Abstract
Patients with CLL have a variable clinical course. Identification of modifiable characteristics related to CLL-specific survival may provide opportunities for therapeutic intervention. The absolute number of T-cell and natural kill(NK)-cells was calculated for 166 consecutive patients with CLL evaluated by flow cytometry at Mayo Clinic <2 months of diagnosis. The size of the T-cell/NK-cell compartment relative to the size of the malignant monoclonal B-cell(MBC) compartment was evaluated by calculating NK:MBC and T:MBC ratios. Patients exhibited substantial variation in the absolute number of T and NK-cells as well as T:MBC and NK:MBC ratios at diagnosis. Higher T:MBC and NK:MBC ratios were observed among patients with early stage and mutated IgVH genes(all P<0.0003). As continuous variables, both T:MBC ratio(p value=0.03) and NK:MBC ratio(p value=0.02) were associated with time to treatment(TTT). On multivariate Cox modeling including stage, CD38, absolute MBC count, NK:MBC ratio, and T:MBC ratio, the independent predictors of TTT were stage, T:MBC ratio, and NK:MBC ratio. These findings suggest measurable characteristics of the host immune system relate to the rate of disease progression in patients with newly diagnosed CLL. These characteristics can be modified and continued evaluation of immunomodulatory drugs, vaccination strategies, and cellular therapies to delay/prevent disease progression are warranted.
Keywords: CLL, prognosis, immune system, T-cells, natural killer cells
Introduction
Chronic lymphocytic leukaemia (CLL) is one of the most common lymphoid malignancies in the United States, with an estimated incidence of 15,000 patients per year. In the modern era, 70-80% of CLL patients are diagnosed with early stage disease identified incidentally on blood counts obtained for unrelated purposes (Call, et al 1994, Molica and Levato 2001, Rozman, et al 1997) . The clinical course of early stage CLL is highly variable with some patients living for decades without requiring treatment and others experiencing a rapidly progressive illness leading to premature death (Shanafelt, et al 2004). Current prognostic indicators (ZAP-70, CD38, IgVH gene mutation status, FISH) are able to identify early-stage CLL patients at high risk of rapid disease progression (Shanafelt, et al 2004) (Kay, et al 2007). These prognostic factors focus on characteristics of the malignant B cell clone and are not currently modifiable factors (although some may be targeted (Castro, et al 2005)). Identification of modifiable characteristics of the host unrelated to the leukaemic B-cell that relate to CLL-specific survival would provide opportunities for therapeutic intervention.
Absolute lymphocyte count (ALC) at diagnosis, a surrogate marker of host immunity (i.e., T-cells/natural killer (NK) cells) has been shown to predict survival in patients with follicular lymphoma (Siddiqui, et al 2006). In addition, we have found that a higher pretreatment ALC is associated with improved response rates, progression free survival, and overall survival in patients with follicular lymphoma treated with either rituximab (Behl, et al 2007) or radioimmunotherapy (Porrata, et al 2007). Notably, the utility of ALC as a prognostic parameter in patients with follicular lymphoma persists on multivariate analysis controlling for other well established prognostic parameters [e.g., FLIPI index (Solal-Celigny, et al 2004)] in all of these circumstances (Behl, et al 2007, Porrata, et al 2007, Siddiqui, et al 2006 ). A limitation of these studies is the lack of lymphocyte subsets analysis to identify the immune effector cell(s) mediating the superior survival.
Unlike follicular lymphoma, in patients with CLL the ALC is primarily a measure of the size of the leukaemic clone rather than normal T/NK cells. To our knowledge, no study has addressed the prognostic importance of the interaction between the malignant B-cell component and the host immune component (T-cells/NK cells) in the ALC. Such interactions between the leukaemic cells and the native immune system could be a potentially important influence on disease progression. We hypothesized that CLL patients with enhanced host immunity may experience a more indolent disease course due to enhanced immune regulation of their disease. In the present study, we evaluated the relationship between the peripheral blood T-cells and NK-cells at the time of diagnosis and clinical outcomes in a cohort of newly diagnosed CLL patients.
Methods
Patient population
We identified 166 consecutive patients in the Mayo Clinic CLL database who were diagnosed with CLL between 2000 and 2002, were evaluated at the Mayo Clinic within 2 months of diagnosis, and had flow cytometry testing of their peripheral blood performed at Mayo Clinic at the time of diagnosis. Patient clinical characteristics (gender, age at diagnosis, stage at diagnosis) were abstracted from the Mayo Clinic CLL database in March of 2007 and clinical outcomes (e.g., treatment status and vital status) were updated through August 2007. Approval for this study was obtained from the Mayo Clinic Institutional Review Board and was in accordance with US federal regulations and the Declaration of Helsinki.
End point
The primary end point of the study was to assess variation in time to treatment (TTT) based on the normal peripheral blood T-cells and NK cells in newly diagnosed CLL patients. The decision to treat patients was based on physician discretion in accord with the NCI 96 CLL Working Group Criteria (Cheson, et al 1996).
Assessment of Absolute T-cell and NK-cell Count at Diagnosis
Immunophenotyping of peripheral blood is required for the diagnosis of CLL and is typically performed by flow cytometry (Cheson, et al 1996). While the primary purpose of such analysis is to look for the immunophenotypic pattern that is characteristic of the leukaemic B-cells in CLL, the standard diagnostic flow cytometry analysis in this study also included monoclonal antibodies against CD3 (Leu-4) and CD16 (Leu-11c) which permitted simultaneous assessment of T-cells and NK-cells, respectively. Multicolor flow cytometric immunophenotyping analysis was performed according to previously described methods using fluorochrome-conjugated antibodies (Hanson, et al 1999). A standard, whole-blood assay with erythrocyte cell lysis was used for preparing the blood specimens. All of the samples were evaluated on a FACS Calibur flow cytometer (Becton Dickinson). We reviewed the existing flow cytometry data to determine the absolute T-cell and NK-cell number at the time of diagnosis. The proportion of B-cells, T-cells, and NK-cells were determined in each case, normalized to 100% lymphocytes and then an absolute count of each lymphocyte subset was calculated using a simultaneously run CBC with automated differential counts, including total lymphocytes.
Prognostic testing
IgVH mutation status, ZAP-70 status, CD38 status, and cytogenetic abnormalities by FISH testing were assessed using methods previously described by our group (Dewald, et al 2003, Jelinek, et al 2001, Shanafelt, et al 2006a, Shanafelt, et al 2006b). Antibodies to CD19 and ZAP-70 were used to detect ZAP-70 expression in CD19+ cells using the gating strategy of Rassenti and colleagues (Rassenti, et al 2004).
Statistical Analysis
Distributions of absolute T and absolute NK were explored both graphically and descriptively. Relationships between continuous measures and categorical prognostic variables (Rai stage, CD38 positive/negative, IgVH mutated/unmutated, etc.) were evaluated using the Kruskal-Wallis test. TTT was defined as time from diagnosis to time of first therapy. For metrics which demonstrated a relationship with TTT as a continuous variable, we also explored various cut points based on either parameter distribution (e.g., median value) or the threshold that best predicted TTT using the method of Contal and O’Quigley (Contal and O’Quigley 1999)(Williams, et al 2006). Differences in TTT between prognostic groups were evaluated using standard Kaplan-Meier methods and logrank statistics.
Univariate and multivariate Cox models were used to identify significant prognostic factors for TTT. The leaps and bounds variable selection approach was used to identify variables for multivariable models (Furnival and Wilson 1974). Insufficient numbers of patients had ZAP-70, IgVH gene mutation status, or FISH results for inclusion of these prognostic factors in the multivariate analysis.
Results
Patients’ characteristics
The baseline characteristics of the cohort are summarized in Table I. The median patient age (66 years old), gender distribution (60% male), and Rai stage at diagnosis (95% Rai 0, I, or II) were typical of newly diagnosed patients with CLL (Call, et al 1994a) (Molica, et al 1999) (Rozman, et al 1997). The ALC at diagnosis was <25 × 109/Liter in 87% of patients. The results of prognostic testing demonstrate this consecutive series of patients tended to have more favorable characteristics with respect to CD38 (66% negative), ZAP-70 (74% negative), IgVH gene mutation status (60% mutated), and cytogenetic abnormalities as assessed by FISH (62% 13q- or normal) as would be expected in a series of patients evaluated at diagnosis as opposed to referral samples of patients evaluated later in the course of their disease.
Table I.
Patient Characteristics
| Characteristic | N = 166 |
|---|---|
|
Age at diagnosis
[median (range), years] |
66 (38, 90) |
|
| |
| Age (years) | |
| <50 | 20 (12%) |
| 50-60 | 36 (22%) |
| 61-70 | 52 (31%) |
| >70 | 58 (35%) |
|
| |
| Gender | |
| Male | 100 (60%) |
| Female | 66 (40%) |
|
| |
| Rai Stage at diagnosis | |
| 0 | 113 (70%) |
| I | 21 (13%) |
| II | 19 (12%) |
| III | 4 (2%) |
| IV | 4 (2%) |
| Missing | 5 |
|
| |
| ALC (×109/L) at diagnosis | |
| <10.0 | 90 (55%) |
| 10.0-25.0 | 53 (32%) |
| >25.0 – 50.0 | 9 (5%) |
| >50.0 | 13 (8%) |
| Missing | 1 |
|
| |
| Hb (g/dL) at diagnosis | |
| <11 | 10 (6%) |
| ≥ 11 | 155 (94%) |
| Missing | 1 |
|
| |
| Platelets (×109/L) at diagnosis | |
| <100 | 6 (4%) |
| 100-150 | 29 (18%) |
| >150 | 130 (79%) |
| Missing | 1 |
|
| |
| CD38 | |
| Positive (≥30%) | 54 (34%) |
| Negative (<30%) | 107 (66%) |
| Missing | 5 |
|
| |
| ZAP-70 | |
| Positive (≥20%) | 12 (26%) |
| Negative (<20%) | 34 (74%) |
| Missing | 120 |
|
| |
| IgVH gene mutation status | |
| Unmutated | 27 (40%) |
| Mutated | 41 (60%) |
| Missing | 98 |
|
| |
| FISH result | |
| 13q- | 26 (37%) |
| Normal | 18 (25%) |
| +12 | 15 (21%) |
| 11q- | 9 (13%) |
| 17p- | 2 (3%) |
| Other (6q-) | 1 (1%) |
| Missing | 95 |
Peripheral blood T and NK cells
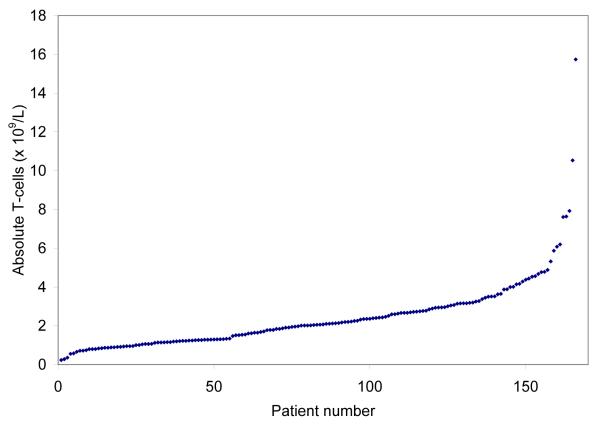
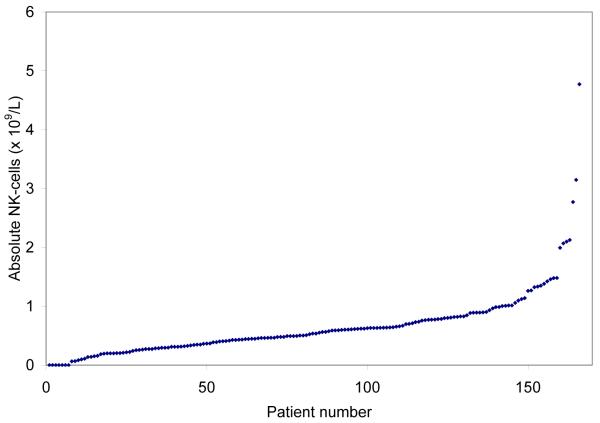
The peripheral blood ALC is comprised of leukaemic monoclonal B-cells (MBC) and normal B-cells, T-cells, and NK-cells. Figs. 1A and 1B show the distribution of absolute T-cell and NK cell counts at diagnosis and demonstrate these metrics are continuous variables. Overall, 89 of 166 (54%) of patients had an increase in absolute T-cell count (upper limit of normal <2.0 × 109/L) and 74 of 166 (45%) an increase in absolute NK-cells count (upper limit of normal <600 × 109/L). The distribution of absolute T-cell and NK-cell number within prognostic categories was balanced (Table II). We next evaluated the size of the T-cell/NK-cell compartment relative to the size of the malignant B-cell clone by calculating the NK-cell to MBC ratio (NK:MBC ratio) and T-cell to MBC ratio (T:MBC ratio; Table III) from absolute T-cell, NK-cell, and MBC counts. Higher NK:MBC and T:MBC ratios were observed among patients with early Rai stage disease (all P ≤ 0.0003) and those with mutated IgVH gene mutation status (all P = 0.0002).
Fig. 1A.
Distribution of Absolute T-cell and NK-cell # at diagnosis
Distribution Absolute T-cell # at diagnosis (×109/L)
Table II.
Absolute T-cell and NK cell number at diagnosis
| Prognostic factors/variable | Number of patients |
Median NK cell count (× 109/L) |
Median T cell count (× 109/L) |
||
|---|---|---|---|---|---|
| All patients | 166 | 0.54 | 2.06 | ||
|
| |||||
| Rai Stage at diagnosis | p=0.13 | p=0.81 | |||
| 0 | 113 | 0.59 | 2.06 | ||
| I/II | 40 | 0.45 | 1.97 | ||
| III/IV | 8 | 0.25 | 2.39 | ||
|
| |||||
| CD38 | p=0.16 | p=0.77 | |||
| Positive (≥30%) | 54 | 0.48 | 2.11 | ||
| Negative (<30%) | 107 | 0.59 | 2.02 | ||
|
| |||||
| ZAP-70 | p=0.71 | p=0.73 | |||
| Positive (≥20%) | 12 | 0.48 | 2.30 | ||
| Negative (<20%) | 34 | 0.59 | 2.19 | ||
|
| |||||
| IgVH gene mutation status | p=0.17 | p=0.23 | |||
| Unmutated | |||||
| Mutated | 27 | 0.45 | 1.64 | ||
| 41 | 0.63 | 2.25 | |||
|
| |||||
| FISH result | p=0.30 | p=0.24 | |||
| 13q- | 26 | 0.53 | 2.17 | ||
| Normal | 18 | 0.56 | 1.62 | ||
| +12 | 15 | 0.54 | 2.73 | ||
| 11q-/17p- | 11 | 0.29 | 1.24 | ||
Table III.
Ratio of T-cell and NK cell to monoclonal B cell at diagnosis
| Prognostic factors/variable | Number of patients |
Median ratio absolute NK/absolute monoclonal B |
Median ratio absolute T/absolute monoclonal B |
||
|---|---|---|---|---|---|
| All patients | 166 | 0.07 | 0.26 | ||
|
| |||||
| Rai Stage at diagnosis | p<0.0001 | p=0.0003 | |||
| 0 | 113 | 0.09 | 0.28 | ||
| I/II | 40 | 0.04 | 0.13 | ||
| III/IV | 8 | 0.01 | 0.06 | ||
|
| |||||
| CD38 | p=0.49 | p=0.58 | |||
| Positive (≥30%) | 54 | 0.07 | 0.27 | ||
| Negative (<30%) | 107 | 0.07 | 0.23 | ||
|
| |||||
| ZAP-70 | p=0.24 | p=0.77 | |||
| Positive (≥20%) | 12 | 0.04 | 0.23 | ||
| Negative (<20%) | 34 | 0.05 | 0.22 | ||
|
| |||||
| IgVH gene mutation status | p=0.0002 | p=0.0002 | |||
| Unmutated | |||||
| Mutated | 27 | 0.02 | 0.08 | ||
| 41 | 0.10 | 0.23 | |||
|
| |||||
| FISH result | p=0.66 | p=0.53 | |||
| 13q- | 26 | 0.05 | 0.19 | ||
| Normal | 18 | 0.04 | 0.12 | ||
| +12 | 15 | 0.04 | 0.22 | ||
| 11q-/17p- | 11 | 0.04 | 0.19 | ||
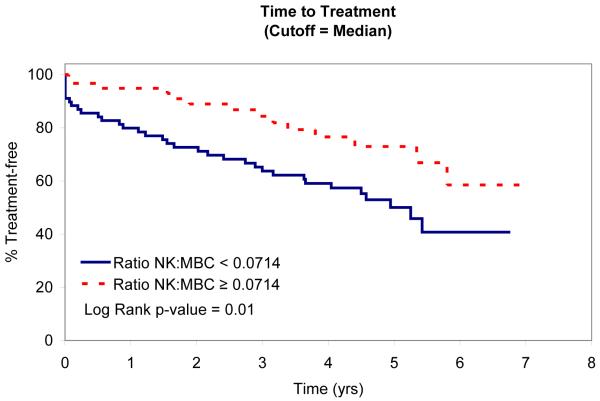
Role of T/NK cells on time-to-treatment
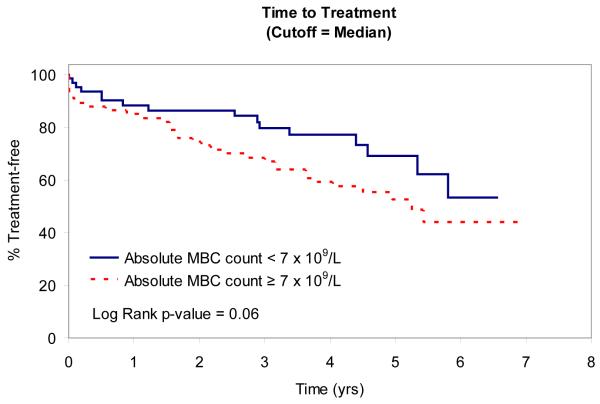
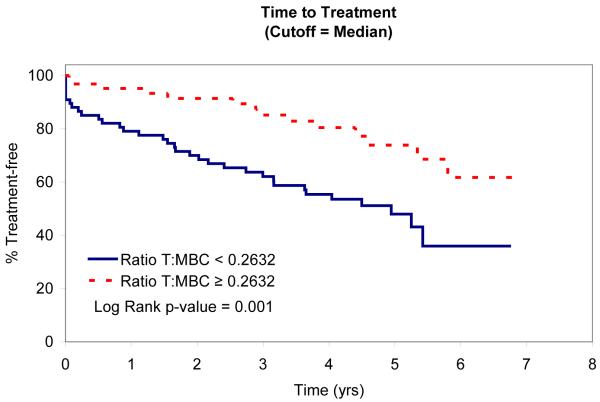
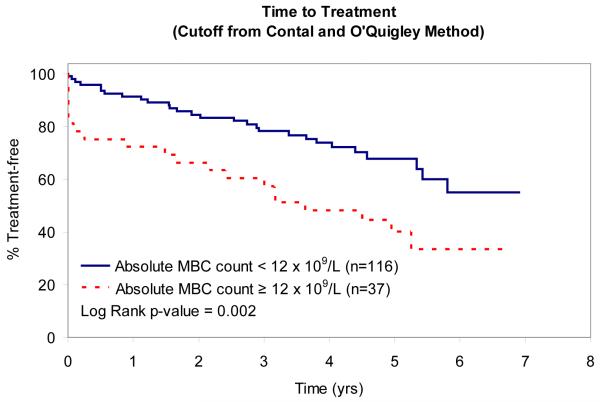
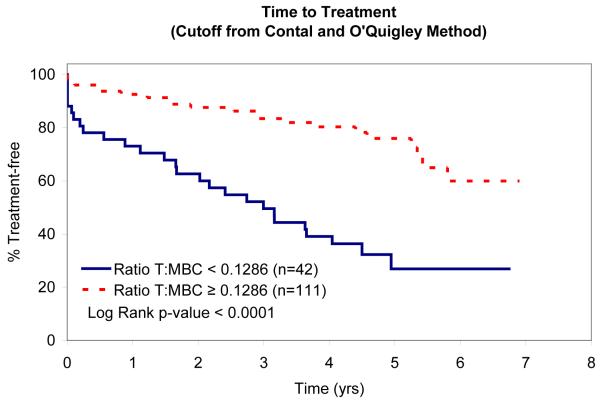
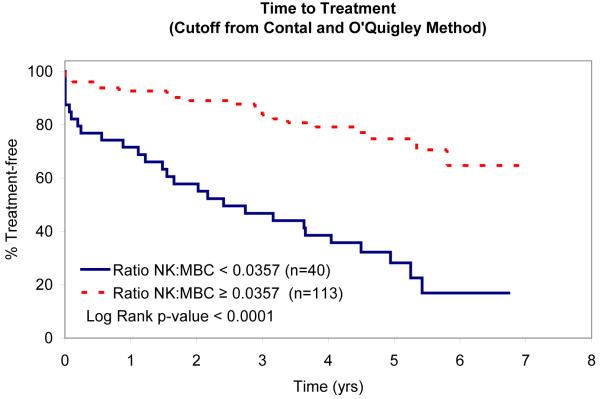
In an attempt to identify factors that influence TTT, we evaluated the relationship between absolute MBC, absolute NK-cell, absolute T-cell, NK:MBC ratio, and T:MBC ratio and TTT among Rai stage 0, I, and II patients (n=153; Table IV). As continuous variables, the T:MBC ratio (P value = 0.03), NK:MBC ratio (P value = 0.02), and absolute MBC count (p<0.0001) were associated with TTT. In contrast, no association between TTT and absolute NK-cell count or absolute T-cell count was identified. Given the relationship between NK:MBC ratio, T:MBC ratio, and absolute MBC count and TTT as continuous variables, we explored the prognostic significance of various thresholds to classify patients’ time to treatment. Using both median values (Fig. 2) and the method of Contal and O’Quigley to find the best cut-off values with which to classify patients (Fig. 3), superior TTT among Rai stage 0, I, and II patients was observed for those with higher NK:MBC or T:MBC ratios at the time of diagnosis and lower absolute MBC count. In these analyses the NK:MBC (hazard ratio=4.41; p=<0.0001) and T:MBC ratios (hazard ratio=3.96; p<0.0001) both outperformed the MBC count (hazard ratio=2.42; p=0.002).
Table IV.
Models for Time to Treatment Among Early Stage Patients
| Univariate Analysis | HR | HR 95% CI | p-value |
|---|---|---|---|
| TTT from diagnosis | |||
| Rai stage (0 vs. 1-2) | 3.05 | 1.74, 5.37 | 0.0001 |
| CD38 | 2.30 | 1.30, 4.04 | 0.004 |
| T:MBC ratio* | 3.96 | 2.24, 7.00 | <0.0001 |
| NK:MBC ratio* | 4.41 | 2.50, 7.77 | <0.0001 |
| MBC count* | 2.42 | 1.37, 4.26 | 0.002 |
|
| |||
| Multivariate Analysis | |||
|
| |||
| TTT from diagnosis | |||
| Rai Stage (0 vs. 1-2) | 2.20 | 1.23, 3.97 | 0.008 |
| CD38 | NS | ||
| T:MBC ratio* | 2.07 | 1.04, 4.09 | 0.04 |
| NK:MBC ratio* | 2.53 | 1.27, 5.0 | 0.009 |
| MBC count* | NS | ||
Categorized low vs high (see text)
Fig. 1B.
Distribution of Absolute T-cell and NK-cell # at diagnosis
Distribution Absolute NK-cell # at diagnosis (×109/L)
Fig. 2A.
Relationship Between TTT and Median MBC and Median T:MBC and NK:MBC ratios
Patient TTT stratified based on the median MBC
Multivariate analysis
Finally, we performed multivariate Cox modeling analysis to identify factors associated with TTT in Rai stage 0-II patients. Absolute MBC, NK:MBC ratio, T:MBC ratio, Rai stage at diagnosis and CD38 status were included in the analysis. Independent predictors of outcome selected by the leaps and bounds variable selection method were NK:MBC ratio, T:MBC ratio, and stage (Table IV).
Discussion
In this observational, cohort study we report wide inter-patient variation in size of the normal T-cell and NK cell compartment at the time of diagnosis. The size of the blood T/NK cell compartments relative to the size of the circulating leukaemic clone was associated with more advanced stage at diagnosis as well as IgVH gene mutation status raising the possibility that CLL patients with greater host immunity may experience a more indolent disease course due to more effective immune regulation of their disease. When considered as continuous variables both the ratio of T:MBC and NK:MBC correlated with TTT and as categorical prognostic parameters both the T:MBC and NK:MBC ratios were more strongly associated with TTT than the MBC. Notably, on multivariate analysis controlling for stage, MBC, and CD38 status, the T:MBC and NK:MBC ratios both remained independent predictors of TTT for patients with early stage CLL.
Many prognostic parameters are available for patients with early stage CLL (Shanafelt, et al 2004) (Kay, et al 2007) and progress will require identification of characteristics that provide insight into disease biology and have the potential for therapeutic targeting. Against this back drop, the results reported here are important for several reasons. First, nearly all currently available factors (ZAP-70, CD38, IgVH, FISH) focus on characteristics of the malignant B cell while the present analysis evaluates the prognostic importance of the non-malignant host immune system. Second, the findings suggest that efforts to enhance host immunity through immunomodulatory drugs, vaccination strategies, and cellular therapies may have the potential to delay/prevent disease progression and are worthy strategies for therapeutic testing. To this end, baseline assessment of host immunity may identify patients more or less likely to benefit from such approaches. Third, the information on T-cells and NK-cells used in this analysis is already collected as part of routine flow cytometry performed for diagnostic purposes in patients with CLL. Accordingly, these tests do not involve development or implementation of an expensive new molecular assay but simply report of information that is already available for most patients. Fourth, the findings also imply that CLL therapies that are toxic to normal T-cells and NK-cells (e.g., purine nucleoside analogues, alemtuzumab) may contribute not only to infectious complications but also have unintended effects on subsequent rates of disease progression. This possibility needs to be taken into account and carefully evaluated in ongoing trials of early treatment for patients with “high risk” CLL.
Our findings are subject to a number of important limitations. First the study evaluated TTT rather than overall survival. Longer follow-up of this cohort will be necessary before correlations with overall survival can be evaluated. Second, because the study was a cohort study of all newly diagnosed patients seen between 5 and 7 years ago rather than individuals participating in a research trial, some recently identified molecular prognostic parameters (e.g., ZAP-70, FISH, IgVH mutational status) were available for a limited number patients of CLL which reduced the prognostic factors that could be included in the multi-factor analysis. Third, our sample size is relatively small and larger studies in independent patient samples are needed before assessment of T:MBC or NK:MBC ratio can be employed as a prognostic tests. Fourth, we recognize that CLL patients who are CMV seropositive have an increased number of CD8+ T-cells (Mackus, et al 2003) and it is possible CMV seropositive patients have a shorter survival (Torres, et al 2006). Although CMV status was not available for the patients in our series, we found a favorable rather than unfavorable clinical outcome for those with increased T-cells (as assessed by the T:MBC ratio) suggesting the results of our study are unlikely due to patients’ CMV status. Finally, our analysis is limited to absolute T-cell and NK cell numbers rather than evaluation of specific T-cell/NK-cell function in terms of their impact on leukaemic B-cell survival or apoptosis regulation which is likely an important aspect of how the host immune system may modulate the rate of disease progression.
Our study has several important strengths. The individuals studied were a consecutive series of newly diagnosed patients. The majority of these individuals had early stage disease (>80% Rai 0-I) – precisely the patient group in need of prognostic tests. All patients had peripheral blood T-cell and NK-cell levels assayed within 2 months of diagnosis and were untreated at the time T-cell and NK-cell assays were performed. The information on T-cell and NK-cell counts were collected as part of routine diagnostic flow-cytometry analysis by a clinical laboratory with no knowledge such results would be used to evaluate patient outcomes providing unbiased assessment. Both the T:MBC and NK:MBC ratios predicted TTT as continuous variable and were an independent predictors of TTT on multivariate analysis controlling for stage and CD38.
In conclusion, measurable characteristics of the normal host immune system at the time of diagnosis appear to relate to the rate of disease progression in patients with CLL. This finding re-enforces the important influence of the host micro-environment on the progression potential of the leukaemic clone in patients with CLL. Continued efforts to evaluate the ability of immunomodulatory drugs, vaccination strategies, and cellular therapies to delay/prevent disease progression are warranted. Additional studies exploring how interactions between the host immune system and the leukaemic clone influence clinical outcomes are needed.
Fig. 2B.
Relationship Between TTT and Median MBC and Median T:MBC and NK:MBC ratios
Patient TTT stratified based on the median T:MBC ratio
Fig. 2C.
Relationship Between TTT and Median MBC and Median T:MBC and NK:MBC ratios
Patient TTT stratified based on the median NK:MBC ratio
Fig. 3A.
Relationship Between TTT and Statistically Derived Thresholds to Classify MBC and T:MBC and NK:MBC ratios
Patient TTT stratified based on the MBC count that best stratifies outcome
Fig. 3B.
Relationship Between TTT and Statistically Derived Thresholds to Classify MBC and T:MBC and NK:MBC ratios
Patient TTT stratified based on the T:MBC Ratio that best stratifies outcome
Fig. 3C.
Relationship Between TTT and Statistically Derived Thresholds to Classify MBC and T:MBC and NK:MBC ratios
Patient TTT stratified based on the NK:MBC Ratio that best stratifies outcome
Acknowledgments
Support through grants from the National Cancer Institute (NCI CA113408; NCI CA94919; NCI CA97274) and Bayer Health Care Pharmaceuticals are gratefully acknowledged.
References
- Behl D, Ristow K, Markovic SN, Witzig TE, Habermann TM, Colgan JP, Inwards DJ, White WL, Ansell SM, Micallef IN, Johnston PB, Porrata LF. Absolute lymphocyte count predicts therapeutic efficacy of rituximab therapy in follicular lymphomas. British Journal of Haematology. 2007;137:409–415. doi: 10.1111/j.1365-2141.2007.06596.x. [DOI] [PubMed] [Google Scholar]
- Call T, Phyilky R, Noel P, Habermann T, Beard C, O’Fallon W, Kurland L. Incidence of chronic lymphocytic leukemia in Olmsted County, Minnesota, 1935 through 1989, with emphasis on changes in initial stage at diagnosis. Mayo Clinic Proceedings. 1994;69:323–328. doi: 10.1016/s0025-6196(12)62215-0. [DOI] [PubMed] [Google Scholar]
- Castro JE, Prada CE, Loria O, Kamal A, Chen L, Burrows FJ, Kipps TJ. ZAP-70 is a novel conditional heat shock protein 90 (Hsp90) client: inhibition of Hsp90 leads to ZAP-70 degradation, apoptosis, and impaired signaling in chronic lymphocytic leukemia. Blood. 2005;106:2506–2512. doi: 10.1182/blood-2005-03-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheson BD, Bennett JM, Grever M, Kay NE, Keating M, O’Brien O, Rai K. National Cancer Institute-Sponsored Working Group Guidelines fo Chronic Lymphocytic Leukemia: Reised Guidelines for Diagnosis and Treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- Contal C, O’Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Computational Statistics and Data Analysis. 1999;30:253–270. [Google Scholar]
- Dewald G, Brockman S, Paternoster S, Bone N, O’Fallon J, allmer C, James C, Jelinek D, Tschumper R, Hanson C, Pruthi R, Witzig T, Call T, Kay N. Chromosome anomalies detected by interphase fluorscence in hybridization: correlation with significant biological features of chronic lymphocytic leukemia. British Journal of Haematology. 2003;121:287–295. doi: 10.1046/j.1365-2141.2003.04265.x. [DOI] [PubMed] [Google Scholar]
- Furnival GM, Wilson RW. Regression by leaps and bounds. Technometrics. 1974;16:499–511. [Google Scholar]
- Hanson CA, Kurtin PJ, Katzmann JA, Hoyer JD, Li CY, Hodnefield JM, Meyers CH, Habermann TM, Witzig TE. Immunophenotypic analysis of peripheral blood and bone marrow in the staging of B-cell malignant lymphoma. Blood. 1999;94:3889–3896. [PubMed] [Google Scholar]
- Jelinek DF, Tschumper RC, Geyer SM, Bone ND, Dewald GW, Hanson CA, Stenson MJ, Witzig TE, Tefferi A, Kay NE. Analysis of clonal B-cell CD38 and immunoglobulin variable region sequence status in relation to clinical outcome for B-chronic lymphocytic leukaemia. Br J Haematol. 2001;115:854–861. doi: 10.1046/j.1365-2141.2001.03149.x. [DOI] [PubMed] [Google Scholar]
- Kay NE, O’Brien S M, Pettitt AR, Stilgenbauer S. The role of prognostic factors in assessing ‘high-risk’ subgroups of patients with chronic lymphocytic leukemia. Leukemia. 2007 doi: 10.1038/sj.leu.2404802. [DOI] [PubMed] [Google Scholar]
- Mackus WJ, Frakking FN, Grummels A, Gamadia LE, De Bree GJ, Hamann D, Van Lier RA, Van Oers MH. Expansion of CMV-specific CD8+CD45RA+CD27-T cells in B-cell chronic lymphocytic leukemia. Blood. 2003;102:1057–1063. doi: 10.1182/blood-2003-01-0182. [DOI] [PubMed] [Google Scholar]
- Molica S, Levato D. What is changing in the natural history of chronic lymphocytic leukemia. Haematologica. 2001;86:8–12. [PubMed] [Google Scholar]
- Molica S, Levato D, Dattilo A. Natural history of early chronic lymphocytic leukemia. A single institution study with emphasis on the impact of disease-progression on overall survival. Haematologica. 1999;84:1094–1099. [PubMed] [Google Scholar]
- Porrata LF, Ristow K, Witzig TE, Tuinistra N, Habermann TM, Inwards DJ, Ansell SM, Micallef IN, Johnston PB, Markovic SN. Absolute lymphocyte count predicts therapeutic efficacy and survival at the time of radioimmunotherapy in patients with relapsed follicular lymphomas. Leukemia. 2007 doi: 10.1038/sj.leu.2404819. [DOI] [PubMed] [Google Scholar]
- Rassenti LZ, Huynh L, Toy TL, Chen L, Keating MJ, Gribben JG, Neuberg DS, Flinn IW, Rai KR, Byrd JC, Kay NE, Greaves A, Weiss A, Kipps TJ. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. New England Journal of Medicine. 2004;351:893–901. doi: 10.1056/NEJMoa040857. [DOI] [PubMed] [Google Scholar]
- Rozman C, Bosch F, Montserrat E. Chronic lymphocytic leukemia: a changing natural history? Leukemia. 1997;11:775–778. doi: 10.1038/sj.leu.2400679. [DOI] [PubMed] [Google Scholar]
- Shanafelt TD, Geyer S, Kay N. Prognosis at diagnosis: integrating molecular biologic insights into clinical practice for patients with CLL. Blood. 2004;103:1202–1210. doi: 10.1182/blood-2003-07-2281. [DOI] [PubMed] [Google Scholar]
- Shanafelt TD, Jelinek D, Tschumper R, Schwager S, Nowakowski G, DeWald GW, Kay NE. Cytogenetic abnormalities can change during the course of the disease process in chronic lymphocytic leukemia. J Clin Oncol. 2006a;24:3218–3219. doi: 10.1200/JCO.2006.06.1077. author reply 3219-3220. [DOI] [PubMed] [Google Scholar]
- Shanafelt TD, Witzig TE, Fink SR, Jenkins RB, Paternoster SF, Smoley SA, Stockero KJ, Nast DM, Flynn HC, Tschumper RC, Geyer S, Zent CS, Call TG, Jelinek DF, Kay NE, Dewald GW. Prospective evaluation of clonal evolution during long-term follow-up of patients with untreated early-stage chronic lymphocytic leukemia. J Clin Oncol. 2006b;24:4634–4641. doi: 10.1200/JCO.2006.06.9492. [DOI] [PubMed] [Google Scholar]
- Siddiqui M, Ristow K, Markovic SN, Witzig TE, Habermann TM, Colgan JP, Inwards DJ, White WL, Ansell SM, Micallef IN, Johnston PB, Call TG, Porrata LF. Absolute lymphocyte count predicts overall survival in follicular lymphomas. British Journal of Haematology. 2006;134:596–601. doi: 10.1111/j.1365-2141.2006.06232.x. [DOI] [PubMed] [Google Scholar]
- Solal-Celigny P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R, Au WY, Bellei M, Brice P, Caballero D, Coiffier B, Conde-Garcia E, Doyen C, Federico M, Fisher RI, Garcia-Conde JF, Guglielmi C, Hagenbeek A, Haioun C, LeBlanc M, Lister AT, Lopez-Guillermo A, McLaughlin P, Milpied N, Morel P, Mounier N, Proctor SJ, Rohatiner A, Smith P, Soubeyran P, Tilly H, Vitolo U, Zinzani PL, Zucca E, Montserrat E. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–1265. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- Torres HA, Kontoyiannis DP, Aguilera EA, Younes A, Luna MA, Tarrand JJ, Nogueras GM, Raad II, Chemaly RF. Cytomegalovirus infection in patients with lymphoma: an important cause of morbidity and mortality. Clin Lymphoma Myeloma. 2006;6:393–398. doi: 10.3816/CLM.2006.n.016. [DOI] [PubMed] [Google Scholar]
- Williams B, Mandrekar J, Mandrekar S, Cha S, Furth A. Technical Report Series N#79: Finding Optimal Cutpoints for Continuous Co-variates with Binary and Time-to-Event Outcomes. M.C.D.o.H.S. Research; Rochester, MN: 2006. [Google Scholar]