 140/2077 (6.7) | 1 | 0.37 | ||
| Yes | 223/3977 (5.6) | 0.9 (0.71-1.13) | ||||
|
| ||||||
|
Duration of current
viraemic episode (months) |
0-6 | 150/2399 (6.3) | 1 | 0.21 | ||
| 6.1-12 | 100/1494 (6.7) | 1.1 (0.83-1.45) | ||||
| 12.1-18 | 48/900 (5.3) | 1.09 (0.77-1.56) | ||||
| >18 | 65/1261 (5.2) | 1.43 (1.03-1.98) | ||||
|
| ||||||
|
Visit number during
episode of virological failure |
1-2 | 168/2731 (6.2) | 1 | <0.01t | 1 | <0.01t |
| 3-4 | 99/1505 (6.6) | 1.35 (1.03-1.77) | 0.69d | 1.45 (1.05-2.02) | 0.23d | |
| ≥5 | 96/1818 (5.3) | 1.65 (1.23-2.19) | 1.88 (1.32-2.68) | |||
|
| ||||||
|
Log10 VL result available at visit, 5403 visits; 1459 patients; 61 clinics |
<3 | 4/202 (2.0) | 0.41 (0.14-1.17) | 0.55 (0.18-1.62) | ||
| 3-3.99 | 84/1945 (4.3) | 1 | <0.01 | 1 | <0.01 | |
| 4-4.99 | 181/2269 (8.0) | 2.81 (2.09-3.77) | 2.49 (1.73-3.59) | |||
| ≥5 | 72/987 (7.29) | 2.87 (2.0-4.13) | 1.94 (1.21-3.12) | |||
|
| ||||||
|
VL slope preceding visit 4659 visits; 1338 patients; 60 clinics |
≥50% increase | 104/1379 (7.5) | 1.19 (0.88-1.6) | 1.04 (0.76-1.43) | ||
| No change | 112/1650 (6.8) | 1 | <0.01 | 1 | 0.09 | |
| ≥50% decline | 64/1630 (3.9) | 0.51 (0.37-0.72) | 0.71 (0.49-1.01) | |||
|
| ||||||
|
CD4 result available at visit (cells/mm3) 5444 visits; 1456 patients; 61 clinics |
>350 | 25/664 (3.8) | 1 | <0.01t | 1 | <0.01t |
| 200-350 | 84/1708 (4.9) | 1.39 (0.86-2.25) | 0.42 d | 1.15 (0.67-1.98) | 0.13d | |
| 100-199 | 117/1844 (6.3) | 1.98 (1.24-3.15) | 1.31 (0.76-2.26) | |||
| <100 | 116/1228 (9.5) | 3.68 (2.29-5.92) | 2.47 (1.4-4.35) | |||
|
| ||||||
|
CD4 slope preceding visit 4689 visits; 1328 patients; 60 clinics |
≥50% increase | 32/850 (3.76) | 0.63 (0.41-0.94) | |||
| No change | 138/2455 (5.62) | 1 | <0.01 | |||
| ≥50% decline | 107/1384 (7.73) | 1.57 (1.18-2.07) | ||||
|
| ||||||
|
Weight change between last and current visit 5165 visits; 1384 patients; 60 clinics |
Loss ≥2kg/m | 14/406 (3.5) | 1.27 (0.7-2.31) | |||
| No change | 116/4351 (2.7) | 1 | 0.62 | |||
| Gain ≥2kg/m | 12/408 (2.9) | 1.23 (0.65-2.32) | ||||
|
| ||||||
|
Clinic contact in 4 months
preceding visit |
Yes | 335/5351 (6.3) | 1 | <0.01 | 1 | <0.01 |
| No | 28/703 (4.0) | 0.4 (0.27-0.61) | 0.31 (0.17-0.59) | |||
|
| ||||||
| Size of clinic at time of visit | <50 | 19/208 (9.1) | 1 | 0.47 | ||
| 50-250 | 150/1935 (7.8) | 0.84 (0.4-1.78) | ||||
| 250-500 | 121/2471 (4.9) | 0.63 (0.26-1.54) | ||||
| >500 | 73/1440 (5.1) | 0.46 (0.16-1.31) | ||||
|
| ||||||
|
Clinic rate of virological
failure |
Low | 61/586 (10.4) | 1 | 0.30 | ||
| High | 302/5468 (5.5) | 0.70 (0.35-1.38) | ||||
LRT for clinic-level clustering in the final multivariable model p<0.001;
LRT for linear trend;
LRT for departure from linear trend
Abbreviations: OR, odds ratio; aOR, adjusted odds ratio; CI, confidence interval; LRT, likelihood ratio test
Multivariable analysis (table 2) showed that independent determinants of switching included the clinic visit being the third or subsequent visit during the viremic period, the visit occurring in 2008 vs. earlier years, the patient being ART-experienced pre-programme, or the most recent VL result being log10 ≥4, or CD4 count <100 cells/mm3. Patients with no clinic contact in the 4 months preceding the visit, or declining VL (trend), were less likely to switch regimens. In the final model there was strong evidence of clinic-level clustering (LRT, p<0.001) which remained after accounting for programme, clinic size and clinic rate of virological failure.
When the final model was stratified by duration of virological failure or programme; or restricted to those known to be ART-naive or with prior viral suppression, the direction of associations remained the same (web appendix, tables 2-5).
Discussion
In this large multi-centre ART programme in South Africa, despite 6-monthly virological monitoring and guidelines recommending a switch after confirmed virological failure, rates of switching to second-line ART were low. Many patients experienced extended periods of viraemia following virological failure, in part due to relatively infrequent VL monitoring and clinic visits. However, other factors including delayed clinic attendance, VL (magnitude and trend), and patients’ immune status appeared to influence healthcare workers’ decisions regarding when to switch regimens.
Although current programme guidelines advise intensifying adherence interventions and switching regimens if a second VL remains high,23, 26 this rarely happened in practice. Instead, following a second raised VL, many patients experienced prolonged periods of viraemia with the rate of viral re-suppression on first-line ART approaching the rate of switching to second-line ART. By the end of follow-up, nearly a quarter of patients remaining on first-line ART were virologically suppressed. Others report high levels of viral re-suppression following a single raised VL,25, 28-31 however these results highlight that even after a second raised viral load, viral re-suppression remains possible due to, for example, a change in patients adherence behaviour or removal of a drug interaction. Excluding non-adherence as a cause of viraemia is challenging; current adherence measurement tools are inadequate32 and strategies to improve adherence can take time and are often context-specific, requiring multi-dimensional interventions.33 These difficulties may be compounded by weaknesses in the healthcare system, for example out-of-date patient contact details, lack of an appointment system or outreach workers may create delays in detecting and tracing patients with raised viral loads or missed appointments.34
When deciding to switch to second-line ART, healthcare workers appear to use the magnitude and trend in VLs, prior ART experience and clinic attendance to evaluate the probability of resistance as opposed to non-adherence. As reported elsewhere, patients with high VLs were more likely to switch regimens;35-37 although there is a suggestion in our study that healthcare workers were less likely to switch patients with a VL >5 log10 as compared to 4-5 log10, perhaps recognising that these patients have a lower likelihood of resistance and may be non-adherent .38-40 Patients whose VL had fallen to 400-1000 copies/ml or whose VL had declined by >50%, suggesting improved adherence, and patients with delayed clinic attendance who were likely to have run out of medication were less likely to be switched. A decision not to switch patients in whom adherence interventions are working or where clinic appointments have been missed appears rational.
Patients with a low CD4 count were more likely to switch to second-line ART, confirming findings from other studies.5, 35-37, 41 A low CD4 count may reflect immunological progression occurring as a result of viral replication due to treatment interruption, non-adherence, or resistance.42-43 There appears to be no consistent relationship between current CD4 count and odds of detecting resistance.38, 40 This may in part relate to the mutations studied; M184V, a mutation which emerges early has been associated with higher concurrent CD4 count,44-45 while some46-47 but not all studies44, 48 have demonstrated an association between low CD4 count and thymidine analogue mutations. Regardless of the reason, patients with a low CD4 count are at high risk of short-term clinical progression,19, 49 and it may be this factor in itself which prompts a decision to switch to second-line ART.
The frequency of clinic visits contributes to prolonged viraemia, with results from one visit only available to influence decision-making at the subsequent visit. One-fifth of patients had no record of clinic attendance following confirmed virological failure, therefore healthcare workers had no opportunity to assess and instigate appropriate interventions. Interestingly, duration of viraemia at the time of visit was not associated with switching regimens, but number of visits was. Regardless of duration of viraemia, healthcare workers may require repeated visits to exclude alternative causes of viraemia and ensure the patient is engaged in care.
Rates of switching and re-suppression varied markedly between clinics, supporting observations by others.4, 36, 50 This heterogeneity was not accounted for by the individual or clinic-level variables measured. Others have demonstrated the role of contextual and programme factors on the timing of ART initiation,51 however their influence on the timing of the switch decision is not fully understood.52 Factors such as variations in workload, available adherence support measures within the clinic, procedures for tracing of patients who have missed appointments, and the influence of contextual factors on individual healthcare workers’ and patients decision-making regarding virological failure needs explored.
While other studies in resource-limited settings have reported on rates and predictors of switching, these are largely from specialist centres, and describe rates of switching following initiation of first-line ART, evaluating predictors either from this time-point or at 6 months.4, 53-55 In contrast our analysis describes rates of switching in patients with virological failure, and explores determinants of switching at the time decisions are made i.e. clinic visit. By taking this approach, rather than a time-to-event analysis, we have avoided the potential bias introduced by competing risks of viral re-suppression, and interval censoring due to frequency of visits. While some visit records may be missing, we do not believe this will introduce bias but rather weaken associations.
This study was conducted using routine programme data and while reasons for stopping treatment, including switching regimens were available, reasons for continuing a failing regimen were not. We were unable to fully explore the role non-adherence might have on the timing of the switch decision as our only available measure of non-adherence was patients’ self-report. This is recognized to be a sub-optimal measure,32 and was often missing. In addition other factors, not captured in routine reporting, may affect decision-making e.g. patient’s readiness to switch regimens, healthcare workers’ concerns regarding limited future regimens and experience in prescribing second-line ART, or health-system factors.52-54, 56-58 Finally, some patients may have switched to bPI for reasons other than treatment failure e.g. toxicity. In a study of the same cohort, the majority of patients switching to second-line ART whilst viraemic did so for treatment failure;59 we therefore believe there is minimal misclassification in our outcome measurement.
Given the large numbers of people living with HIV in sub-Saharan Africa, the need for life-long treatment, the high cost of second- and third-line drugs, and limited resources, it is vital that the currently available ART regimens are used effectively. These results highlight that even in programmes with access to virological monitoring healthcare workers encounter difficulties in determining which patients are likely to have resistance and should switch regimens, and which need further adherence support. Current tools for measuring non-adherence are inadequate 32 and alternative approaches, including targeted resistance testing warrants further investigation.60 For some patients attempts to improve non-adherence will be unsuccessful; patients will remain viraemic and at risk of resistance accumulation. Switching such patients to a bPI regimen, which is more tolerant of non-adherence, is one strategy; however the cost-effectiveness in resource-limited settings needs explored. Evidence to support decisions, together with clear algorithms for the management of virological failure, particularly where there is ongoing sub-optimal adherence are needed; this becomes particularly pertinent in light of increasing task-shifting of HIV care to nurses and field-workers.
Supplementary Material
Acknowledgements
We would like to thank the patients and healthcare teams at the participating sites, and the staff at Aurum Institute for their assistance with this study.
Financial disclosures: Funding for this study was provided through a Wellcome Trust Research Fellowship (VJ; Grant number 087261/Z/08/Z). The community programme was funded by the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR; Grant number 5U2GPS000811) and the workplace programme by the employers. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of interest: VJ: none, KLF: none, SC: none, GJC: none, AP: none, ADG: none
References
- 1.World Health Organization. UNAIDS. UNICEF [Accessed 14th June 2011];Towards Universal Access: scaling up priority HIV/AIDS interventions in the health sector: Progress report. 2010 Available at: http://www.who.int/hiv/pub/2010progressreport/en/index.html.
- 2.Barth RE, van der Loeff MF, Schuurman R, Hoepelman AI, Wensing AM. Virological follow-up of adult patients in antiretroviral treatment programmes in sub-Saharan Africa: a systematic review. Lancet Infect Dis. 2010 Mar;10(3):155–166. doi: 10.1016/S1473-3099(09)70328-7. [DOI] [PubMed] [Google Scholar]
- 3.Renaud-Thery F, Duncome C, Kerr S, Thierry S, Perriens J. Systematic Review of First-line Treatment Failure and Attrition Rates [Abstract 827]. Paper presented at: 17th Conference on Retroviruses and Opportunistic Infections; San Francisco. 2010. [Google Scholar]
- 4.Keiser O, Tweya H, Boulle A, et al. Switching to second-line antiretroviral therapy in resource-limited settings: comparison of programmes with and without viral load monitoring. AIDS. 2009 Sep 10;23(14):1867–1874. doi: 10.1097/QAD.0b013e32832e05b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou J, Li PC, Kumarasamy N, et al. Deferred modification of antiretroviral regimen following documented treatment failure in Asia: results from the TREAT Asia HIV Observational Database (TAHOD) HIV Med. 2010 Jan;11(1):31–39. doi: 10.1111/j.1468-1293.2009.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang LW, Harris J, Humphreys E. Optimal monitoring strategies for guiding when to switch first-line antiretroviral therapy regimens for treatment failure in adults and adolescents living with HIV in low-resource settings. Cochrane Database Syst Rev. 2010;4 doi: 10.1002/14651858.CD008494. CD008494. [DOI] [PubMed] [Google Scholar]
- 7.Laurent C, Kouanfack C, Laborde-Balen G, et al. Monitoring of HIV viral loads, CD4 cell counts, and clinical assessments versus clinical monitoring alone for antiretroviral therapy in rural district hospitals in Cameroon (Stratall ANRS 12110/ESTHER): a randomised non-inferiority trial. Lancet Infect Dis. 2011 Nov;11(11):825–833. doi: 10.1016/S1473-3099(11)70168-2. [DOI] [PubMed] [Google Scholar]
- 8.Mugyenyi P, Walker AS, Hakim J, et al. Routine versus clinically driven laboratory monitoring of HIV antiretroviral therapy in Africa (DART): a randomised non-inferiority trial. Lancet. 2010 Jan 9;375(9709):123–131. doi: 10.1016/S0140-6736(09)62067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mermin J, Ekwaru JP, Were W, et al. Utility of routine viral load, CD4 cell count, and clinical monitoring among adults with HIV receiving antiretroviral therapy in Uganda: randomised trial. BMJ. 2011;343:d6792. doi: 10.1136/bmj.d6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jourdain G, Ngo-Giang-Huong N, Le Coeur S, et al. PHPT-3: A Randomized Clinical Trial Comparing CD4 vs Viral Load ART Monitoring/Switching Strategies in Thailand [Abstract 44]. Paper presented at: 18th Conference on Retroviruses and Opportunistic Infections; Boston. 2011. [Google Scholar]
- 11.Wilson D, Keiluhu AK, Kogrum S, et al. HIV-1 viral load monitoring: an opportunity to reinforce treatment adherence in a resource-limited setting in Thailand. Trans R Soc Trop Med Hyg. 2009 Jun;103(6):601–606. doi: 10.1016/j.trstmh.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Gupta RK, Hill A, Sawyer AW, et al. Virological monitoring and resistance to first-line highly active antiretroviral therapy in adults infected with HIV-1 treated under WHO guidelines: a systematic review and meta-analysis. Lancet Infect Dis. 2009 Jul;9(7):409–417. doi: 10.1016/S1473-3099(09)70136-7. [DOI] [PubMed] [Google Scholar]
- 13.Cozzi-Lepri A, Phillips AN, Martinez-Picado J, et al. Rate of accumulation of thymidine analogue mutations in patients continuing to receive virologically failing regimens containing zidovudine or stavudine: implications for antiretroviral therapy programs in resource-limited settings. J Infect Dis. 2009 Sep 1;200(5):687–697. doi: 10.1086/604731. [DOI] [PubMed] [Google Scholar]
- 14.Phillips AN, Pillay D, Garnett G, et al. Effect on transmission of HIV-1 resistance of timing of implementation of viral load monitoring to determine switches from first to second-line antiretroviral regimens in resource-limited settings. AIDS. 2011 Feb 3;25(6):843–850. doi: 10.1097/QAD.0b013e328344037a. [DOI] [PubMed] [Google Scholar]
- 15.Ajose O, Mookerjee S, Mills EJ, Boulle A, Ford N. Treatment outcomes of patients on second-line antiretroviral therapy in resource-limited settings: a systematic review and meta-analysis. AIDS. 2012 Feb 4; doi: 10.1097/QAD.0b013e328351f5b2. [DOI] [PubMed] [Google Scholar]
- 16.Long L, Fox M, Sanne I, Rosen S. The high cost of second-line antiretroviral therapy for HIV/AIDS in South Africa. AIDS. 2010 Mar 27;24(6):915–919. doi: 10.1097/QAD.0b013e3283360976. [DOI] [PubMed] [Google Scholar]
- 17.Murri R, Lepri AC, Cicconi P, et al. Is moderate HIV viremia associated with a higher risk of clinical progression in HIV-infected people treated with highly active antiretroviral therapy: evidence from the Italian cohort of antiretroviral-naive patients study. J Acquir Immune Defic Syndr. 2006 Jan 1;41(1):23–30. doi: 10.1097/01.qai.0000188337.76164.7a. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organisation . WHO Consultation on ART Failure in the Context of a Public Health Approach : meeting report. World Health Organisation; Geneva: 2008. [Accessed on 15th September 2011]. Available at: http://www.who.int/hiv/pub/arv/art_meeting_art_fail.pdf. [Google Scholar]
- 19.Ledergerber B, Lundgren JD, Walker AS, et al. Predictors of trend in CD4-positive T-cell count and mortality among HIV-1-infected individuals with virological failure to all three antiretroviral-drug classes. Lancet. 2004 Jul 3-9;364(9428):51–62. doi: 10.1016/S0140-6736(04)16589-6. [DOI] [PubMed] [Google Scholar]
- 20.Kousignian I, Abgrall S, Duval X, Descamps D, Matheron S, Costagliola D. Modeling the time course of CD4 T-lymphocyte counts according to the level of virologic rebound in HIV-1-infected patients on highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2003 Sep 1;34(1):50–57. doi: 10.1097/00126334-200309010-00007. [DOI] [PubMed] [Google Scholar]
- 21.Phillips AN, Pillay D, Miners AH, Bennett DE, Gilks CF, Lundgren JD. Outcomes from monitoring of patients on antiretroviral therapy in resource-limited settings with viral load, CD4 cell count, or clinical observation alone: a computer simulation model. Lancet. 2008 Apr 26;371(9622):1443–1451. doi: 10.1016/S0140-6736(08)60624-8. [DOI] [PubMed] [Google Scholar]
- 22.Walensky RP, Ciaranello AL, Park JE, Freedberg KA. Cost-effectiveness of laboratory monitoring in sub-Saharan Africa: a review of the current literature. Clin Infect Dis. 2010 Jul 1;51(1):85–92. doi: 10.1086/653119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization [Accessed on 15th September 2011];Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. 2010 Available at: http://www.who.int/hiv/pub/arv/adult2010/en/ [PubMed]
- 24.Keiser O, Tweya H, Braitstein P, et al. Mortality after failure of antiretroviral therapy in sub-Saharan Africa. Trop Med Int Health. 2010 Feb;15(2):251–258. doi: 10.1111/j.1365-3156.2009.02445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orrell C, Kaplan R, Wood R, Bekker LG. Virological breakthrough: a risk factor for loss to followup in a large community-based cohort on antiretroviral therapy. AIDS Res Treat. 2011;2011:469127. doi: 10.1155/2011/469127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Department of Health, South, Africa Clinical Guidelines for the Management of HIV & AIDS in Adults and Adolescents. 2010.
- 27.Coviello V, Boggess M. Cumulative Incidence estimation in the presence of competing risks. The STATA Journal. 2004;4(2):103–112. [Google Scholar]
- 28.Hoffmann CJ, Charalambous S, Sim J, et al. Viremia, resuppression, and time to resistance in human immunodeficiency virus (HIV) subtype C during first-line antiretroviral therapy in South Africa. Clin Infect Dis. 2009 Dec 15;49(12):1928–1935. doi: 10.1086/648444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castelnuovo B, Sempa J, Agnes KN, Kamya MR, Manabe YC. Evaluation of WHO Criteria for Viral Failure in Patients on Antiretroviral Treatment in Resource-Limited Settings. AIDS Res Treat. 2011;2011:736938. doi: 10.1155/2011/736938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brinkhof MW, Boulle A, Weigel R, et al. Mortality of HIV-infected patients starting antiretroviral therapy in sub-Saharan Africa: comparison with HIV-unrelated mortality. PLoS Med. 2009 Apr 28;6(4):e1000066. doi: 10.1371/journal.pmed.1000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Messou E, Chaix ML, Gabillard D, et al. Association between medication possession ratio, virologic failure and drug resistance in HIV-1-infected adults on antiretroviral therapy in Cote d’Ivoire. J Acquir Immune Defic Syndr. 2011 Apr;56(4):356–364. doi: 10.1097/QAI.0b013e3182084b5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gill CJ, Hamer DH, Simon JL, Thea DM, Sabin LL. No room for complacency about adherence to antiretroviral therapy in sub-Saharan Africa. AIDS. 2005 Aug 12;19(12):1243–1249. doi: 10.1097/01.aids.0000180094.04652.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simoni JM, Amico KR, Smith L, Nelson K. Antiretroviral adherence interventions: translating research findings to the real world clinic. Curr HIV/AIDS Rep. 2010 Feb;7(1):44–51. doi: 10.1007/s11904-009-0037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braitstein P, Daggy J, Easterbrook P, et al. Retaining adults in HIV care: impact of key program characteristics on patient loss to follow-up (LTFU) in the IeDEA East Africa Consortium [Abstract 1015]. Presented at 18th Conference on Retroviruses and Opportunistic Infections; Boston, USA. 2011. [Google Scholar]
- 35.Lee KJ, Dunn D, Porter K, et al. Treatment switches after viral rebound in HIV-infected adults starting antiretroviral therapy: multicentre cohort study. AIDS. 2008 Oct 1;22(15):1943–1950. doi: 10.1097/QAD.0b013e32830e4cf3. [DOI] [PubMed] [Google Scholar]
- 36.Davies MA, Moultrie H, Eley B, et al. Virologic failure and second-line antiretroviral therapy in children in South Africa--the IeDEA Southern Africa collaboration. J Acquir Immune Defic Syndr. 2011 Mar 1;56(3):270–278. doi: 10.1097/QAI.0b013e3182060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petersen ML, van der Laan MJ, Napravnik S, Eron JJ, Moore RD, Deeks SG. Long-term consequences of the delay between virologic failure of highly active antiretroviral therapy and regimen modification. AIDS. 2008 Oct 18;22(16):2097–2106. doi: 10.1097/QAD.0b013e32830f97e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marconi VC, Sunpath H, Lu Z, et al. Prevalence of HIV-1 drug resistance after failure of a first highly active antiretroviral therapy regimen in KwaZulu Natal, South Africa. Clin Infect Dis. 2008 May 15;46(10):1589–1597. doi: 10.1086/587109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bangsberg DR, Moss AR, Deeks SG. Paradoxes of adherence and drug resistance to HIV antiretroviral therapy. J Antimicrob Chemother. 2004 May;53(5):696–699. doi: 10.1093/jac/dkh162. [DOI] [PubMed] [Google Scholar]
- 40.Prosperi MC, Mackie N, Di Giambenedetto S, et al. Detection of drug resistance mutations at low plasma HIV-1 RNA load in a European multicentre cohort study. J Antimicrob Chemother. 2011 Aug;66(8):1886–1896. doi: 10.1093/jac/dkr171. [DOI] [PubMed] [Google Scholar]
- 41.Danel C, Gabillard D, Inwoley A, et al. Medium-term probability of success of antiretroviral treatment after early warning signs of treatment failure in West African adults. AIDS Res Hum Retroviruses. 2009 Aug;25(8):783–793. doi: 10.1089/aid.2009.0018. [DOI] [PubMed] [Google Scholar]
- 42.Kranzer K, Ford N. Unstructured treatment interruption of antiretroviral therapy in clinical practice: a systematic review. Trop Med Int Health. 2011 Jul 1; doi: 10.1111/j.1365-3156.2011.02828.x. [DOI] [PubMed] [Google Scholar]
- 43.Seyler C, Adje-Toure C, Messou E, et al. Impact of genotypic drug resistance mutations on clinical and immunological outcomes in HIV-infected adults on HAART in West Africa. AIDS. 2007 May 31;21(9):1157–1164. doi: 10.1097/QAD.0b013e3281c615da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Zyl GU, van der Merwe L, Claassen M, Zeier M, Preiser W. Antiretroviral resistance patterns and factors associated with resistance in adult patients failing NNRTI-based regimens in the western cape, South Africa. J Med Virol. 2011 Oct;83(10):1764–1769. doi: 10.1002/jmv.22189. [DOI] [PubMed] [Google Scholar]
- 45.Doualla-Bell F, Gaseitsiwe S, Ndungu T, et al. Mutations and polymorphisms associated with antiretroviral drugs in HIV-1C-infected African patients. Antivir Chem Chemother. 2004 Jul;15(4):189–200. doi: 10.1177/095632020401500402. [DOI] [PubMed] [Google Scholar]
- 46.Orrell C, Walensky RP, Losina E, Pitt J, Freedberg KA, Wood R. HIV type-1 clade C resistance genotypes in treatment-naive patients and after first virological failure in a large community antiretroviral therapy programme. Antivir Ther. 2009;14(4):523–531. [PMC free article] [PubMed] [Google Scholar]
- 47.Hosseinipour MC, van Oosterhout JJ, Weigel R, et al. The public health approach to identify antiretroviral therapy failure: high-level nucleoside reverse transcriptase inhibitor resistance among Malawians failing first-line antiretroviral therapy. AIDS. 2009 Jun 1;23(9):1127–1134. doi: 10.1097/QAD.0b013e32832ac34e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sigaloff KC, Hamers RL, Wallis CL, et al. Unnecessary antiretroviral treatment switches and accumulation of HIV resistance mutations; two arguments for viral load monitoring in Africa. J Acquir Immune Defic Syndr. 2011 Jun 18; doi: 10.1097/QAI.0b013e318227fc34. [DOI] [PubMed] [Google Scholar]
- 49.Srasuebkul P, Lim PL, Lee MP, et al. Short-term clinical disease progression in HIV-infected patients receiving combination antiretroviral therapy: results from the TREAT Asia HIV observational database. Clin Infect Dis. 2009 Apr 1;48(7):940–950. doi: 10.1086/597354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee K, Lyall H, Walker A, et al. Wide disparity in switch to second-line therapy in HIV infected children in CHIPS. Paper presented at: Eighth International Congress on Drug Therapy in HIV Infection; Glasgow, United Kingdom. 2006. [Google Scholar]
- 51.Nash D, Wu Y, Elul B, Hoos D, El Sadr W. Program-level and contextual-level determinants of low-median CD4+ cell count in cohorts of persons initiating ART in eight sub-Saharan African countries. AIDS. 2011 Jul 31;25(12):1523–1533. doi: 10.1097/QAD.0b013e32834811b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Srasuebkul P, Calmy A, Zhou J, Kumarasamy N, Law M, Lim PL. Impact of drug classes and treatment availability on the rate of antiretroviral treatment change in the TREAT Asia HIV Observational Database (TAHOD) AIDS Res Ther. 2007;4(1):18. doi: 10.1186/1742-6405-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Landier J, Akonde A, Pizzocolo C, et al. Switch to second-line ART in West African routine care: incidence and reasons for switching. AIDS Care. 2011 Jan;23(1):75–78. doi: 10.1080/09540121.2010.498867. [DOI] [PubMed] [Google Scholar]
- 54.Orrell C, Harling G, Lawn SD, et al. Conservation of first-line antiretroviral treatment regimen where therapeutic options are limited. Antivir Ther. 2007;12(1):83–88. [PubMed] [Google Scholar]
- 55.Luca D, Marazzi MC, Mancinelli S, et al. Prognostic value of virological and immunological responses after 6 months of antiretroviral treatment in adults with HIV-1 infection in Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2011 Nov 30; doi: 10.1097/QAI.0b013e31824276e9. [DOI] [PubMed] [Google Scholar]
- 56.Hawkins C, Murphy RL. Management of antiretroviral failure and resistance in developing countries. Curr Opin HIV AIDS. 2009 Nov;4(6):538–544. doi: 10.1097/COH.0b013e328331d2fb. [DOI] [PubMed] [Google Scholar]
- 57.Harries AD, Zachariah R, van Oosterhout JJ, et al. Diagnosis and management of antiretroviral-therapy failure in resource-limited settings in sub-Saharan Africa: challenges and perspectives. Lancet Infect Dis. 2010 Jan;10(1):60–65. doi: 10.1016/S1473-3099(09)70321-4. [DOI] [PubMed] [Google Scholar]
- 58.Pitts M, J G, Koelmeyer R. If it ain’t broke, don’t fix it: the impact of patient and doctor concerns on commencing and changing antiretroviral treatment. 6th IAS Conference on HIV Pathogenesis, Treatment and Prevention; Rome, Italy. 2011. Vol Abstract: CDB364. [Google Scholar]
- 59.Johnston V, Fielding K, Charalambous S, et al. Second-line Antiretroviral Therapy in a Workplace and Community-based Treatment Programme in South Africa: determinants of virological outcome. PLoS ONE. 2012 doi: 10.1371/journal.pone.0036997. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosen S, Long L, Sanne I, Stevens WS, Fox MP. The net cost of incorporating resistance testing into HIV/AIDS treatment in South Africa: a Markov model with primary data. J Int AIDS Soc. 2011 May 15;14(1):24. doi: 10.1186/1758-2652-14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.