Abstract
Context
Patients with depression and poorly controlled diabetes mellitus, coronary heart disease (CHD), or both have higher medical complication rates and higher health care costs, suggesting that more effective care management of psychiatric and medical disease control might also reduce medical service use and enhance quality of life.
Objective
To evaluate the cost-effectiveness of a multicondition collaborative treatment program (TEAM-care) compared with usual primary care (UC) in outpatients with depression and poorly controlled diabetes or CHD.
Design
Randomized controlled trial of a systematic care management program aimed at improving depression scores and hemoglobin A1c (HbA1c), systolic blood pressure (SBP), and low-density lipoprotein cholesterol (LDL-C) levels.
Setting
Fourteen primary care clinics of an integrated health care system.
Patients
Population-based screening identified 214 adults with depressive disorder and poorly controlled diabetes or CHD.
Intervention
Physician-supervised nurses collaborated with primary care physicians to provide treatment of multiple disease risk factors.
Main Outcome Measures
Blinded assessments evaluated depressive symptoms, SBP, and HbA1c at baseline and at 6, 12, 18, and 24 months. Fasting LDL-C concentration was assessed at baseline and at 12 and 24 months. Health plan accounting records were used to assess medical service costs. Quality-adjusted life-years (QALYs) were assessed using a previously developed regression model based on intervention vs UC differences in HbA1c, LDL-C, and SBP levels over 24 months.
Results
Over 24 months, compared with UC controls, intervention patients had a mean of 114 (95% CI, 79 to 149) additional depression-free days and an estimated 0.335 (95% CI, −0.18 to 0.85) additional QALYs. Intervention patients also had lower mean outpatient health costs of $594 per patient (95% CI, −$3241 to $2053) relative to UC patients.
Conclusions
For adults with depression and poorly controlled diabetes, CHD, or both, a systematic intervention program aimed at improving depression scores and HbA1c, SBP, and LDL-C levels seemed to be a high-value program that for no or modest additional cost markedly improved QALYs.
With the aging of the US population and enhanced medical treatments, more people are living with multiple medical conditions. As the number of chronic diseases increase, there is an increased risk of functional decline, adverse drug interactions, poor adherence to complex medical regimens, duplicative medical tests, conflicting medical advice, unnecessary hospitalizations, and worsening mortality.1–5 Patients with multiple chronic illnesses also have the highest medical costs. The 27% of adult Americans with 2 or more chronic medical illnesses now account for approximately 65% of total health care spending.6 Yet, most quality improvement efforts developed to enhance disease control and outcomes of chronic illness have focused on single conditions, such as diabetes or congestive heart failure. New interventions are needed that focus on primary care patients with commonly occurring multiple illnesses associated with adverse outcomes.
Diabetes and coronary heart disease (CHD)7 are 2 of the most common conditions in primary care, and depression co-occurs in up to 20% of patients with these conditions.8,9 Patients with comorbid depression and diabetes or CHD have poorer self-care (ie, lower adherence to diet, exercise, smoking cessation, and taking medications),10 a higher medical symptom burden,11 greater functional impairment,12 and an increased risk of complications and mortality.13 Given these data, it is not surprising that comorbid depression has been shown to increase medical costs in patients with diabetes and CHD by 50% to 70%.14
An important health policy question is whether interventions that improve medical disease control and depression outcomes could also improve quality of life and cost-effectiveness of medical care. This article describes a 2-year cost-effectiveness analysis of a team-based intervention integrated into primary care found to improve medical and depression disease control in patients with comorbid depression and diabetes, heart disease, or both.15
METHODS
The TEAMcare study was a randomized trial of a systematic program to improve disease control of depression, hemoglobin A1c (HbA1c), systolic blood pressure (SBP), and low-density lipoprotein cholesterol (LDL-C) in patients with poorly controlled diabetes mellitus, CHD, or both and comorbid depression.15 Participants were identified by a population-based screening program. Study methods are described in detail elsewhere15 and are briefly summarized herein.
SETTING AND PARTICIPANTS
Group Health (GH) is an integrated health care system with more than 650 000 enrollees in Washington State. Between May 1, 2007, and October 31, 2008, we recruited patients and primary care physicians (PCPs) from 14 GH primary care clinics. Institutional review boards from the University of Washington and GH approved this study.
PATIENTS
We identified patients with diabetes, CHD, or both with evidence of 1 ormore poorly controlled disease parameters (HbA1c ≥8.5%, SBP >140 mm Hg, or LDL-C level >130 mg/dL [to convert to millimoles per liter, multiply by 0.0259]) from GH electronic records. Patients with evidence of poor disease control were eligible if they had a depression score of at least 10 on the Patient Health Questionnaire-9.16 The exclusion criteria were terminal illness, severe hearing loss, residence in long-term care, planning bariatric surgery, ongoing psychiatric care, bipolar disorder or schizophrenia, antipsychotic or mood stabilizer medication use, and mental confusion suggesting dementia. Eligible patients completed a baseline interview and gave oral consent for laboratory tests before an in-person visit, at which time they provided written informed consent. Patients were randomized into the intervention group receiving the TEAMcare protocol (n=106) or the usual care (UC) group (n=108).15 Usual care patients were advised to consult their PCP to receive care for depression, diabetes, and CHD.
ENHANCED UC
All the PCPs in the 14 primary care clinics were invited, and 151 (92%) agreed to participate. For each intervention and UC patient, PCPs received initial notification of depression and poor medical disease control and laboratory test results at baseline and at 6, 12, 18, and 24 months. The PCPs were responsible for medication management of intervention and UC patients. Although GH patients can either self-refer or be referred by PCPs for mental health care, GH has few mental health professionals providing services in primary care.
INTERVENTION: MULTICONDITION COLLABORATIVE CARE MANAGEMENT (TEAMcare)
The patient-centered, team-based collaborative care management intervention for patients with multiple chronic conditions used a combination of principles from collaborative care depression interventions17 and the chronic care model18 and integrated a treat-to-target medication strategy initially developed for diabetes.19 One consistent treatment approach was applied systematically across 3 chronic illnesses (diabetes, depression, and CHD). A physician-supervised nurse care manager was added to the primary care team to enhance patient self-management, treatment intensification, coordination, and continuity of care. The nurse care manager worked closely with each patient’s PCP to optimize systematic management of chronic illnesses.
Nurse care managers worked with patients and PCPs to identify clinical goals and to develop individualized care plans. They educated patients and used behavioral activation, motivational interviewing, and problem-solving strategies to help patients perform specific self-care activities (ie, self-monitoring of BP and improving adherence to medication, diet, and exercise regimens). Nurses tracked patient progress using a care management electronic information system and reviewed their caseloads weekly with a consulting psychiatrist and internist or family physician. Treatment recommendations based on the physician caseload review and treat-to-target algorithms19 were communicated by care managers to the PCPs. These weekly systematic case reviews with physician consultants enhanced care coordination and ensured accountability for follow-up to guideline-level disease management and achieving clinical goals. Nurse care managers proactively monitored patients with visits or telephone calls (initially 2–3 contacts a month), administered the Patient Health Questionnaire-9 depression questionnaire, and reviewed home BP or glucose control and laboratory test results. Frequency of later contacts depended on clinical response. Once patients achieved clinical targets (depression, HbA1c, SBP, and LDL-C), they worked with care managers to formulate a maintenance plan for follow-up with their primary care team. During the maintenance phase, care managers followed up the patients with telephone calls every 4 to 6 weeks and offered more frequent contacts or visits for those who did not meet clinical targets or had relapses in depressive symptoms. Intervention contacts and active monitoring continued for 12 months after randomization.
PATIENT-LEVEL OUTCOMES
Patients in the intervention and UC groups were contacted by telephone for blinded assessment of clinical outcomes 6, 12, 18, and 24 months after randomization and had clinical and laboratory assessments of BP and HbA1c at 6, 12, 18, and 24 months and of LDL-C at 12 and 24 months. Analyses of clinical outcomes used linear regression models adjusting for baseline measures. Models across 18- and 24-month time points were estimated using generalized estimating equations to account for correlation over time.
Effectiveness was defined in 2 ways during follow-up: the number of “depression-free days” and the number of quality-adjusted life-years (QALYs). As in our previous studies, depression-free days were measured by using methods developed by Lave et al20 that integrate depression symptoms scores over time to estimate days free of depression symptoms during follow-up. Using these methods, depression-free days were calculated using a threshold score of 0.5 on the 20 depression items from the Symptom Checklist–90 (SCL-20)21 to define depression-free and a threshold score of 1.7 to define fully symptomatic depression.22 These thresholds have been shown to correlate with Hamilton depression scores of 7 and 20, respectively, based on previous research.23
The increase in QALYs associated with TEAMcare vs UC was estimated in 2 ways: (1) based on changes in differences of depression-free days between intervention and UC patients over 24 months and (2) based on changes in HbA1c, SBP, and LDL-C levels from baseline to 24 months. This latter QALY measure captures the combined impact of these 3 risk factor differences between intervention and UC patients on future quality of life due to increased longevity and avoidance of nonfatal morbidities that decrease life’s value.24
Previous literature25–27 has suggested that going from fully symptomatic to full remission of depression is associated with an increase in quality of life by 0.2 to 0.4 points on a scale from 0 (no quality) to 1(full quality). To determine the incremental QALYs associated with the intervention, we divided the 2-year difference in depression-free days by 365 and then multiplied by the lower (0.2) and upper (0.4) bound increase in QALYs associated with full remission of depression.
A previously developed regression model24 was used to generate estimated QALYs based on age, sex, microalbuminuria, and HbA1c, LDL-C, and SBP levels over 24 months. For each individual, an estimated QALY was derived by multiplying the patient-level data by the regression coefficients.
The potential beneficial effects on QALYs from improvement in depression and disease control are complementary. Improvement in depression outcomes is likely to have amore immediate effect on improvement in quality of life, whereas improvement in medical disease control has a long-term effect on improvement in future quality of life by potentially increasing longevity and decreasing morbidity.
The cost analysis was calculated from the perspective of the health care system. Use and cost of services provided by GH were measured using the health care plan’s computerized cost accounting records. This system uses general ledger costs to calculate actual budget-based cost (not charges) for all services provided or purchased by GH. Costs for intervention services provided by the study, including supervision by physicians, were calculated using actual salary and fringe benefit rates plus 30% overhead (eg, administrative support and clinic space). Resulting unit costs were $79 for each in-person nurse visit (typically 30 minutes) and $31 for each telephone contact (typically 10–15 minutes). These estimates include time required for recordkeeping and outreach efforts. Approximately 45 minutes of nurse time was allowed for each 10- to 15-minute telephone contact. Intervention costs also included a fixed $100 for each participant for costs of physician supervision (supervision occurred 3 of 4 weeks per month at an estimated hourly cost of $140 per physician) and information system support.
The a priori analysis plan for this study focused on total 24-month outpatient costs as the primary numerator in the cost-effectiveness analysis. Because of limited power, we planned to describe only the percentage of intervention and control patients with 1 or more hospitalizations and total 24-month inpatient costs. Outpatient costs were defined as outpatient services provided or purchased by GH and all services provided by intervention staff. All the analyses were intention to treat and were based on original treatment assignment. The primary analysis compared effectiveness and cost for the full 24-month follow-up period, but we also present data for the first and second 12-month periods separately. Primary analyses examined incremental effectiveness, incremental total outpatient costs, and incremental cost-effectiveness ratios for participants contributing any follow-up data (eg, completed ≥1 blinded outcome assessment or remained enrolled for ≥3 months). These analyses used repeated observations of laboratory data (HbA1c, SBP, and LDL-C), depressive symptoms based on the SCL-20, and costs based on each interview period, with individuals included in the study until either costs or effectiveness information was missing. Patients who died during follow-up had complete cost data but incomplete effectiveness data. Because patients died between interviews, the analysis excluded costs accrued between the final interview and the date of death. Complete cost data were available only for participants who survived the 2-year period and remained enrolled in the GH health plan. There were no significant intervention vs UC differences in 2-year mortality or of patients disenrolling from GH during the 2 years.
The Breusch-Pagan/Cook-Weisberg test was used to examine the assumptions of homoscedasticity for the study groups on outpatient and inpatient total costs. Estimated incremental total outpatient costs were adjusted for age, sex, and total outpatient costs during the 12 months before baseline. Estimated gain in depression-free days was adjusted for age, sex, and baseline depression score. Estimated changes in HbA1c, SBP, and LDL-C levels over 24 months were adjusted for baseline values of these parameters. The primary analysis used linear regression with 95% CIs for incremental cost and incremental cost-effectiveness ratios based on the Fieller theorem.28
To determine the probability that intervention was associated with a cost-effectiveness acceptability threshold29 of less than $20 000 per QALY for interventions that are recommended for rapid dissemination,30 we performed a bootstrapping procedure with 10 000 replications. This bootstrapping procedure was performed by creating 10 000 estimates of the mean outpatient costs and QALYs adjusted for age, sex, and 12-month costs for the intervention and UC groups. The incremental costs were then divided by the incremental QALYs.
Given that the business case for the TEAMcare intervention may be more favorable for fee-for-service health care systems that can bill Medicare and other insurers for diabetes nurse visits, we conducted an additional 24-month incremental cost-effectiveness sensitivity analysis using the 2011 Medicare fee schedule rate that allows reimbursement of $54 per visit for up to 10 nurse educator visits.
Total inpatient medical costs were defined as the sum of costs for all medical/surgical, mental health, and substance abuse admissions. Estimated incremental inpatient costs were adjusted for age, sex, and total inpatient costs in the 12 months before baseline. The sample size was not sufficient to provide accurate comparisons of inpatient health services costs, so those are presented as descriptive analyses only. Initial analyses indicated that the distribution of inpatient cost data was highly skewed and that intervention patients had higher inpatient costs than did UC patients in the 12 months before baseline. Propensity analysis is recommended in cost-effectiveness analyses of randomized trial results when there is a relatively small sample size, cost data are highly skewed, and covariates of prognostic importance (such as the previous 12-month inpatient costs) are imbalanced.31 For each individual, the propensity to be a member of the intervention group was calculated using logistic regression, with the outcome being in the intervention vs UC group and the predictors being age, sex, and the previous year’s inpatient costs. These propensity scores were converted into weights using the reciprocal of the weight for the UC group and 1/(1−weight) for the intervention group. These values were then used to weight the ordinary least squares regression examining the relationship between treatment group and inpatient costs. The beta coefficient associated with group status is interpreted as the adjusted incremental cost.
To ascertain that the estimated propensity score appropriately balanced all baseline covariates across treatment group, we ran 3 regressions in which each baseline covariate was regressed on the treatment indicator weighted by the inverse propensity of treatment assignment.32,33 We confirmed that proper balancing was achieved based on the fact that the coefficients on the treatment indicators were not significantly different from zero (balancing test) and the absolute coefficient divided by the standard deviation of that baseline variable (balancing ratio) was less than 0.5.34
The first weighted analysis of inpatient costs used the intention-to-treat sample, and the second removed the extreme outliers (the 3.5% of the sample [n=8] whose input costs were >3 SD above the mean), which substantially improved the normality of the distribution.
RESULTS
Patients assigned to the intervention and UC groups were comparable in demographic and baseline clinical characteristics except for trends toward higher outpatient and inpatient costs in intervention compared with UC patients (Table 1).
Table 1.
Characteristics of the 214 Study Patients
| Intervention Group (n = 106) |
Usual Care Group (n = 108) |
|
|---|---|---|
| Age, mean (SD), y | 57.4 (10.5) | 56.3 (12.1) |
| Female sex, % | 48 | 56 |
| ≥1 y of college, % | 61 | 56 |
| Nonwhite or Hispanic race/ethnicity, % | 25 | 22 |
| Employment, % | ||
| Part time or full time | 53 | 59 |
| Retired | 34 | 26 |
| Unemployed or disabled | 10 | 13 |
| Homemaker | 3 | 2 |
| ≥1 Antidepressant prescription filled in the previous 12 mo, No. (%) | 61 (57) | 57 (53) |
| PHQ-9 score | ||
| Mean (SD) | 14.7 (3.8) | 13.9 (3.1) |
| Range | 10.0–26.0 | 10.0–23.0 |
| Depression for ≥2 y, % | 72 | 76 |
| SCL-20 score | ||
| Mean (SD) | 1.7 (0.6) | 1.7 (0.6) |
| Range | 0.2–3.25 | 0.3–2.95 |
| Hemoglobin A1c, mean (SD), % | 8.1 (2.0) | 8.0 (1.9) |
| LDL-C, mean (SD), mg/dL | 106.5 (35.3) | 109.0 (36.5) |
| Systolic blood pressure, mean (SD), mm Hg | 136 (18.4) | 132 (17.2) |
| Diabetes, with or without coronary heart disease, % | 89 | 82 |
| Coronary heart disease, % | 23 | 30 |
| Body mass index, mean (SD)a | 36.9 (8.3) | 36.6 (8.5) |
| Outpatient costs in the previous 12 mo, mean (95% CI), $ | 10 026 (8312–11 741) | 9663 (8070–11 254) |
| Inpatient costs in the previous 12 mo, mean (95% CI), $ | 3210 (1553–4868) | 2748 (1453–4043) |
Abbreviations: LDL-C, low-density lipoprotein cholesterol; PHQ-9, Patient Health Questionnaire-9; SCL-20, the 20 depression items from the Symptom Checklist–90.
SI conversion factor: To convert LDL-C to millimoles per liter, multiply by 0.0259.
Calculated as weight in kilograms divided by height in meters squared.
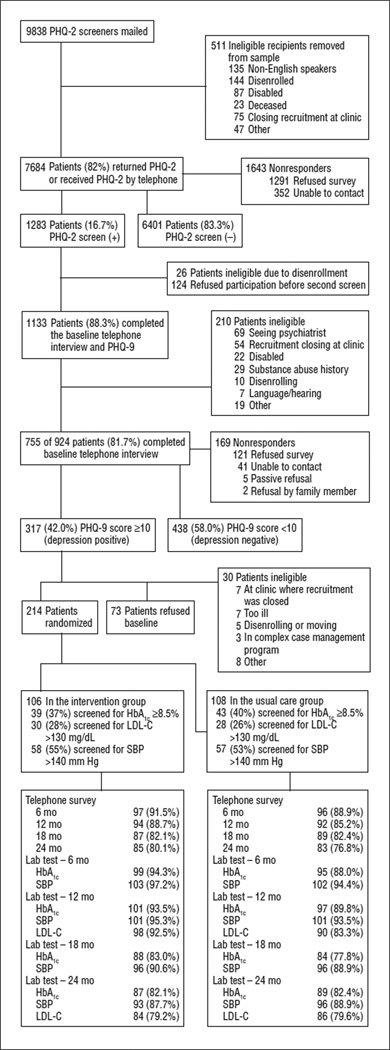
The proportions of randomized participants completing the telephone surveys and the proportions of patients completing laboratory and clinical assessments (HbA1c, SBP, and LDL-C) at each follow-up are shown in the Figure. There were no significant differences between intervention and UC patients.
Figure.
CONSORT flowchart. HbA1c indicates hemoglobin A1c; LDL-C, low-density lipoprotein cholesterol (to convert to millimoles per liter, multiply by 0.0259); PHQ-2 and -9, Patient Health Questionnaire-2 and -9; and SBP, systolic blood pressure.
More than 99% of intervention patients completed an initial visit, and 88% had at least 4 in-person visits with the nurse. Intervention patients had a mean of 10.0 in-person visits and 10.8 telephone visits with the nurse case manager during the 12-month period. The estimated intervention cost per patient, including all nurse contacts, physician supervision, and the information support system was $1224.
The 24-month course of SCL-20 depression scores and HbA1c, LDL-C, and SBP levels are given in Table 2. As previously reported, compared with controls, patients in the intervention group had significantly greater overall improvement across depression scores and HbA1c, LDL-C, and SBP levels during the first 12 months. During the 18- and 24-month periods, SCL-20 depression scores remained significantly lower in the intervention group compared with control patients. In contrast, HbA1c, LDL-C, and SBP levels did not show sustained differences between the intervention and UC groups at postintervention 18- and 24-month follow-ups.
Table 2.
Intervention vs Usual Care: 24-Month Changes in SCL-20 Depression Scores and HbA1c, LDL-C, and Systolic Blood Pressure Levels
| Intervention Group | Usual Care Group | ||||
|---|---|---|---|---|---|
| Mean (SD) | 12- and 24-mo Change From BL |
Mean (SD) | 12- and 24-mo Change From BL SCL-20 score |
Estimated Group Differences (95% CI) |
|
| SCL-20 score | |||||
| Intervention perioda | |||||
| Baseline | 1.74 (0.59) | 1.65 (0.60) | |||
| 6 mo | 0.84 (0.68) | 1.26 (0.72) | |||
| 12 mo | 0.83 (0.68) | 0.91 | 1.14 (0.66) | 0.51 | −0.41 (−0.56 to −0.26)b |
| Postintervention period | |||||
| 18 mo | 0.87 (0.65) | 1.08 (0.61) | |||
| 24 mo | 0.88 (0.66) | 0.86 | 1.04 (0.69) | 0.61 | −0.24 (−0.40 to −0.08)b |
| HbA1c, % | |||||
| Intervention period | |||||
| Baseline | 8.14 (2.03) | 8.04 (1.87) | |||
| 6 mo | 7.42 (1.23) | 7.87 (1.93) | |||
| 12 mo | 7.33 (1.21) | 0.81 | 7.81 (1.90) | 0.23 | −0.56 (−0.85 to −0.27)b |
| Postintervention period | |||||
| 18 mo | 7.74 (1.61) | 7.88 (1.99) | |||
| 24 mo | 7.86 (1.70) | 0.28 | 7.66 (1.73) | 0.38 | −0.14 (−0.50 to 0.22)b |
| LDL-C, mg/dL | |||||
| Intervention period | |||||
| Baseline | 106.8 (35.4) | 109.4 (36.7) | |||
| 12 mo | 91.9 (36.7) | 14.9 | 101.4 (36.6) | 8.0 | −9.1 (−17.5 to −0.8)c |
| Postintervention period | |||||
| 24 mo | 98.5 (37.8) | 8.3 | 98.7 (36.6) | 10.7 | −0.6 (−9.7 to 8.6)c |
| Systolic blood pressure, mm Hg | |||||
| Intervention period | |||||
| Baseline | 135.7 (18.4) | 131.9 (17.0) | |||
| 6 mo | 131.9 (15.2) | 133.5 (20.4) | |||
| 12 mo | 131.0 (18.2) | 4.7 | 132.3 (17.4) | −0.4 | −3.4 (−6.9 to 0.1)b |
| Postintervention period | |||||
| 18 mo | 131.3 (18.5) | 132.0 (19.5) | |||
| 24 mo | 131.4 (17.3) | 4.3 | 130.2 (17.1) | 1.7 | −1.1 (−5.2 to 2.9)b |
Abbreviations: BL, baseline; HbA1c, hemoglobin A1c; LDL-C, low-density lipoprotein cholesterol; SCL-20, the 20 depression items from the Symptom Checklist–90.
SI conversion factor: To convert LDL-C to millimoles per liter, multiply by 0.0259.
Intervention period results have been reported previously15 and are replicated.
Values are from a generalized estimating equation model predicting either a 6- or 12-month outcome or an 18- or 24-month outcome controlling for baseline.
Values are from a linear regression model predicting either a 12- or 24-month outcome controlling for baseline.
Unadjusted analyses found that TEAMcare compared with UC was associated with an increment of 47 (95% CI, 13 to 79) depression-free days in year 1 and 55 (95% CI, 17 to 93) depression-free days in year 2 for a 2-year increment of 102 (95% CI, 38 to 172) depression-free days. Adjustment for baseline differences in age, sex, and SCL-20 scores increased the increment in depression-free days associated with TEAMcare to 114 (95% CI, 79 to 149) (Table 3).
Table 3.
Incremental Effectiveness, Outpatient Costs, and Cost-effectiveness of Intervention vs Usual Care
| Incremental 24-mo Value, Mean (95% CI) | |||||
|---|---|---|---|---|---|
| DFDsa | QALYsb | Total Outpatient Costs, $c |
Outpatient Costs/DFD, $ |
Outpatient Costs/QALY, $ |
|
| Primary analysis | 114 (79 to 149) | 0.335 (−0.18 to 0.85) | −594 (−3421 to 2053) | −5.26 (−29.76 to 19.17) | −1773 (−2878 to 2878) |
| Sensitivity analysis based on Medicare reimbursement of $54 per diabetes nurse visit | 114 (79 to 149) | 0.335 (−0.18 to 0.85) | −1116 (−3768 to 1536) | −9.88 (−34.97 to 14.16) | −3297 (−4014 to 2722 |
Abbreviations: DFD, depression-free day; QALY, quality-adjusted life-year.
The incremental depression-free estimate was adjusted for age, sex, and baseline depression score.
The QALY estimate was based on baseline to 24-month changes in hemoglobin A1c, systolic blood pressure, and low-density lipoprotein cholesterol levels.
Estimated incremental 24-month total outpatient costs were adjusted for age, sex, and 12-month prebaseline total outpatient costs.
The test for heteroscedasticity for total 24-month outpatient costs was nonsignificant (, P = .87), indicating that the groups did not violate the assumption of homoscedasticity. As seen in Table 4, the unadjusted total outpatient costs for intervention compared with UC patients were $363 higher in the 12 months before baseline. Unadjusted costs in the first 12-month period and the 12- to 24-month postbaseline periods were $1061 higher and $258 higher, respectively, for intervention compared with UC patients. The 24-month unadjusted total outpatient costs were $1319 higher in intervention compared with UC patients.
Table 4.
Unadjusted Intervention vs Usual Care Costs
| Costs, Arithmetic Mean (95% CI), $ | ||||
|---|---|---|---|---|
| 12 mo Before | Year 1 | |||
| Intervention | Usual Care | Intervention | Usual Care | |
| Emergency | 790 (505–1075) | 653 (402–904) | 1068 (510–1627) | 588 (300–876) |
| Laboratory and radiology | 663 (497–828) | 680 (534–826) | 840 (629–1052) | 788 (574–1002) |
| Pharmacy | 2348 (1799–2898) | 2235 (1780–2690) | 3087 (2505–3669) | 2991 (2190–3791) |
| Primary care | 1236 (1057–1415) | 1339 (1152–1525) | 1281 (1078–1484) | 1421 (1098–1743) |
| Specialty care | 2461 (1926–2996) | 2385 (1851–2920) | 2802 (2082–3523) | 2847 (2055–3640) |
| Mental health | 76 (18–134) | 154 (26–281) | 227 (121–335) | 178 (53–304) |
| Ambulatory surgery | 483 (234–732) | 807 (476–1138) | 777 (441–1113) | 723 (436–1009) |
| Othera | 1969 (1231–2708) | 1410 (877–1943) | 1144 (677–1612) | 1835 (1129–2540) |
| Intervention | NA | NA | 1204 (1092–1314) | NA |
| Outpatient total | 10 026 (8312–11741) | 9663 (8070–11254) | 12 432 (10 377–14 487) | 11 371 (8885–13859) |
| Inpatient total | 3210 (1553–4868) | 2748 (1453–4043) | 4756 (2207–5305) | 2581 (937–4224) |
| Year 2 | Year 1 + Year 2 | |||
| Intervention | Usual Care | Intervention | Usual Care | |
| Emergency | 859 (268–1450) | 641 (290–922) | 1927 (936–2918) | 1229 (721–1736) |
| Laboratory and radiology | 702 (495–909) | 683 (530–835) | 1542 (1203–1881) | 1471 (1169–1773) |
| Pharmacy | 2714 (2071–3357) | 2348 (1793–2904) | 5801 (4606–6995) | 5339 (4048–6630) |
| Primary care | 1085 (871–1299) | 1216 (935–1498) | 2366 (2001–2732) | 2637 (2106–3168) |
| Specialty care | 2343 (1561–3125) | 2451 (1769–3133) | 5146 (3887–6404) | 5299 (4042–6555) |
| Mental health | 147 (61–234) | 97 (29–166) | 375 (210–540) | 276 (117–343) |
| Ambulatory surgery | 520 (271–769) | 563 (296–830) | 1297 (854–1741) | 1285 (818–1753) |
| Othera | 805 (437–1173) | 918 (525–1311) | 1950 (1212–2688) | 2753 (1849–3657) |
| Intervention | NA | NA | 1204 (1092–1514) | NA |
| Outpatient total | 9175 (7079–11272) | 8917 (7076–10760) | 21 607 (17 723–25 493) | 20 288 (16 378–24 199) |
| Inpatient total | 3980 (1219–6741) | 4955 (2079–7810) | 8736 (4116–13357) | 7525 (4042–11008) |
Abbreviation: NA, not applicable.
Alternative health care, dialysis, durable medical equipment, and physical and occupational therapy.
After adjustment for age, sex, and previous 12-month outpatient costs, TEAMcare patients had mean 2-year total outpatient costs that were $594 (95% CI, −$3241 to $2053) lower than those of UC patients (Table 3). The incremental cost-effectiveness ratio based on these adjusted results shows a mean cost per depression-free day that was less than zero but with a wide CI (−$5.26 per depression-free day; 95% CI, −$29.76 to $19.17). Point estimates for the cost-effectiveness ratio were in the dominant range (intervention had lower costs and greater effectiveness), but 95% CIs included the possibility that the intervention could increase total outpatient costs.
The sensitivity analysis that allowed for reimbursement for diabetes nurse visits at $54 per visit for up to 10 visits showed even more favorable incremental 24-month total outpatient cost savings of $1116 (95% CI, −$3768 to $1536), as well as cost savings of $9.88 (95% CI, −$34.97 to $14.16) per depression-free day and $3297 (95% CI, −$4014 to $2722) per QALY (Table 3).
We also estimated the increment in QALYs associated with the TEAMcare intervention vs UC based on changes in HbA1c, SBP, and LDL-C levels from baseline through 24 months. At baseline, the QALY estimates were similar for the intervention (arithmetic mean=12.5; 95% CI, 11.8 to 13.1) and UC (arithmetic mean=12.6;95%CI, 11.8 to 13.3) groups. The incremental change in QALYs based on these 3 medical disease control measures was 0.335 (95% CI, −0.18 to 0.85) (Table 3). Combining this QALY estimate with the adjusted cost estimate for incremental outpatient costs of −$594 also resulted in a negative mean cost per QALY with a wide CI (−$1773, 95% CI, −$2878 to $2878). The cost-effectiveness acceptability analysis found that there was a 99.7% probability that the total 24-month outpatient costs would be less than $20 000 per QALY.
Because of the extensive literature showing the adverse impact of depression on QALYs,25–27 we estimated the increment in QALYs associated with intervention vs UC improvements in depression over 24 months. Based on the available estimates of depression and QALYs, the increase in depression-free days of 114 associated with the TEAMcare intervention corresponds to an estimate of 0.062 (95% CI, 0.043 to 0.082) to 0.125 (95% CI, 0.086 to 0.163) additional QALYs.
The test for heteroscedasticity for the inpatient costs was significant (, P = .05), indicating that the groups violated the assumption of homoscedasticity. The secondary analyses that examined unadjusted inpatient costs found that intervention patients compared with controls had $462 higher inpatient costs in the 12 months before baseline (Table 4). In the first 12-month period, intervention patients compared with UC patients had unadjusted mean total inpatient costs that were $2175 higher and in the 12- to 24-month period had unadjusted mean total inpatient costs that were $965 lower than those of controls. The unadjusted 24-month inpatient costs were $1211 higher in intervention patients compared with controls. Secondary adjusted analyses examined intervention vs UC differences in total inpatient costs controlling for age, sex, and previous 12-month inpatient costs. Removing 8 outliers resulted in a more normal distribution of inpatient visits with a nonsignificant test for heteroscedasticity (, P = .83). The propensity analysis that excluded these 8 patients who were greater than 3 SD from the mean found a mean cost savings of $2404 (95% CI, −$6237 to $1428) in intervention compared with UC patients. The propensity analysis that included all the randomized patients found that the intervention was associated with an incremental cost of $926 (95% CI, −$4949 to $6801) compared with UC in 24-month total inpatient costs. Further analysis found that all 8 patients (6 intervention and 2 UC patients) who were greater than 3 SD from the mean in total inpatient costs had 4 or more hospitalizations. On the other hand, there were no differences in the 24-month follow-up in percentage of intervention vs UC patients without a hospitalization (68.0% of intervention patients and 70.4% of UC patients) or percentage of patients with 1 to 3 hospitalizations (27.3% of intervention patients vs 27.8% of UC patients).
COMMENT
Similar to other collaborative care trials22,35 that focused only on improving quality of depression care and depression outcomes, we found a significant and sustained effect of the multicondition collaborative care intervention on depression-free days. During a 24-month period, intervention compared with UC patients had a mean of 3 to 4 more months free of depression. However, improved disease control parameters (HbA1c, SBP, and LDL-C levels) during the active intervention phase were not sustained after the case management intervention ended. It is likely that a longer maintenance period of case management would be needed to sustain blood glucose, BP, and LDL-C differences. The United Kingdom Prospective Diabetes Study trial,36 which randomized 4209 patients with type 2 diabetes mellitus to either dietary restriction or intensive medication therapy for glucose control, also found that between-group differences in HbA1c levels favoring intensive treatment were lost after the first year. However, the intervention group showed continued reduction in microvascular risk and risk reduction for myocardial infarction or death from any cause during 10 years of posttrial follow-up.36
Based on 24-month changes in HbA1c, SBP, and LDL-C levels, we estimated an increase of 0.335 in QALYs associated with intervention vs UC. This is likely a conservative estimate given that previous literature22,23,25–27 and our own estimates have found a substantial increase in QALYs associated with improvements in depression status alone. Comorbid depression has been shown to significantly impact functioning and quality of life in patients with chronic medical illness after controlling for medical disease control and severity of medical illness.37 In patients with diabetes, comorbid depression has been shown to increase the risk of macrovascular and microvascular complications13 and dementia38 over 5 years, which often leads to decrements in QALYs. Thus, improvements in quality of treatment and outcomes of depression and diabetes or CHD are likely to extend far beyond the immediate intervention period.
During the 2-year study, we estimated that the TEAMcare intervention was associated with reducing total outpatient costs by $594 compared with UC, with a wide CI. The incremental cost-effectiveness ratio found a mean cost savings of $1773 per QALY, also with a wide CI. Based on a liberal estimate of intervention costs (ie, based on the higher end of the CI), the intervention could cost up to $2878 per QALY. The sensitivity analysis, which included the ability to bill for diabetes nurses’ services at $54 per visit for up to 10 visits, found a 24-month mean outpatient cost savings of $1116, with a cost savings per QALY of $3297, suggesting a higher return on investment in fee-for-service care. Treatments that show cost-effectiveness ratios less than $20 000 per QALY are recommended for rapid dissemination into health care systems.30 The present cost-effectiveness acceptability curve analysis found a 99.7% probability that this intervention would cost less than $20 000 per QALY.
This analysis of incremental costs took the perspective of the health insurer or health care plan. As would be expected in an aging population with chronic illnesses, disenrollment from the health plan was low. Thus, health insurers may realize long-term benefits for improved depression and quality-of-life outcomes after short-term investment in improved depression, diabetes, and CHD treatment. Several trials39,40 that have tested collaborative depression care approaches to improve quality of depression care and outcomes of depression in aging populations with chronic illnesses have found improved quality of life and potential cost savings for up to 5 years compared with UC.
Health care reform efforts have emphasized the importance of patient-centered approaches and continuity of care for patients with multiple chronic illnesses.18 Previous studies41 have shown that most people (particularly aging populations) prefer receiving mental health care in primary care. In line with these observations, TEAMcare was associated with improved satisfaction with depression and medical care compared with UC.15
There are several limitations in interpreting these data. This study was completed in 1 health plan and 1 geographic region of the United States, and the results may not generalize to other populations or health plans. The TEAMcare intervention may be more difficult to implement in less organized health care systems or in fee-for-service clinics. Financial incentives for improving disease control of populations with chronic illnesses, such as proposed in primary care medical home and accountable care organizations,42 may help defray costs for changes in systems of care that are necessary to implement TEAMcare. Health care use patterns may also differ in this sample of patients from those of patients involved in other health plans with differing financial incentives. Because GH had implemented quality improvement programs in UC for patients with diabetes, intervention vs UC differences may be greater in less organized systems of care. Although we estimate that the intervention program was associated with potential savings in total ambulatory costs over 2 years, CIs are wide and include the possibility of increased costs associated with the intervention. The presence of extreme outliers in inpatient cost data results in uncertainty regarding the effects on inpatient costs and total costs (inpatient and outpatient). The changes in QALYs estimated from depression-free days were based on estimates in the literature and not on using multiattribute utility scales, such as the EuroQOL.
In conclusion, the intervention was associated with important benefits in depression-free days and QALYs. The results of the cost-effectiveness analyses based on outpatient costs suggest that the TEAMcare intervention is a high-value intervention (based on the criteria of costing less that $20 000 per QALY30) that should be rapidly disseminated into primary care systems.
Acknowledgments
Financial Disclosure: Dr Katon has received support to serve on advisory boards from Eli Lilly and Wyeth and has received honoraria for lectures from Pfizer, Forest, Eli Lilly, and Wyeth. Dr Lin served on an advisory board for Physicians Postgraduate Press, receives honoraria for lectures, and has received funds for preparing a manuscript for the following companies: Institute of Clinical Systems Improvement, Prescott Medical, and Health-Star Communications (Eli Lilly). Dr Ciechanowski serves on the editorial boards of Diabetic Living and Diabetes Forecast, owns Samepage and has a patent for his company, receives lecture fees from Rewarding Health, and receives travel fees from Roche Diagnostics. Dr Von Korff receives support from grants to the Group Health Research Institute from Johnson & Johnson Inc for developing a risk score for chronic pain prognosis and a study of acetaminophen use.
Funding/Support: This research was supported by grants MH041739 and MH069741 from the Services Division of the National Institute of Mental Health (Dr Katon).
Footnotes
Trial Registration: clinicaltrials.gov Identifier: NCT00468676
REFERENCES
- 1.Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002;162(20):2269–2276. doi: 10.1001/archinte.162.20.2269. [DOI] [PubMed] [Google Scholar]
- 2.Warshaw G. Introduction advances and challenges in care of older people with chronic illness. Generation. 2006;30(3):5–10. [Google Scholar]
- 3.Lee TA, Shields AE, Vogeli C, Gibson TB, Woong-Sohn M, Marder WD, Blumenthal D, Weiss KB. Mortality rate in veterans with multiple chronic conditions. J Gen Intern Med. 2007;22(suppl 3):403–407. doi: 10.1007/s11606-007-0277-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogeli C, Shields AE, Lee TA, Gibson TB, Marder WD, Weiss KB, Blumenthal D. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med. 2007;22(suppl 3):391–395. doi: 10.1007/s11606-007-0322-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson G, Horvath J. Chronic Conditions: Making the Case for Ongoing Care: Robert Wood Johnson Foundation’s Partnership for Solutions. Baltimore, MD: Johns Hopkins University; 2002. [Google Scholar]
- 6.Thorpe KE, Ogden LL, Galactionova K. Chronic conditions account for rise in Medicare spending from 1987 to 2006. Health Aff (Millwood) 2010;29(4):718–724. doi: 10.1377/hlthaff.2009.0474. [DOI] [PubMed] [Google Scholar]
- 7.Schäfer I, von Leitner EC, Schön G, Koller D, Hansen H, Kolonko T, Kaduszkiewicz H, Wegscheider K, Glaeske G, van den Bussche H. Multimorbidity patterns in the elderly: a new approach of disease clustering identifies complex interrelations between chronic conditions. PLoS One. 2010;5(12):e15941. doi: 10.1371/journal.pone.0015941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2006;23(11):1165–1173. doi: 10.1111/j.1464-5491.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- 9.Carney RM, Freedland KE. Depression in patients with coronary heart disease. Am J Med. 2008;121(11) suppl 2:S20–S27. doi: 10.1016/j.amjmed.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Lin EH, Katon W, Von Korff M, Rutter C, Simon GE, Oliver M, Ciechanowski P, Ludman EJ, Bush T, Young B. Relationship of depression and diabetes selfcare, medication adherence, and preventive care. Diabetes Care. 2004;27(9):2154–2160. doi: 10.2337/diacare.27.9.2154. [DOI] [PubMed] [Google Scholar]
- 11.Ludman EJ, Katon W, Russo J, Von Korff M, Simon G, Ciechanowski P, Lin E, Bush T, Walker E, Young B. Depression and diabetes symptom burden. Gen Hosp Psychiatry. 2004;26(6):430–436. doi: 10.1016/j.genhosppsych.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Von Korff M, Katon W, Lin EH, Simon G, Ludman E, Oliver M, Ciechanowski P, Rutter C, Bush T. Potentially modifiable factors associated with disability among people with diabetes. Psychosom Med. 2005;67(2):233–240. doi: 10.1097/01.psy.0000155662.82621.50. [DOI] [PubMed] [Google Scholar]
- 13.Black SA, Markides KS, Ray LA. Depression predicts increased incidence of adverse health outcomes in older Mexican Americans with type 2 diabetes. Diabetes Care. 2003;26(10):2822–2828. doi: 10.2337/diacare.26.10.2822. [DOI] [PubMed] [Google Scholar]
- 14.Simon GE, Katon WJ, Lin EH, Ludman E, Von Korff M, Ciechanowski P, Young BA. Diabetes complications and depression as predictors of health service costs. Gen Hosp Psychiatry. 2005;27(5):344–351. doi: 10.1016/j.genhosppsych.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Katon WJ, Lin EH, Von Korff M, Ciechanowski P, Ludman EJ, Young B, Peterson D, Rutter CM, McGregor M, McCulloch D. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363(27):2611–2620. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med. 2006;166(21):2314–2321. doi: 10.1001/archinte.166.21.2314. [DOI] [PubMed] [Google Scholar]
- 18.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff (Millwood) 2001;20(6):64–78. doi: 10.1377/hlthaff.20.6.64. [DOI] [PubMed] [Google Scholar]
- 19.Riddle MC, Rosenstock J, Gerich J Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26(11):3080–3086. doi: 10.2337/diacare.26.11.3080. [DOI] [PubMed] [Google Scholar]
- 20.Lave JR, Frank RG, Schulberg HC, Kamlet MS. Cost-effectiveness of treatments for major depression in primary care practice. Arch Gen Psychiatry. 1998;55(7):645–651. doi: 10.1001/archpsyc.55.7.645. [DOI] [PubMed] [Google Scholar]
- 21.Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist (HSCL). A measure of primary symptom dimensions. Mod Probl Pharmacopsychiatry. 1974;7(0):79–110. doi: 10.1159/000395070. [DOI] [PubMed] [Google Scholar]
- 22.Katon W, Unützer J, Fan MY, Williams JW, Jr, Schoenbaum M, Lin EH, Hunkeler EM. Cost-effectiveness and net benefit of enhanced treatment of depression for older adults with diabetes and depression. Diabetes Care. 2006;29(2):265–270. doi: 10.2337/diacare.29.02.06.dc05-1572. [DOI] [PubMed] [Google Scholar]
- 23.Simon GE, Von Korff M, Heiligenstein JH, Revicki DA, Grothaus L, Katon W, Wagner EH. Initial antidepressant choice in primary care. Effectiveness and cost of fluoxetine vs tricyclic antidepressants. JAMA. 1996;275(24):1897–1902. [PubMed] [Google Scholar]
- 24.Schmittdiel J, Vijan S, Fireman B, Lafata JE, Oestreicher N, Selby JV. Predicted quality-adjusted life years as a composite measure of the clinical value of diabetes risk factor control. Med Care. 2007;45(4):315–321. doi: 10.1097/01.mlr.0000254582.85666.01. [DOI] [PubMed] [Google Scholar]
- 25.Wells KB, Sherbourne CD. Functioning and utility for current health of patients with depression or chronic medical conditions in managed, primary care practices. Arch Gen Psychiatry. 1999;56(10):897–904. doi: 10.1001/archpsyc.56.10.897. [DOI] [PubMed] [Google Scholar]
- 26.Unützer J, Patrick DL, Diehr P, Simon G, Grembowski D, Katon W. Quality-adjusted life years in older adults with depressive symptoms and chronic medical disorders. Int Psychogeriatr. 2000;12(1):15–33. doi: 10.1017/s1041610200006177. [DOI] [PubMed] [Google Scholar]
- 27.Revicki DA, Wood M. Patient-assigned health state utilities for depression-related outcomes: differences by depression severity and antidepressant medications. J Affect Disord. 1998;48(1):25–36. doi: 10.1016/s0165-0327(97)00117-1. [DOI] [PubMed] [Google Scholar]
- 28.Willan AR, Lin DY, Manca A. Regression methods for cost-effectiveness analysis with censored data. Stat Med. 2005;24(1):131–145. doi: 10.1002/sim.1794. [DOI] [PubMed] [Google Scholar]
- 29.Fenwick E, Byford S. A guide to cost-effectiveness acceptability curves. Br J Psychiatry. 2005;187:106–108. doi: 10.1192/bjp.187.2.106. [DOI] [PubMed] [Google Scholar]
- 30.Gold M, Siegel J, Russel L, Weinstein M. Cost-effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 31.Basu A, Polsky D, Manning WG. Use of Propensity Scores in Nonlinear Response Models: The Case for Health Care Expenditures. Cambridge, MA: National Bureau of Economic Research; 2008. NBER working paper series w14086. [Google Scholar]
- 32.Hirano KI, Imbens GW, Ridder G. Efficient estimation of average treatment effects using the estimated propensity score. Econometrica. 2003;71(4):1161–1189. [Google Scholar]
- 33.Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(10):33–38. [Google Scholar]
- 34.Rosenbaum P. Propensity Score. 2nd ed. Vol 5. New York, NY: John Wiley & Sons Inc; 1998. [Google Scholar]
- 35.Katon WJ, Schoenbaum M, Fan MY, Callahan CM, Williams J, Jr, Hunkeler E, Harpole L, Zhou XH, Langston C, Unützer J. Cost-effectiveness of improving primary care treatment of late-life depression. Arch Gen Psychiatry. 2005;62(12):1313–1320. doi: 10.1001/archpsyc.62.12.1313. [DOI] [PubMed] [Google Scholar]
- 36.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 37.Katon WJ. Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues Clin Neurosci. 2011;13(1):7–23. doi: 10.31887/DCNS.2011.13.1/wkaton. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katon WJ, Lin EH, Williams LH, Ciechanowski P, Heckbert SR, Ludman E, Rutter C, Crane PK, Oliver M, Von Korff M. Comorbid depression is associated with an increased risk of dementia diagnosis in patients with diabetes: a prospective cohort study. J Gen Intern Med. 2010;25(5):423–429. doi: 10.1007/s11606-009-1248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katon WJ, Russo JE, Von Korff M, Lin EH, Ludman E, Ciechanowski PS. Long-term effects on medical costs of improving depression outcomes in patients with depression and diabetes. Diabetes Care. 2008;31(6):1155–1159. doi: 10.2337/dc08-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Unutzer J, Katon WJ, Fan MY, Schoenbaum MC, Lin EH, Della Penna RD, Powers D. Long-term cost effects of collaborative care for late-life depression. Am J Manag Care. 2008;14(2):95–100. [PMC free article] [PubMed] [Google Scholar]
- 41.Bartels SJ, Coakley EH, Zubritsky C, Ware JH, Miles KM, Areán PA, Chen H, Oslin DW, Llorente MD, Costantino G, Quijano L, McIntyre JS, Linkins KW, Oxman TE, Maxwell J, Levkoff SE PRISM-E Investigators. Improving access to geriatric mental health services: a randomized trial comparing treatment engagement with integrated versus enhanced referral care for depression, anxiety, and at-risk alcohol use. Am J Psychiatry. 2004;161(8):1455–1462. doi: 10.1176/appi.ajp.161.8.1455. [DOI] [PubMed] [Google Scholar]
- 42.Katon W, Unützer J. Consultation psychiatry in the medical home and accountable care organizations: achieving the triple aim. Gen Hosp Psychiatry. 2011;33(4):305–310. doi: 10.1016/j.genhosppsych.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]