Abstract
OBJECTIVE
To examine whether treatment with guideline-recommended care (surgery and chemotherapy) is associated with mortality differences between black and white women with advanced epithelial ovarian cancer.
METHODS
We conducted an observational cohort study using the Surveillance, Epidemiology, and End Results (SEER) linked to Medicare claims for 1995-2007. We evaluated long-term survival for 4,695 black and white women with stage III or IV epithelial ovarian cancer with Kaplan-Meier analysis and Cox regression, and then in patients matched by propensity score to create two similar cohorts for comparison. We investigated the association between race, stage, and survival among women who were treated with guideline-recommended care and those who received incomplete treatment.
RESULTS
Black women with advanced epithelial ovarian cancer were more likely to die than white women; unadjusted hazard ratio (HR):1.27 (95% confidence interval [CI]: 1.10-1.46). Black women were less likely than white women to receive guideline-recommended care (54% vs. 68%, p<0.001) and women who did not receive recommended treatment had lower survival than women who received recommended care. Cox proportional hazards models demonstrated no black versus white differences in mortality among women who were treated with guideline-recommended care; adjusted HR:1.04 (95% CI: 0.85-1.26) or among women who received incomplete treatment; adjusted HR:1.09 (95% CI: 0.89-1.34). The survival analysis of patients matched by propensity score confirmed these analyses.
Conclusions
Differences in rates of treatment with guideline-recommended care are associated with black–white mortality disparities among women with advanced epithelial ovarian cancer.
Introduction
Epithelial ovarian cancer is the 5th leading cause of cancer deaths among women in the United States and significant racial and ethnic disparities exist in ovarian cancer mortality.1 Black women are more likely to die from ovarian cancer than white women in this country. Between 1975 and 2005, the 5-year survival rate for U.S. white women with advanced ovarian cancer improved from 37% to 45% but declined for black women from 43% to 38%.2 This discrepancy is particularly striking because the overall improvement in survival is largely attributed to the introduction of platinum-based chemotherapy.3
The higher mortality experienced by black women with ovarian cancer in this country is thought to be due to the fact that black women are more likely to present with advanced disease.4 Using Surveillance, Epidemiology, and End Results (SEER) data, investigators found that blacks are more likely to be diagnosed at a later stage and this difference is most pronounced for stage IV disease which accounts for 41% of black compared with 34% of white women with ovarian cancer (p<.0001).4 Survival is directly related to stage of disease at the time of diagnosis with a 5-year survival rate of 89% for women with Stage I disease and declining to 11% for women with the most advanced disease, Stage IV.5 Black–white mortality disparities may also be explained by differences in the receipt of treatment, with blacks less likely to undergo ovarian cancer specific procedures (i.e., hysterectomy, colon resection, and lymphadenectomy), and less likely to be operated on by high-volume surgeons.6
Many of the studies investigating racial disparities in ovarian cancer mortality have used data from single institutions, case studies, and voluntary registries.7,8 The few research studies using population based samples such as Surveillance, Epidemiology, and End Results (SEER) database are limited because these data do not contain information on chemotherapy or comorbidities.5 Studies using SEER-Medicare linked data that contain reliable information on both surgery and chemotherapy have found that blacks, those with higher comorbidity scores, and older age are less likely to receive surgery and chemotherapy.9,10 None of these studies, however, examined whether differences in rates of treatment with guideline-recommended care was associated with black–white mortality disparities in advanced ovarian cancer survival using propensity score matching methods.
Current guidelines from the National Comprehensive Cancer Network recommend that primary treatment for most patients with advanced ovarian cancer should include cytoreductive surgery and at least six cycles of systemic chemotherapy.11 We sought to examine whether treatment with guideline-recommended care is associated with mortality differences between U.S. white and black women with advanced epithelial ovarian cancer.
METHODS
We used the Surveillance, Epidemiology, and End Results (SEER) linked to Medicare claims (Medicare Enrollment Database) for 1995-2007. The SEER database includes cancer registries representing approximately 15% to 25% of the United States population during this study period.2 Validity and reliability of the combined SEER-Medicare database have been studied extensively.12-16 The Mount Sinai School of Medicine Institutional Review Board (Program for the Protection of Human Subjects) approved this study.
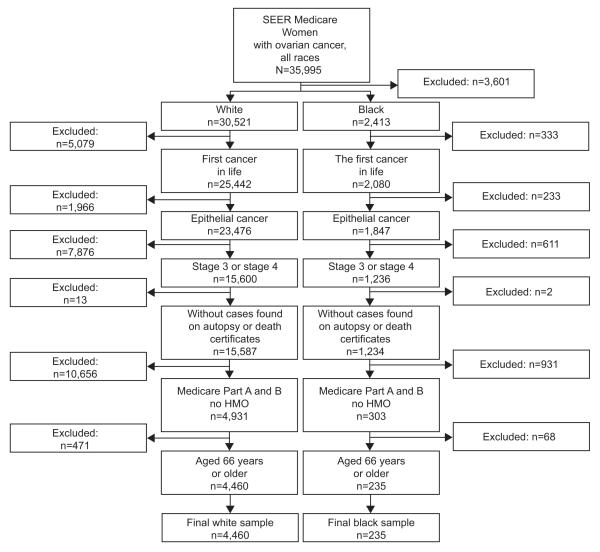
Using the linked SEER-Medicare database, we identified women diagnosed with ovarian cancer from January 1, 1996 - December 31, 2007 (N=35,995). Among them, there were 32,934 white and blacks. We excluded women with more than one primary cancer and women with nonepithelial ovarian cancer, as well as women diagnosed on autopsy or death certificate and women with stage I or II ovarian cancer as classified by the American Joint Commission on Cancer. We excluded women not enrolled in Medicare parts A and B and women enrolled in an HMO who do not have Medicare fee for service claims. We excluded women less than 66, to provide one year of data on comorbidities that can influence treatment decisions. Treatment information was obtained from Medicare parts A and B claims. All steps of study population selection are shown in Figure 1.
Figure 1.
Population selection. Surveillance, epidemiology,and end results (SEER).
To identify treatment, we searched Medicare claims from 30 days prior to diagnosis and up to 120 days after the date of diagnosis. Since SEER reports only month and year of the diagnoses, the diagnosis date was assigned the 15th day of the month of diagnosis. We identified primary surgical treatment in MEDPAR files using ICD-9 procedure and HCPCS codes. Surgical resection codes were determined based on literature review and expert gynecologic oncologic and biller’s opinion. 17 We identified treatment with chemotherapy using inpatient, outpatient, physician claims, and DME (Durable Medical Equipment) files. We classified chemotherapy treatment in Medicare claims as the 1st chemotherapy that occurred within 180 days prior to surgery and 90 days after surgical treatment. The chemotherapy claims from all sources within three days were grouped into one. The number of chemotherapy cycles were identified from the initial chemotherapy date and by counting number of cycles.
We classified treatment into two categories: complete versus incomplete. All women who were treated with guideline-recommended care (cytoreductive surgery and six or more cycles of chemotherapy) were classified as receiving complete treatment. Incomplete treatment included women who did not receive treatment and those who received suboptimal treatment. Suboptimal treatment included surgery only, chemotherapy only, and surgery plus 5 or less cycles of chemotherapy. We conducted additional analyses varying the definition of complete treatment as surgery plus 4 or more cycles of chemotherapy; as findings are similar, we report the standard six cycles as complete.
Sociodemographic and clinical variables of age, SEER geographic region, year of diagnosis, tumor grade, and histology were derived from SEER. Data on race for these analyses was ascertained from the Medicare Enrollment Database an accurate source to assess race.18-20 Comorbidity score was determined using Medicare claims for the 12 months prior to ovarian cancer diagnosis to calculate the Charlson comorbidity index.21,22 Bivariate analyses were performed using chi-square tests for categorical variable and t-test for continuous variables. Multivariable logistic regression was performed to investigate the association between treatment with guideline-recommended care and race. Kaplan-Meier survival analysis methods were used to compare the overall survival of black versus white women with advanced epithelial ovarian cancer and to compare the overall survival of women who received complete treatment versus women who received incomplete treatment. Adjustment for multiple comparisons for the logrank test was done with Sidak test. Cox proportional hazard regression models assessed the association between overall survival and race, and overall survival and treatment group after controlling for sociodemographics, tumor characteristics, and comorbidity. We used the Martingale methods to check the proportional hazard assumption.
We created a sample of black and white patients with similar characteristics using propensity score matching.23 All baseline sociodemographic and clinical factors including age, comorbidity, stage, histology, grade, year of diagnosis, and SEER region were included in a logistic model predicting black race. Once the model was fitted, we used a 1:2 scheme without replacement to match black and white patients by propensity scores. We used a paired t test or McNemar test, as appropriate to assess whether baseline characteristics of black and white patients were well-balanced in the matched cohort.(Appendix 1) In addition, standardized difference between blacks and white women prior and after the matching were calculated. Rates of complete treatment were compared for the matched pairs of blacks and whites using McNemar chi square test. Survival of black and white patients were compared using a marginal Cox model with a robust sandwich variance. estimator.24,25 All statistical analysis was performed using the SAS system software version 9.2 (SAS Institute Inc, Cary, NC).
RESULTS
Our final sample included 4,695 women: 235 blacks and 4,460 whites. Of the 4,695 patients, 5% (n=235) were black and the remainder were white (Table 1). Black women were more likely to present with Stage IV disease (42.1% vs. 33.5%, p=.007), have higher mean Charlson comorbidity scores (0.93 vs. 0.56, p<.001), and one or more comorbidities (52.8% vs. 35.0%, p<.001). Blacks were more likely to come from the SEER geographic regions of the Midwest and the South and to have unknown tumor grade than whites.
Table 1.
Patient Baseline Characteristics
| Demographics | Whites n=4,460 |
Blacks n=235 |
P |
|---|---|---|---|
| Mean age at diagnosis | 74.9 | 74.4 | NS |
| Age groups | 0.025 | ||
| Age 66-69 | 22.5% | 26.8% | |
| Age 70-74 | 28.1% | 33.2% | |
| Age 75-79 | 27.0% | 18.7% | |
| Age 80-84 | 15.3% | 12.8% | |
| Age 85 or greater | 7.0% | 8.5% | |
| Stage | 0.007 | ||
| Stage III | 66.5% | 57.9% | |
| Stage IV | 33.5% | 42.1% | |
| Comorbidities | |||
| CHF | 5.2% | 9.4% | 0.006 |
| Diabetes | 12.7% | 29.9% | <.001 |
| Diabetes with sequelae | 2.1% | 6.4% | <.001 |
| Charlson Comorbidity Score | 0.56 | 0.93 | <.001 |
| Comorbidity Groups | <.001 | ||
| No Comorbidity | 65.0% | 47.2% | |
| 1 Comorbidity | 22.3% | 30.6% | |
| 2 Comorbidities | 7.7% | 11.9% | |
| 3+ Comorbidities | 5.0% | 10.2% | |
| Histology | 0.351 | ||
| Adenocarcinoma | 81.8% | 81.3% | |
| Clear Cell | 1.9% | ≤4.7% | |
| Endometroid | 8.1% | 7.7% | |
| Mucinous | 3.7% | 6.0% | |
| Other Epithelian | 4.6% | ≤4.7% | |
| Treatment | <.0001 | ||
| Complete Treatment | 67.8% | 54.0% | |
| Incomplete Treatment | 32.2% | 46.0% | |
| - Surgery only | 18.3% | 28.1% | <0.001 |
| - Surgery + 5 or less Chemotherapy cycles |
13.9% | 17.9% | NS |
| Debulking surgery (SEER code) | 29.1% | 26.7% | NS |
| Grade | NS | ||
| Grade 1 | 3.1% | ≤4.7% | |
| Grade 2 | 15.9% | 17.0% | |
| Grade 3 | 50.6% | 44.7% | |
| Grade 4 | 13.0% | ≤12.0% | |
| Unknown grade | 17.4% | 24.3% | |
| SEER Region | <.001 | ||
| Northeast | 20.9% | 16.2% | |
| Midwest | 19.5% | 30.6% | |
| South | 15.2% | 30.2% | |
| West | 44.5% | 23.0% | |
NS, not significant; SEER, Surveillance, Epidemiology, and End Results.
Black women were less likely to receive complete treatment (surgery and six or more cycles of chemotherapy) as compared with white women (54% vs. 68%, p<.001). In a multivariable model predicting complete treatment that controlled for age, comorbidity, stage, and tumor characteristics, blacks were less likely than whites to be treated with guideline-recommended care (adjusted odds ratio 0.65; 95% CI: 0.48-0.88). Black women were more likely than whites to receive only surgery (28% vs. 18%), p<.001).
Overall, black women were more likely to die than white women, unadjusted hazard ratio (HR) of 1.27, 95% confidence interval (CI):1.10-1.46. Women who received complete treatment were less likely to die than women who received incomplete treatment, unadjusted HR 0.32, 95% CI: 0.30-0.34. Survival analyses stratified for treatment, revealed no racial mortality difference among women who received incomplete treatment: unadjusted HR of 1.13, 95% CI: 0.93-1.38 and among women who received complete treatment group: unadjusted HR of 1.11, 95% CI: 0.91-1.34, see Figure 2. In a Cox model adjusting for age, comorbidity, stage, and tumor characteristics, black women were more likely to die than white women (adjusted HR of 1.17; 95% CI:1.02-1.35). After adjusting for treatment, the hazard ratio for black women was no longer significantly elevated: adjusted HR of 1.10, 95% CI: 0.95-1.26, see Table 2.
Figure 2.
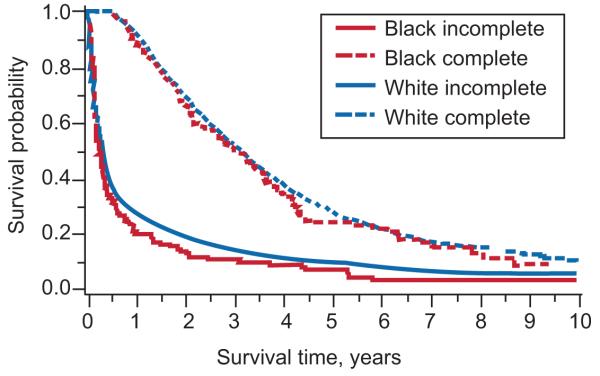
Survival analysis for treatment by race (P value for race is 0.10; P value for treatment <0.001).
Number at risk: Complete treatment
White: 3,022, 2,094, 963, 438, 208, 85
Black: 127, 84, 38, 19, less than or equal to 11, less than or equal to 11.
Number at risk: Incomplete treatment
White: 1,438, 273, 147, 76, 53, 28
Black: 108, 15, less than or equal to 11, less than or equal to 11, less than or equal to 11, less than or equal to 11.
Table 2.
Effect of Treatment on Black–White Mortality Differences in Advanced Ovarian Cancer
| Type of Model | Hazard Ratio for Black (Compared With White Women) With Advanced Ovarian Cancer |
95% Confidence Interval |
|---|---|---|
| Unadjusted | 1.27 | 1.10-1.46 |
| Adjusted for demographic, comorbidity, stage, and tumor characteristics |
1.17 | 1.02-1.35 |
| Adjusted for demographic, comorbidity, stage, tumor characteristics, and treatment |
1.10 | 0.95-1.26 |
| Confirmatory Analyses of Propensity Matched Cohorts | ||
| Not adjusted for treatment | 1.18 | 1.00-1.38 |
| Adjusted for treatment | 1.06 | 0.84-1.34 |
Analyses using propensity matched cohorts confirmed these results. The standardized differences between whites and blacks in this matched cohort were less than 10% for the majority of variables.26 (Appendix 1) The final propensity score model yielded good model discrimination (c-statistic was 0.75). The propensity score matching analyses demonstrated that blacks were less likely to receive complete treatment than whites (54% versus 66% respectively, p<.03). Similar to the analyses using the full data set, blacks were more likely to die than whites in survival analyses among the propensity matched cohorts: HR of 1.18, 95% CI: 1.00-1.38). However, there was no difference in mortality between black and white women in the survival analysis of propensity matched cohorts after adjustment for treatment; adjusted HR of 1.06, 95% CI: 0.84-1.34 (Table 2).
In the complete treatment group older age, later stage, increasing comorbidity, increasing or unknown grade were associated with higher mortality (Table 3). Among women in the incomplete treatment group, older age, later stage, increasing comorbidity, mucinous histology, and increasing or unknown grade increased risk of mortality.
Table 3.
Cox Model Predicting Mortality
| Hazards Ratio | 95% CI | P | |
|---|---|---|---|
| Received Complete Treatment | |||
| Black vs. White | 1.04 | 0.85-1.26 | NS |
| Age at diagnosis | 1.03 | 1.02-1.03 | <.001 |
| Stage IV vs. stage III | 1.18 | 1.08-1.28 | <.001 |
| Charlson comorbidity score | 1.14 | 1.09-1.19 | <.001 |
| Histology: mucinous vs. serous | 1.14 | 0.87-1.50 | NS |
| Clear cell vs. serous | 0.86 | 0.62-1.20 | NS |
| Endometroid vs. serous | 0.82 | 0.71-0.95 | 0.001 |
| Other epithelial vs. serous | 0.79 | 0.64-0.97 | 0.024 |
| Grade 2 vs. Grade 1 | 1.44 | 1.09-1.91 | 0.011 |
| Grade 3 vs. Grade 1 | 1.36 | 1.04-1.78 | 0.027 |
| Grade 4 vs. Grade 1 | 1.38 | 1.04-1.84 | 0.028 |
| Unknown Grade* vs. Grade 1 | 1.71 | 1.29-2.27 | <0.001 |
| Midwest vs. Northeast | 1.09 | 0.96-1.24 | NS |
| South vs. Northeast | 1.08 | 0.95-1.24 | NS |
| West vs. Northeast | 0.94 | 0.84-1.05 | NS |
| Year of diagnoses | 0.99 | 0.98-1.00 | NS |
| Received Incomplete Treatment | |||
| Black vs. White | 1.09 | 0.89-1.34 | NS |
| Age at diagnosis | 1.02 | 1.01-1.03 | <.001 |
| Stage IV vs. stage III | 1.49 | 1.33-1.66 | <.001 |
| Charlson comorbidity score | 1.10 | 1.05-1.15 | <.001 |
| Histology: mucinous vs. serous | 1.46 | 1.19-1.79 | <.001 |
| Clear cell vs. serous | 0.71 | 0.49-1.05 | NS |
| Endometroid vs. serous | 0.92 | 0.75-1.12 | NS |
| Other epithelial vs. serous | 1.14 | 0.90-1.44 | NS |
| Grade 1 vs. grade 3 | 1.40 | 1.02-1.90 | 0.035 |
| Grade 2 vs. grade 3 | 1.63 | 1.2 -2.18 | 0.001 |
| Grade 4 vs. grade 3 | 1.53 | 1.10-2.13 | 0.011 |
| Unknown grade vs. grade 3 | 1.70 | 1.26-2.30 | <0.001 |
| Midwest vs. Northeast | 0.95 | 0.81-1.12 | NS |
| South vs. Northeast | 0.98 | 0.82-1.16 | NS |
| West vs. Northeast | 0.89 | 0.78-1.03 | NS |
| Year of diagnoses | 1.01 | 1.00-1.03 | NS |
CI, confidence interval; NS, not significant.
DISCUSSION
Black–white mortality disparities in survival from advanced epithelial ovarian cancer among Medicare beneficiaries are associated with treatment differences. Black women are less likely to receive recommended surgical and chemotherapy treatment for advanced epithelial ovarian cancer and women who receive incomplete treatment are more likely to die from this disease. Lower rates of surgery have been suggested to explain higher mortality of black women from advanced epithelial ovarian cancer.6,7,27 While others have documented lower rates of chemotherapy among black women, these studies did not take into account potential selection bias inherent in observational data. This is a serious omission given racial differences in comorbidity, stage, and age at diagnosis, all factors that affect physician and patient decision making and also affect survival. Our data demonstrate that black women are more likely than white women to receive surgery only but are often not receiving recommended post surgical chemotherapy. This study advances current knowledge controlling for serious potential confounders of race’s effect on receipt of cancer treatment and on mortality. We found no racial difference in mortality among women with equivalent risk factors and similar treatments.
Reasons as to why black women are less likely to receive postsurgical chemotherapy are unclear. Women with greater comorbidity were less likely to receive chemotherapy.10 However, whether this association is driven by physician or patient preference or sound clinical judgment is uncertain. Our data sources do not allow us to determine the degree to which patient refusal versus providers not referring patients for chemotherapy explain this finding. Research studying causes of racial disparities in breast cancer found that both patient and system issues affect racially disparate treatment effects.28,29 Black women were less likely to receive adjuvant chemotherapy post breast surgery. While surgeons referred patients at similar rates, system failures, cases in which patients were referred to oncologists, did not refuse treatment, yet care did not ensue, primarily accounted for the racial disparities in receipt of adjuvant chemotherapy. Black women were less likely to know the benefits of adjuvant treatment, were more mistrustful of the care delivery system, and less likely to receive adjuvant treatment. Whether system failures, physician recommendations, or patient preferences explain lower treatment rates in black women with advanced epithelial ovarian cancer is not known.
This study shares limitations posed by observational administrative data. Ascertainment of treatment and comorbidities is subject to the accuracy of coding. Although Medicare data and chart review have high level of agreement for surgery and chemotherapy, the accuracy is lower for diagnostic codes of comorbid illness and treatment complications.14 Although there are not effective screening instruments for ovarian cancer detection, whites continue to be diagnosed at earlier stages than blacks. To reduce the potential effect of earlier detection we limited our study sample to women with later stage cancers. Given that Medicare starts at age 65 for the vast majority of people, we were unable to investigate outcomes among a younger age group of women. Because this is an observational study, there are potentially unmeasured characteristics associated with race that may have biased our results. However, our nearly identical findings of both Cox proportional hazards models and propensity scores analyses suggest that treatment differences do contribute to racial disparities in advanced epithelial ovarian cancer mortality among women older than 65 years.
Black women have higher mortality from a number of different types of cancer and ovarian cancer is no exception. While the mortality gap has been largely attributed to the fact that blacks are more likely to present at a later stage as compared with white women, our findings suggest that treatment differences account for a portion of the racial disparity in ovarian cancer mortality. There was no mortality gap between blacks and whites treated with guideline-recommended care nor between blacks and whites who received incomplete treatment. We found that a larger proportion of black than white women did not receive recommended care. Future research needs to investigate patient, provider, and system factors that may explain this finding. The silver lining of our disturbing findings lies in the real possibility of remediating a significant racial disparity in cancer mortality by ensuring guideline concordant treatment is provided to all who can benefit from it.
Acknowledgments
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Supported by the National Cancer Institute (1RO1CA157176-01).
APPENDIX
Appendix 1.
Patients’ Characteristics Before and After Matching by Propensity Score
| Before Matching | After Matching | |||||||
|---|---|---|---|---|---|---|---|---|
| White (n=4,460) |
Black (n=235) |
P | Standardized Difference |
White (n=470) |
Black (n=235) |
P | Standardized Difference |
|
| Mean age at diagnosis |
74.9 | 74.4 | 0.252 | 13 | 74.7 | 74.4 | 0.144 | 0 |
| Age 66-69 | 22.5% | 26.8% | 0.128 | 16 | 27.7% | 26.8% | 0.599 | 2 |
| Age 70-74 | 28.1% | 33.2% | 0.093 | 6 | 36.4% | 33.2% | 0.541 | 7 |
| Age 75-79 | 27.0% | 18.7% | 0.005 | 19 | 18.3% | 18.7% | 0.763 | 1 |
| Age 80-84 | 15.3% | 12.8% | 0.285 | 7 | 10.9% | 12.8% | 0.758 | 6 |
| Age 85 or greater | 7.0% | 8.5% | 0.393 | 2 | 6.8% | 8.5% | 0.353 | 6 |
| Stage III | 66.5% | 57.9% | 0.007 | 15 | 62.1% | 57.9% | 0.094 | 9 |
| Stage IV | 33.5% | 42.1% | 0.007 | 15 | 37.9% | 42.1% | 0.094 | 9 |
| Charlson Comorbidity Score |
0.56 | 0.93 | <.001 | 38 | 0.87 | 0.928 | 0.577 | 0 |
| No comorbidity | 65.0% | 47.2% | <.001 | 39 | 49.4% | 47.2% | 0.354 | 4 |
| 1 comorbidity | 22.3% | 30.6% | 0.003 | 20 | 27.7% | 30.6% | 0.225 | 7 |
| 2 comorbidities | 7.7% | 11.9% | 0.021 | 12 | 14.3% | 11.9% | 0.206 | 7 |
| 3+ comorbidities | 5.0% | 10.2% | <0.001 | 25 | 8.7% | 10.2% | 0.384 | 5 |
| Histology | ||||||||
| Serous | 81.8% | 81.3% | 0.841 | 4 | 89.4% | 81.3% | 0.134 | 1 |
| Clear cell | 1.9% | ≤4.7% | 0.445 | 18 | ≤4.7% | ≤4.7% | 0.564 | 13 |
| Endometroid | 8.1% | 7.7% | 0.812 | 9 | ≤4.7% | 7.7% | 0.369 | 2 |
| Mucinous | 3.7% | 6.0% | 0.074 | 6 | ≤4.7% | 6.0% | 0.670 | 0 |
| Other epithelial | 4.6% | ≤4.7% | 0.189 | 0 | ≤4.7% | ≤4.7% | 0.317 | 0 |
| Grade | ||||||||
| 1 | 3.1% | ≤4.7% | 0.665 | 2 | ≤4.7% | ≤4.7% | 0.046 | 1 |
| 2 | 15.9% | 17.0% | 0.646 | 6 | 14.4% | 17.0% | 0.297 | 7 |
| 3 | 50.6% | 44.7% | 0.076 | 12 | 43.0% | 44.7% | 0.473 | 3 |
| 4 | 13.0% | 11.5% | 0.4934 | 5 | 14.3% | ≤12.0% | 0.047 | 8 |
| 9* | 17.4% | 24.3% | 0.007 | 15 | 26.0% | 24.3% | 0.606 | 4 |
| Northeast | 20.9% | 16.2% | 0.080 | 8 | 13.8% | 16.2% | 0.869 | 7 |
| Midwest | 19.5% | 30.6% | <.001 | 23 | 34.0% | 30.6% | 0.249 | 7 |
| South | 15.2% | 30.2% | <.001 | 37 | 28.5% | 30.2% | 0.599 | 4 |
| West | 44.5% | 23.0% | <.001 | 48 | 23.6% | 23.0% | 0.414 | 2 |
| Year of diagnoses | 2002.3 | 2001.3 | 0.473 | 5 | 2001.7 | 2001.3 | 0.213 | 0 |
| 1995-1999 | 27.9% | 31.1% | 0.299 | 7 | 27.0% | 31.1% | 0.189 | 9 |
| 2000-2003 | 38.9% | 37.9% | 0.747 | 2 | 36.4% | 37.9% | 0.906 | 3 |
| 2004-2007 | 33.1% | 31.1% | 0.510 | 4 | 36.6% | 31.1% | 0.179 | 12 |
| Probability | 0.048 | 0.093 | <.001 | 73 | 0.091 | 0.091 | 0.263 | 0 |
Cell type not determined
Footnotes
Financial Disclosure: Dr. Wisnivesky is a member of the research board of EHE International, he has received consulting honorarium from Merck Pharmaceutical, UBS, and IMS Health and a research grant from GlaxoSmithKline. The other authors did not report any potential conflicts of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.National Cancer Institute General Information About Ovarian Epithelial Cancer. 2012 Nov 7; Available at: http://www.cancer.gov/cancertopics/pdq/treatment/ovarianepithelial/HealthProfessional#Section_416. Retrieved.
- 2.National Cancer Institute Surveillance Epidemiology and End Results (SEER) Program. 2012 Nov 7; Available at: http://www.seer.cancer.gov/csr/1975_2009_pops09/browse_csr.php?section=21&page=sect_21_table.08.html. Retrieved.
- 3.Terplan M, Temkin S, Tergas A, Lengyel E. Does equal treatment yield equal outcomes? The impact of race on survival in epithelial ovarian cancer. Gynecol Oncol. 2008 Nov;111(2):173–178. doi: 10.1016/j.ygyno.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan JK, Zhang M, Hu JM, Shin JY, Osann K, Kapp DS. Racial disparities in surgical treatment and survival of epithelial ovarian cancer in United States. J Surg Oncol. 2008 Feb 1;97(2):103–107. doi: 10.1002/jso.20932. [DOI] [PubMed] [Google Scholar]
- 5.Barnholtz-Sloan JS, Tainsky MA, Abrams J, et al. Ethnic differences in survival among women with ovarian carcinoma. Cancer. 2002 Mar 15;94(6):1886–1893. doi: 10.1002/cncr.10415. [DOI] [PubMed] [Google Scholar]
- 6.Bristow RE, Zahurak ML, Ibeanu OA. Racial disparities in ovarian cancer surgical care: a population-based analysis. Gynecol Oncol. 2011 May 1;121(2):364–368. doi: 10.1016/j.ygyno.2010.12.347. [DOI] [PubMed] [Google Scholar]
- 7.Bristow RE, Ueda S, Gerardi MA, Ajiboye OB, Ibeanu OA. Analysis of racial disparities in stage IIIC epithelial ovarian cancer care and outcomes in a tertiary gynecologic oncology referral center. Gynecol Oncol. 2011 Aug;122(2):319–323. doi: 10.1016/j.ygyno.2011.04.047. [DOI] [PubMed] [Google Scholar]
- 8.Farley JH, Tian C, Rose GS, Brown CL, Birrer M, Maxwell GL. Race does not impact outcome for advanced ovarian cancer patients treated with cisplatin/paclitaxel: an analysis of Gynecologic Oncology Group trials. Cancer. 2009 Sep 15;115(18):4210–4217. doi: 10.1002/cncr.24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du XL, Sun CC, Milam MR, Bodurka DC, Fang S. Ethnic differences in socioeconomic status, diagnosis, treatment, and survival among older women with epithelial ovarian cancer. Int J Gynecol Cancer. 2008 Jul-Aug;18(4):660–669. doi: 10.1111/j.1525-1438.2007.01081.x. [DOI] [PubMed] [Google Scholar]
- 10.Thrall MM, Gray HJ, Symons RG, Weiss NS, Flum DR, Goff BA. Trends in treatment of advanced epithelial ovarian cancer in the Medicare population. Gynecol Oncol. 2011 Jul;122(1):100–106. doi: 10.1016/j.ygyno.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.NCCN . Practice guidelines in Oncology v2.2010 Oavrian FAllopian Tube and Primary Peritoneal Carinomas. 2010. 2010. [Google Scholar]
- 12.Warren JLP, Harlan LCP, Fahey A, et al. Utility of the SEER-Medicare Data to Identify Chemotherapy Use. Medical Care. 2002;40((8)(Supplement)):IV-55–IV-61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 13.Warren JLP, Klabunde CNP, Schrag DP, Bach PBMD, Riley GFM. Overview of the SEER-Medicare Data: Content, Research Applications, and Generalizability to the United States Elderly Population. Medical Care. 2002;40((8)(Supplement)):IV-3–IV-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 14.Cooper GSMD, Virnig BP, Klabunde CNP, Schussler NBS, Freeman JPa, Warren JLP. Use of SEER-Medicare Data for Measuring Cancer Surgery. Medical Care. 2002;40((8)(Supplement)):IV-43–IV-48. doi: 10.1097/00005650-200208001-00006. [DOI] [PubMed] [Google Scholar]
- 15.Potosky ALP, Warren JLP, Riedel ERMA, Klabunde CNP, Earle CCMD, Begg CBP. Measuring Complications of Cancer Treatment Using the SEER-Medicare Data. Medical Care. 2002;40((8)(Supplement)):IV-62–IV-68. doi: 10.1097/00005650-200208001-00009. [DOI] [PubMed] [Google Scholar]
- 16.Virnig BAP, Warren JLP, Cooper GSMD, Klabunde CNP, Schussler NBS, Freeman JP. Studying Radiation Therapy Using SEER-Medicare-Linked Data. Medical Care. 2002;40((8)(Supplement)):IV-49–IV-54. doi: 10.1097/00005650-200208001-00007. [DOI] [PubMed] [Google Scholar]
- 17.Earle CC, Schrag D, Neville BA, et al. Effect of surgeon specialty on processes of care and outcomes for ovarian cancer patients. J Natl Cancer Inst. 2006 Feb 1;98(3):172–180. doi: 10.1093/jnci/djj019. [DOI] [PubMed] [Google Scholar]
- 18.Arday SL, Arday DR, Monroe S, Zhang J. HCFA’s racial and ethnic data: Current accuracy and recent improvements. Health Care Financing Review. Summer Summer. 2000;21(4):107–116. 2000. [PMC free article] [PubMed] [Google Scholar]
- 19.Bach PBMDM, Guadagnoli EP, Schrag DMDMPH, Schussler NBS, Warren JLP. Patient Demographic and Socioeconomic Characteristics in the SEER-Medicare Database: Applications and Limitations. Medical Care. 2002;40((8)(Supplement)):IV-19–IV-25. doi: 10.1097/00005650-200208001-00003. [DOI] [PubMed] [Google Scholar]
- 20.Zaslavsky AM, Ayanian JZ, Zaborski LB. The Validity of Race and Ethnicity in Enrollment Data for Medicare Beneficiaries. Health Services Research. 2012;47(3pt2):1300–1321. doi: 10.1111/j.1475-6773.2012.01411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. Journal of Clinical Epidemiology. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997 Oct 15;127(8 Pt 2):757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 24.Ying G, Liu C. Statistical analysis of clustered data using SASsystem; NorthEast SAS UsersGroup (NESUG) Proceedings; 2006.pp. 1–13. [Google Scholar]
- 25.Gharibvand L, Liu L. Analysis of Survival Data with Clustered Events; SAS Global Forum Proceedings SaDA; 2009; Paper 237. [Google Scholar]
- 26.Oakes J, Kaufman J, editors. Methods in Social Epidemiology. Jon Wiley & Sons, Inc.; San Francisco: 2006. [Google Scholar]
- 27.Terplan M, Smith EJ, Temkin SM. Race in ovarian cancer treatment and survival: a systematic review with meta-analysis. Cancer Causes Control. 2009 Sep;20(7):1139–1150. doi: 10.1007/s10552-009-9322-2. [DOI] [PubMed] [Google Scholar]
- 28.Bickell NA, LePar F, Wang JJ, Leventhal H. Lost opportunities: physicians’ reasons and disparities in breast cancer treatment. J Clin Oncol. 2007 Jun 20;25(18):2516–2521. doi: 10.1200/JCO.2006.09.5539. [DOI] [PubMed] [Google Scholar]
- 29.Bickell NA, Weidmann J, Fei K, Lin JJ, Leventhal H. Underuse of breast cancer adjuvant treatment: patient knowledge, beliefs, and medical mistrust. J Clin Oncol. 2009 Nov 1;27(31):5160–5167. doi: 10.1200/JCO.2009.22.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]