Abstract
To determine if gene expression of An. gambiae is modulated in response to o’nyong-nyong virus (ONNV) infection, we utilized cDNA microarrays including about 20 000 cDNAs. Gene expression levels of ONNV-infected female mosquitoes were compared to that of the uninfected control females harvested at 14 days postinfection. In response to ONNV infection, expression levels of 18 genes were significantly modulated, being at least two-fold up- or down-regulated. Quantitative real-time PCR analysis (qRT-PCR) further substantiated the differential expression of six of these genes in response to ONNV infection. These genes have similarity to a putative heat shock protein 70, DAN4, agglutinin attachment subunit, elongation factor 1 alpha and ribosomal protein L35. One gene, with sequence similarity to mitochondrial ribosomal protein L7, was down-regulated in infected mosquitoes. The expression levels and annotation of the differentially expressed genes are discussed in the context of host/virus interaction including host translation/replication factors, and intracellular transport pathways.
Keywords: Anopheles gambiae, o’nyong-nyong virus, gene expression, cDNA microarray
Introduction
Anopheles gambiae is the primary vector of malaria parasites in sub-Saharan Africa, where almost 90% of the world’s malaria cases occur (WHO, 1998). However, An. gambiae is also the primary vector of the alphavirus o’nyong-nyong (ONNV) (Williams et al., 1965; Powers et al., 2000). ONNV epidemic data have implicated An. gambiae and Anopheles funestus as important vector species (Williams et al., 1965). This is an unusual vector–virus relationship because mosquito-borne viruses are typically transmitted by Culicine mosquitoes. ONNV is the only arbo-virus commonly transmitted by An. gambiae and the molecular basis of this unique relationship is unknown. ONNV is an alphavirus which is an enveloped, single-stranded (+) RNA virus with a genome of approximately 12 kb and serologically classified in the Semliki Forest virus complex (Karabatsos, 1975; Levinson et al., 1990). ONNV was first identified during an epidemic in Uganda in 1959, which ultimately infected over 2 million patients across East Africa from 1959 to 1961 (Johnson, 1988). ONNV symptoms in human include fever, severe back and joint pain, lymphadenopathy and an itchy rash (Corbet et al., 1961). After a 35-year absence, an ONNV epidemic occurred in Southern Uganda (Rwaguma et al., 1997).
Alphaviruses including Sindbis (SINV) and ONNV infect mosquitoes after ingestion of blood from a viremic vertebrate, replicate in midgut epithelial cells, and then disseminate through the haemoecoel to the salivary glands and other target tissues, e.g. fat body and thoracic muscle (Pierro et al., 2003). The tissue tropism of SINV differs in Aedes albopictus and Aedes aegypti mosquitoes (Jackson et al., 1993; Bowers et al., 1995), suggesting that the susceptibility of mosquitoes to alphavirus infection may be based on the existence of tissue-specific factors such as cellular proteins. The infection and replication events for alphaviruses are well understood (Strauss & Strauss, 1994). Following binding to the cell membrane and endocytosis, the RNA genome is released into the cytoplasm, and is translated into the four non-structural proteins that form the viral RNA-dependent RNA polymerase. This RNA polymerase may interact with host protein subunits which construct a replication complex and replicate the RNA genome into (−) RNA genome. (−) RNA genome produces both the (+) RNA genome and the 26S subgenome. By modulating the host translation machinery, 26S subgenomic RNAs are translated into the capsid and envelope proteins (Lemm & Rice, 1993; Strauss & Strauss, 1994). Cellular proteins are therefore critical factors for the susceptibility of mosquitoes to alphaviruses, but factors such as the modulation of host protein subunits required for viral transcription/replication/translation in the mosquito might also be essential for successful viral replication. For successful replication, the alphavirus needs effectively to modulate gene expression of host cells. We hypothesized that by comparing the expression profile of genes of ONNV-infected and uninfected An. gambiae, candidate genes that are critical for susceptibility of An. gambiae to ONNV would be identified.
To investigate the gene expression profile for An. gambiae resulting from ONNV infection, we conducted cDNA microarray experiments utilizing about 20 000 cDNAs prepared from a normalized library of An. gambiae and two immune competent cell lines (G. K. Christophides, unpublished data; http://www.komar.embl-heidelberg.de/). As cDNA microarray experiments by their nature are combined with the many sources of error associated with the microarray technology itself (Chuaqui et al., 2002), new RNA samples were used to verify the universality of the cDNA microarray results. All candidate genes from cDNA microarray experiment were further examined by qRT-PCR analysis, which has been demonstrated as a reliable technique to quantify mRNA over a broad range of mRNA expression levels (Freeman et al., 1999; Walker, 2001).
Consequently, we have identified seven transcripts that are differentially expressed in ONNV-infected female An. gambiae compared to non-infected control mosquitoes at 14 days postinfection (p.i.). Using An. gambiae and ONNV as a model, this study provides the first genome-wide molecular identification of mosquito genes, associated with arboviral infection and replication in the arthropod vector.
Results and discussion
Genome-wide screening of differentially expressed transcripts of ONNV-infected blood-fed female An. gambiae relative to naive blood-fed females was conducted at 14 days p.i. At 14 days p.i. ONNV is disseminated into the haemocoel from the midgut, infecting other tissues in the mosquito such as fat body, salivary gland and ovaries. The disseminated ONNV would therefore be able to be transmitted to humans (Vanlandingham et al., 2005).
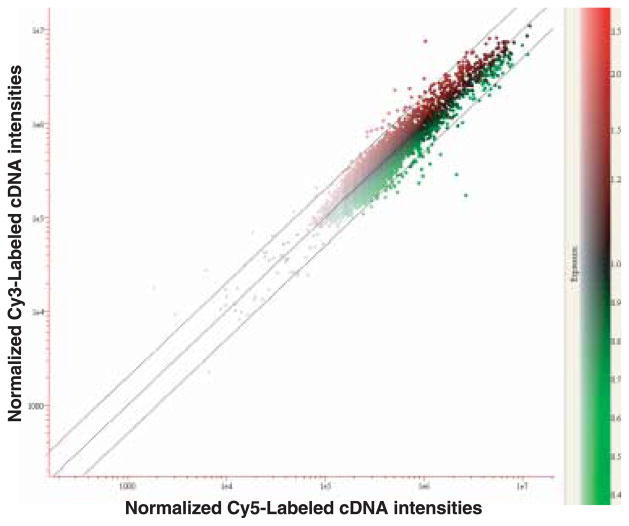
After signal intensities for hybridized cDNAs were filtered, a total of 10 116 cDNAs from the four slides with signal intensity values less than 300 pixels were excluded from further analysis. From cDNA microarray analysis using approximately 18 640 cDNAs, 64 genes were identified as being at least two-fold up-regulated in ONNV-infected compared to uninfected mosquitoes at 14 days p.i. In addition, 38 genes displayed at least two-fold down-regulation in ONN-infected compared to non-infected mosquitoes at 14 days p.i. (Fig. 1).
Figure 1.
Scatterplot analysis of variation in gene expression. The RNA from the non-infected was labelled with Cy5 and the ONNV-infected with Cy3. Red and green spots represent up- and down-regulated gene expression in ONNV-infective compared to non-infected control mosquitoes, respectively. The middle diagonal line indicates one-fold (no change) and the upper and lower diagonal lines show either two-fold up- or down-regulated gene expression, respectively. The x- and y-axes are in logarithmic scales.
Among the two-fold up- and down-regulated genes, differential expression of individual genes was determined using a paired Student’s t-test comparing the signals derived from the ONNV-infected vs. uninfected mosquitoes across replicates. Of the 102 unique cDNA clones that the statistical preliminary screening procedure showed to be differentially expressed in response to ONNV infection, 18 genes were associated with significant signal intensity differences at the 95% confidence level. Following further screening by qRT-PCR analysis, this infection-induced effect at the 95% confidence level was confirmed in seven of the 18 genes. The elimination of 11 genes from our data set may reflect the differential sensitivities of the techniques or sample variation. The conformation that seven genes were differentially expressed using two analytical methods provides confidence that this is an infection-specific phenomenon. As methodology improves, the correlation between the techniques may be increased.
The ribosomal protein S4 (RpS4) was chosen as an internal control for two reasons. Firstly, expression levels in our cDNA microarray experiment were relatively consistent. Secondly, when analysed by qRT-PCR, Student’s t-test determined that the mRNA expression levels of RpS4 in uninfected and infected mosquitoes collected at 24 h, 48 h and 14 days p.i. were not significantly different (data not shown). We therefore concluded that the RpS4 gene can be used as an internal control at early and late stages of ONNV. As shown in Table 1 and Fig. 2, qRT-PCR analysis of RpS4 transcript levels at 14 days p.i. detected no significant difference between the relative transcript levels derived from the ONNV-infected vs. uninfected mosquitoes. We are therefore confident that the differential expression observed for other genes was related to ONNV infection and not attributed to variation in sample loading.
Table 1.
qRT-PCR analysis of differentially expressed genes of An. gambiae in ONNV infection
| Gene ID (GenBank Accession No.) | Primer sequence (5′ to 3′) | Fold change (P-value, SD) | Amplicon size (bp) | Amplification efficiencya (Rb) |
|---|---|---|---|---|
| RpS4 (AJ283756) | Forward: GCTGCCGCTGGTGATCTT | |||
| Reverse: TCGTCACCTCGCTGTTGGT | 1.2 (0.259, 0.42) | 65 | 0.90 (0.99) | |
| AdeL (AL692204) | Forward: CGTGCAGAATGGCCGAA | |||
| Reverse: CGTGCTCAGCGGCGA | 5.4 (0.031, 3.0) | 59 | 0.90 (0.99) | |
| RpL35 (BX466552) | Forward: GCAACCAGCACGAGAAGCA | |||
| Reverse: GAACAGGACGTTCTTGCGGT | 3.5 (0.002, 0.96) | 68 | 1.1 (0.99) | |
| Hsp 70 (AL930714) | Forward: GCGATCCAGGCCGACAT | |||
| Reverse: TCTTTGGCTTGCCCTCGAT | 29.2 (0.02, 16.4) | 64 | 0.97 (0.99) | |
| Dan4 (AL694117) | Forward: GGTAGGAAAAATGCGGGACAA | |||
| Reverse: TTATCGTGCAGCCGCAATC | 6.0 (0.004, 3.24) | 66 | 1.1 (0.99) | |
| EF-1α (N/Dc) | Forward: GGACAGATCAGCAACGGATACA | |||
| Reverse: TCGGAGAACTTGCACGCA | 4.8 (0.01, 0.10) | 73 | 1.1 (0.99) | |
| Agglutinin (N/D) | Forward: CGCTGACCGCTACGGTGT | |||
| Reverse: AATCGGCTGTTCTCGTTGGA | 6.4 (0.01, 1.14) | 67 | 0.91 (0.99) | |
| MRpL7 (N/D) | Forward: GGTGGTGAAGACGACGTTCA | |||
| Reverse: CCTTGATCAGGGCCACCTT | −2.0 (0.04, 0.09) | 71 | 1.1 (0.99) |
Amplification efficiencies were calculated from the slope of standard curves as E = 10[−1/slope] − 1. 100% PCR efficiency corresponds to an amplification efficiency of 1 (Applied Biosystems Application Note);
Regression coefficient of linear standard curve.
Accession number has not yet been designated.
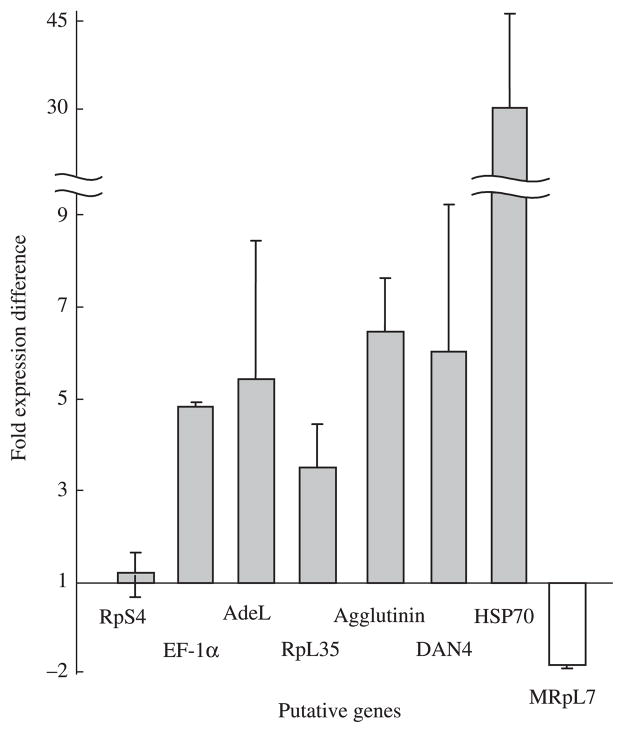
Figure 2.
qRT-PCR analysis of differentially expressed genes of An. gambiae in ONNV infection. For cDNA construction total RNA was isolated from ONNV-infected and non-infected control mosquitoes. Biological replicates were used to generate fold changes and error bars for qRT-PCR experiments (n = 3). Each bar represents standard deviation. The fold changes were calculated to the average transcript level of ONNV infected mosquitoes divided by that of non-infected control mosquitoes. However, there was a fold change decrease in the value of MRpL7 (white bar), therefore, the value was negatively inversed. RpS4 gene (black bar) shows no significant loading variation of the samples.
By combining a high-density cDNA microarray and qRT-PCR analysis we identified seven genes whose expression is modulated by ONNV infection. To verify the amplification of our qRT-PCR primers, the amplification efficiency values of the primers of each cDNA and the regression coefficient of its standard curve were evaluated (Table 1). Amplification efficiency values of the primers were all within 1 ± 0.1, the range of manufacturer’s recommendation, and the regression coefficient of the standard curve of each primers were all greater than 0.99. Sequence analyses of the seven unique transcripts determined that they all shared sequence homology with genes predicted in the An. gambiae genome (Table 2). The results of BlastN, BlastX and BlastP against Nr, EST sequence database and the Universal Protein database, respectively, are shown in Table 2. The products of the seven genes are seemingly involved in modulation of translation/replication factors and intracellular transport pathways. As discussed below, these biological processes may be critical for successful ONNV infection in An. gambiae.
Table 2.
Confirmed list of differentially expressed genes in ONNV-infected An. gambiae identified by cDNA microarray experiments
| Gene ID | Ensembl Gene ID | BlastN (Nr) | BlastX (dbEST) | BlastP (dbUniprot) | Gene ontology | P-value (Meana, SDb) | Fold change |
|---|---|---|---|---|---|---|---|
| 2× Up-regulated genes | 2.2 | ||||||
| AdeL | ENSANGG00000012419 | No description | Adenylosuccinase (8e–39) | Adenylosuccinase (8.1e–213) | purine biosynthesis | 0.014 (814344, 412362) | 2.2 |
| RpL35 | ENSANGG00000015069 | Ribosomal protein L35 (3e–59) | Ribosomal protein L35 (1e–12) | Ribosomal protein L35 (3.4e–45) | protein biosynthesis | 0.041 (1993477, 1545856) | 2.2 |
| HSP 70 | ENSANGG00000017398 | No description | Molecular chaperone Hsc70–4 (6e–38) | Heat Shock Protein 70 (0) | protein folding | 0.014 (1067897, 527168) | 2.6 |
| ENSANGG00000010404 | |||||||
| ENSANGG00000022650 | |||||||
| DAN4 | ENSANGG00000014767 | No description | No description | Yeast, DAN4 precursor (8.8e–08) | integral to membrane | 0.039 (291445, 149264) | 2.4 |
| EF-1α | ENSANGG00000015883 | EF-1 alpha (0) | EF-1 alpha (0) | EF-1 alpha (0) | translational elongation | 0.022 (1146203, 685794) | 2.0 |
| Agglutinin | ENSANGG00000011280 | No description | No description | A-agglutinin attachment subunit (4.6e–07) | membrane adhesion | 0.004 (1046330, 335650) | 2.0 |
| 2× Down-regulated genes | |||||||
| MRpL7 | ENSANGG00000012624 | No description | Mitochondrial ribosomal protein L7/L12 (3e–24) | Mitochondrial ribosomal protein L7/L12 (4.6e–34) | mitochondrion | 0.035 (−293774, 314742) | −2.2 |
Means of differences of signal intensities;
SD: Standard deviations of differences of signal intensities.
Initially, the qRT-PCR data showed a 4.8-fold higher expression of EF-1α in ONNV infected females at 14 days p.i. (Table 1, Fig. 2). Host protein subunits are frequently used for replication and transcription of viral RNAs by RNA viruses (Lai, 1998). EF-1α binds to the 3′ UTRs of West Nile virus (WNV) and a range of RNA viruses (Joshi et al., 1986; Barrera et al., 1993; Blackwell & Brinton, 1997). The sequence of EF-1α is highly conserved between different organisms, and the WNV 3′ (+) RNA binds to EF-1α in mammalian, chicken and mosquito cell extracts, suggesting that EF-1α is used by flaviviruses in divergent host species (Brinton, 2001). As alphaviruses and flaviviruses are both (+) RNA viruses, and relatively closely related (Schlesinger & Schlesinger, 2001), the implication is that functional roles of EF-1α may be highly conserved between ONNV and WNV in different host species.
The 26S RNA of alphaviruses, e.g. ONNV, Semliki Forest Virus (SFV) and Sindbis (SIN) is translated to produce a structural polyprotein consisting of capsid, E3, E2, 6K and E1 proteins (Kaariainen & Ahola, 2002). The capsid protein associates transiently with the 60S subunit of the translating ribosome, and is believed to be transferred to the RNA genome during the assembly of the nucleocapsid (Ranki et al., 1979; Ulmanen et al., 1979). From the qRT-PCR analysis the RpL35 shows a 3.5-fold up-regulation in gene expression in ONNV infection at 14 days p.i. (Table 1, Fig. 2). The up-regulated expression of RpL35 gene suggests that RpL35 may interact with capsid protein in order to transfer the RNA genome and assemble the nucleocapsid.
From the qRT-PCR data, HSP70 shows a 29.2-fold increase in gene expression in ONNV infection at 14 days p.i. (Table 1, Fig. 2). As ONNV infection in vertebrate cells are cytopathic, the elevated gene expression of heat shock protein that we observed may protect the vector host cell from ONNV-induced molecular damage (Morimoto & Santoro, 1998). HSP70 may also play a role in ONNV entry. ONNV (like other alphaviruses) is suggested to use an active clathrin-dependent endocytic pathway for infection (DeTulleo & Kirchhausen, 1998). HSP70 causes dissociation of clathrin from the vesicle surface, and enables it to recycle onto membranes (Newmyer & Schmid, 2001; Young et al., 2003).
DAN4 and agglutinin also displayed significantly higher transcript levels, 6.0- and 6.4-fold changes in ONNV infection at 14 days p.i., respectively (Table 1, Fig. 2). Similarity of DAN4 and agglutinin could not be detected by BlastX and BlastN searches against the Nr and EST database, respectively. However, significant sequence similarities were detected against the Universal protein database. Despite this limitation, the gene ontology of DAN4 and agglutinin suggest that they may interact with endosome and lysosome membranes. As the four non-structural proteins and RNAs of alphaviruses are associated with the membranes of modified endosomes and lysosomes of the replication complex (Kaariainen & Ahola, 2002), DAN4 and agglutinin may therefore be involve in a membrane attachment of the replication complex of ONNV. Further analyses, including functional studies, are necessary to determine the role of each protein in ONNV replication.
Conclusion
We have presented data from cDNA microarray and qRT-PCR analyses as a first attempt to evaluate gene expression profiles in the mosquito vector An. gambiae during infection with ONNV. Gene expression studies of An. gambiae in response to ONNV infection may provide new insight into potential mechanisms through which specific host proteins of the arthropod vector can influence the outcome of arboviral infection. Identification of these proteins may be important in determining the host range and virulence of the virus (Strauss & Strauss, 1999). The understanding of host determinants of vector competence with respect to ONNV infection in An. gambiae may be broadly applicable to other vector–virus relationships, and may identify molecular methods to intervene in the transmission of ONNV and other arboviruses in nature. From a practical viewpoint, the identification of the binding domains of host and virus proteins, for example, might be utilized to empirically design a vaccine to block the essential interactions between virus and host proteins, and may represent possible drug targets. A characterized binding domain may also be a good target for effector molecules which may be introduced into the mosquito genome to block virus replication. Furthermore, given the development of ONNV as an expression vector in An. gambiae (Brault et al., 2004; Keene et al., 2004), the elucidation of molecular interactions between An. gambiae and ONNV may provide new approaches by which infection efficiency and expression levels can be optimized. This will eventually help us to better perform in vivo gene expression studies in An. gambiae and other Anopheline mosquitoes.
Experimental procedures
Virus
The SG650 strain of ONNV was obtained from the World Reference Center for Arboviruses at the University of Texas Medical Branch (Galveston, TX). Strain SG650 was isolated from human serum in Uganda in 1996 (Lanciotti et al., 1998) and has been passed once in Vero cells (GenBank Accession Number AF079456). Stock virus was produced following a single passage in Vero cells maintained at 37 °C in Leibovitz L-15 medium with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin. Cell supernatant was harvested when 75% of the cells showed cytopathic effect (3 + CPE), aliquoted, and stored at −80 °C for use in all experiments.
Mosquitoes
The G3 strain of An. gambiae was reared at 27 °C and 80% relative humidity under a 16 h light: 8 h dark photoperiod, as previously described (Higgs 2004). Adults were supplied with a cotton wool pad soaked in a 10% sucrose solution ad libitum and fed on anaesthetized hamsters once per week for egg production.
Virus infections of mosquitoes
Protocols to infect An. gambiae with ONNV SG650 have been optimized to give infection rates of greater than 90% (Vanlandingham et al., 2005). Four-day-old adult female An. gambiae mosquitoes were divided into two groups, one fed an infectious blood meal, the second fed a meal prepared with uninfected tissue culture supernatant. Fresh virus was grown from stock and harvested from Vero cells when 75% of the cells showed CPE. The viral supernatant was mixed with an equal volume of defibrinated sheep blood (Colorado Serum Company, Denver, CO). As a phagostimulant, adenosine triphosphate at a final concentration of 2 mM was added to the blood meal. Mosquitoes were fed in a Biosafety Level 2 insectary. Blood meals were heated to 37 °C and placed in a Hemotek feeding apparatus (Discovery Workshops, Accrington, UK) and mosquitoes were allowed to feed for 1 h (Cosgrove et al., 1994). Fully engorged females were separated from unfed females and were placed into new cartons. Cartons were placed in a sealed, humidified plastic box and maintained with 10% sucrose in a Precision model 818 environmental chamber (Precision, Winchester, VA) at 27 °C, with a 12 h light: 12 h dark cycle.
Mosquito sampling
Based on previous data (Vanlandingham et al., 2005), samples of approximately 50 mosquitoes were harvested and stored at −80 °C for three time points postinfection (p.i.) representing different phases in the infection cycle: 24 h (early and restricted midgut infection), 48 h (established midgut infection with more active viral replication), and 14 days (disseminated infection with multiple tissue involvement). Identical numbers of uninfected mosquitoes were harvested simultaneously. After titration of three experimental mosquitoes collected at 14 days p.i. had confirmed that all were infected with ONNV (Vanlandingham et al., 2005), RNA was extracted as described below.
Mosquito Microarray Consortium-1 (MMC-1) microarray construction
Inserts of the 16 000 NAP1 cDNA clones were amplified by a two-step PCR amplification using T3 and T7 primers and bacterial cultures as primary templates. The resulting PCR products were purified through Millipore, dried and resuspended in 3× SSC. The amplicons were arrayed on aminosilane-coated glass slides with the Omnigrid microarray spotter (GeneMachines, San Carlos, CA). The slides were then incubated at 60 °C for 3 h and 100 °C for 10 min to cross-link spotted DNAs. MMC-1 microarray also includes the 4000 cDNA clones from two immune competent An. gambiae cell lines (Dimopoulos et al., 2002).
cDNA microarray target preparation and hybridization
Total RNA samples were extracted from three batches of approximately 50 ONNV-infected adult female mosquitoes 14 days p.i. and from non-infected female mosquitoes, which were used as control, using the Trizol Reagent (Invitrogen, Carlsbad, CA). To remove genomic DNA contamination, RNA samples were treated with 1.0 μl DNase I following the manufacturer’s instruction (50–375 units/μl; Invitrogen). RNA labelling and hybridization were performed in triplicate using the 3DNA Array 50 kit according to the methods of Dana et al. (2005). Briefly, to make the first strand of cDNA, the RNA from the non-infected mosquitoes was labelled with Cy5 specific primer and the ONNV-infected with Cy3 specific primer. Competitive hybridizations between infected and non-infected samples were carried out following the two-step hybridization: (1) cDNA hybridization to the amplified DNA probes spotted on the slides; (2) hybridization of fluorescent dendrimers (Cy3 and Cy5) onto cDNA (Genisphere, Hatfield, PA). The cDNA hybridizations were performed at 45 °C overnight. The slides were washed after hybridization. For postwashing, the array was air dried via centrifugation for 3 min at 156 g. The 3DNA hybridizations were performed at 53 °C for 2 h and 0.5 mM DTT was added to the first two wash solutions to protect the fluorochromes from oxidation.
Microarray data acquisition and statistical analysis
Following hybridization and washing, microarray slides were scanned successively at two wavelengths, 532 and 635 nm, using the Affymetrix 428 Array Scanner (Affymetrix, Santa Clara, CA). Raw signal intensities were acquired using the adaptive circle algorithm and spot intensities were quantified using JAGUAR 2.0 software (Affymetrix). Average signal intensities were normalized using the Lowess curve for intensity-dependent normalization followed by a per-gene median normalization using the GENESPRING software (version 5.1; Silicon Genetics, Redwood City, CA). Signal intensities for hybridized cDNAs were filtered and only gene products with raw signal intensity values greater than 300 pixels were included. In addition, genes with more than two-fold expression difference between the ONNV-infected and non-infected samples were utilized in further analysis. Statistical significance of differences in the expression of individual genes was determined by using a paired Student’s t-test between the signals derived from the ONNV-infected and non-infected mosquitoes across three replicates for each gene queried, and a P-value less than 0.05 was considered to be a significant gene expression change.
Sequence analysis
Seven confirmed sequences of significant gene expression changes were analysed using BLASTX, BLASTN and BLASTP (Altschul et al., 1997) against the nr (non-redundant), EST database (http://www.ncbi.nlm.nih.gov/BLAST/), and Universal protein database (http://www.pir.uniprot.org/), respectively. Gene Ontology (GO) (Ashburner, 2000) annotation was recorded to each sequence on the basis of highest BLAST hit with an E-value ≤ 10−6.
Quantitative real-time PCR Analysis (qRT-PCR)
To validate the cDNA microarray results, qRT-PCR was performed using an ABI 7700 Sequence Detection System (Applied Biosystems, Foster City, CA). Standard curves were generated for each transcript tested using 10-fold serial dilutions of An. gambiae genomic DNA ranging from 116 to 0.0116 ng per reaction. All reactions were performed in triplicate in a total volume of 25 μl containing 12.5 μl of SYBR Green PCR Master Mix, 300 nmol of each primer at the following conditions: 50 °C for 2 min, 95 °C for 10 min followed by 50 cycles of denaturation at 95 °C for 15 s, annealing and extension at 60 °C for 1 min. To enhance universality of the results, RNA samples were extracted from mosquitoes at 14 days p.i. which were not used in cDNA microarray experiments. Sequences of gene-specific primer sets are given in Table 2. Statistical significance of differences in the expression of individual genes was determined by using a Student’s t-test between the relative transcript values derived from the ONNV-infected and non-infected mosquitoes across three replicates for each gene, and a P-value less than 0.05 was considered to be a significant gene expression change.
Acknowledgments
This project was supported by the U01-AI48846 from NIH/ NIAID to F.H.C. and by NIH grant number AI47877 and DARPA grant cooperative agreement N00178-02-2-9002 with the Chemical Biological Radiological Sciences & Technology Defense Branch of the Naval Surface Warfare Center, Dahlgren, VA, awarded to S.H. We thank J. Huang, C. Hong and K. Tsetsarkin for technical assistance.
References
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M. A biologist’s view of the Drosophila genome annotation assessment project. Genome Res. 2000;10:391–393. doi: 10.1101/gr.10.4.391. [DOI] [PubMed] [Google Scholar]
- Barrera I, Schuppli D, Sogo JM, Weber H. Different mechanisms of recognition of bacteriophage Q beta plus and minus strand RNAs by Q beta replicase. J Mol Biol. 1993;232:512–521. doi: 10.1006/jmbi.1993.1407. [DOI] [PubMed] [Google Scholar]
- Blackwell JL, Brinton MA. Translation elongation factor-1 alpha interacts with the 3′ stem-loop region of West Nile virus genomic RNA. J Virol. 1997;71:6433–6444. doi: 10.1128/jvi.71.9.6433-6444.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers DF, Abell BA, Brown DT. Replication and tissue tropism of the alphavirus Sindbis in the mosquito Aedes albopictus. Virology. 1995;212:1–12. doi: 10.1006/viro.1995.1447. [DOI] [PubMed] [Google Scholar]
- Brault AC, Foy BD, Myles KM, Kelly CL, Higgs S, Weaver SC, et al. Infection patterns of o’nyong nyong virus in the malaria-transmitting mosquito, Anopheles gambiae. Insect Mol Biol. 2004;13:625–635. doi: 10.1111/j.0962-1075.2004.00521.x. [DOI] [PubMed] [Google Scholar]
- Brinton MA. Host factors involved in West Nile virus replication. Ann NY Acad Sci. 2001;951:207–219. doi: 10.1111/j.1749-6632.2001.tb02698.x. [DOI] [PubMed] [Google Scholar]
- Chuaqui RF, Bonner RF, Best CJ, Gillespie JW, Flaig MJ, Hewitt SM, et al. Post-analysis follow-up and validation of microarray experiments. Nat Genet. 2002;32 (Suppl):509–514. doi: 10.1038/ng1034. [DOI] [PubMed] [Google Scholar]
- Corbet PS, Williams MC, Gillett JD. O’Nyong-Nyong fever: an epidemic virus disease in East Africa. IV. Vector studies at epidemic sites. Trans R Soc Trop Med Hyg. 1961;55:463–480. doi: 10.1016/0035-9203(61)90095-5. [DOI] [PubMed] [Google Scholar]
- Cosgrove JB, Wood RJ, Petric D, Evans DT, Abbott RH. A convenient mosquito membrane feeding system. J Am Mosq Control Assoc. 1994;10:434–436. [PubMed] [Google Scholar]
- Dana AN, Hong YS, Kern MK, Hillenmeyer ME, Harker BW, Lobo NF, et al. Gene expression patterns associated with blood-feeding in the malaria mosquito Anopheles gambiae. BMC Genomics. 2005;6:5. doi: 10.1186/1471-2164-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeTulleo L, Kirchhausen T. The clathrin endocytic pathway in viral infection. Embo J. 1998;17:4585–4593. doi: 10.1093/emboj/17.16.4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos G, Christophides GK, Meister S, Schultz J, White KP, Barillas-Mury C, Kafatos FC. Genome expression analysis of Anopheles gambiae: responses to injury, bacterial challenge, and malaria infection. Proc Natl Acad Sci USA. 2002;99:8814–8819. doi: 10.1073/pnas.092274999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman WM, Walker SJ, Vrana KE. Quantitative RT-PCR: pitfalls and potential. Biotechniques. 1999;26:124–125. doi: 10.2144/99261rv01. [DOI] [PubMed] [Google Scholar]
- Higgs S. Care, maintenance, and experimental infection of mosquitoes. In: Marquardt, et al., editors. The Biology of Disease Vectors. Elsevier Academic Press; 2004. pp. 733–739. [Google Scholar]
- Jackson AC, Bowen JC, Downe AE. Experimental infection of Aedes aegypti (Diptera: Culicidae) by the oral route with Sindbis virus. J Med Entomol. 1993;30:332–337. doi: 10.1093/jmedent/30.2.332. [DOI] [PubMed] [Google Scholar]
- Johnson BK. O’nyong-nyong virus disease. In: Monath TP, editor. The Arbo-viruses: Epidemiology and Ecology. Vol. 3. CRC Press; Boca Raton, FL: 1988. pp. 217–223. [Google Scholar]
- Joshi RL, Ravel JM, Haenni AL. Interaction of turnip yellow mosaic virus Val-RNA with eukaryotic elongation factor EF-1 [alpha]. Search for a function. EMBO J. 1986;5:1143–1148. doi: 10.1002/j.1460-2075.1986.tb04339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaariainen L, Ahola T. Functions of alphavirus non-structural proteins in RNA replication. Prog Nucleic Acid Res Mol Biol. 2002;71:187–222. doi: 10.1016/S0079-6603(02)71044-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabatsos N. Antigenic relationships of group A arbo-viruses by plaque reduction neutralization testing. Am J Trop Med Hyg. 1975;24:527–532. doi: 10.4269/ajtmh.1975.24.527. [DOI] [PubMed] [Google Scholar]
- Keene KM, Foy BD, Sanchez-Vargas I, Beaty BJ, Blair CD, Olson KE. RNA interference acts as a natural antiviral response to O’nyong-nyong virus (Alphavirus; Togaviridae) infection of Anopheles gambiae. Proc Natl Acad Sci USA. 2004;101:17240–17245. doi: 10.1073/pnas.0406983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MM. Cellular factors in the transcription and replication of viral RNA genomes: a parallel in DNA-dependent RNA transcription. Virology. 1998;244:1–12. doi: 10.1006/viro.1998.9098. [DOI] [PubMed] [Google Scholar]
- Lanciotti RS, Ludwig ML, Rwaguma EB, Lutwama JJ, Kram TM, Karabatsos N, et al. Emergence of epidemic O’nyong-nyong fever in Uganda after a 35-year absence: genetic characterization of the virus. Virology. 1998;252:258–268. doi: 10.1006/viro.1998.9437. [DOI] [PubMed] [Google Scholar]
- Lemm JA, Rice CM. Assembly of functional Sindbis virus RNA replication complexes: requirement for coexpression of P123 and P34. J Virol. 1993;67:1905–1915. doi: 10.1128/jvi.67.4.1905-1915.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson RS, Strauss JH, Strauss EG. Complete sequence of the genomic RNA of O’nyong-nyong virus and its use in the construction of alphavirus phylogenetic trees. Virology. 1990;175:110–123. doi: 10.1016/0042-6822(90)90191-s. [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Santoro MG. Stress-inducible responses and heat shock proteins: new pharmacologic targets for cytoprotection. Nat Biotechnol. 1998;16:833–838. doi: 10.1038/nbt0998-833. [DOI] [PubMed] [Google Scholar]
- Newmyer SL, Schmid SL. Dominant-interfering Hsc70 mutants disrupt multiple stages of the clathrin-coated vesicle cycle in vivo. J Cell Biol. 2001;152:607–620. doi: 10.1083/jcb.152.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierro DJ, Myles KM, Foy BD, Beaty BJ, Olson KE. Development of an orally infectious Sindbis virus transducing system that efficiently disseminates and expresses green fluorescent protein in Aedes aegypti. Insect Mol Biol. 2003;12:107–116. doi: 10.1046/j.1365-2583.2003.00392.x. [DOI] [PubMed] [Google Scholar]
- Powers AM, Brault AC, Tesh RB, Weaver SC. Re-emergence of Chikungunya and O’nyong-nyong viruses: evidence for distinct geographical lineages and distant evolutionary relationships. J General Virol. 2000;81:471–479. doi: 10.1099/0022-1317-81-2-471. [DOI] [PubMed] [Google Scholar]
- Ranki M, Ulmanen I, Kaariainen L. Semliki Forest virus-specific nonstructural protein is associated with ribosomes. FEBS Lett. 1979;108:299–302. doi: 10.1016/0014-5793(79)81232-6. [DOI] [PubMed] [Google Scholar]
- Rwaguma EB, Lutwama JJ, Sempala SD, Kiwanuka N, Kamugisha J, Okware S, et al. Emergence of epidemic O’nyong-nyong fever in southwestern Uganda, after an absence of 35 years. Emerg Infect Dis. 1997;3:77. doi: 10.3201/eid0301.970112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger S, Schlesinger MJ. Togaviridae: the viruses and their replication. In: Knipe DM, et al., editors. Fundamental Virology. Lippincott, Williams & Wilkins; PA: 2001. pp. 567–583. [Google Scholar]
- Strauss JH, Strauss EG. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss JH, Strauss EG. Viral RNA replication. With a little help from the host. Science. 1999;283:802–804. doi: 10.1126/science.283.5403.802. [DOI] [PubMed] [Google Scholar]
- Ulmanen I, Soderlund H, Kaariainen L. Role of protein synthesis in the assembly of Semliki forest virus nucleocapsid. Virology. 1979;99:265–276. doi: 10.1016/0042-6822(79)90006-0. [DOI] [PubMed] [Google Scholar]
- Vanlandingham DL, Hong C, Tsetsarkin K, McElroy KL, Powers AM, Lehane M, Higgs S. Differential infectivities of o’nyong-nyong and chikungunya viruses in Anopheles gambiae and Aedes aegypti mosquitoes. Am J Trop Med Hyg. 2005;72:616–621. [PubMed] [Google Scholar]
- Walker NJ. Real-time and quantitative PCR: applications to mechanism-based toxicology. J Biochem Mol Toxicol. 2001;15:121–127. doi: 10.1002/jbt.8. [DOI] [PubMed] [Google Scholar]
- WHO. Fact Sheet no 94. WHO; Geneva: 1998. [Google Scholar]
- Williams MC, Woodall JP, Corbet PS, Gillett JD. O’nyong-Nyong Fever: An Epidemic Virus Disease in East Africa. 8. Virus Isolations from Anopheles Mosquitoes. Trans R Soc Trop Med Hyg. 1965;59:300–306. doi: 10.1016/0035-9203(65)90012-x. [DOI] [PubMed] [Google Scholar]
- Young JC, Barral JM, Ulrich Hartl F. More than folding: localized functions of cytosolic chaperones. Trends Biochem Sci. 2003;28:541–547. doi: 10.1016/j.tibs.2003.08.009. [DOI] [PubMed] [Google Scholar]