Abstract
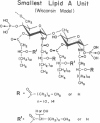
Certain Escherichia coli mutants altered in phosphatidylglycerol metabolism accumulate fatty acyl derivatives of glucosamine 1-phosphate. Especially prominent is 2,3-diacylglucosamine 1-phosphate (previously designated lipid X), which may be an early precursor of lipid A. We have examined the activity of lipid X (Mr = 711.9) and several related compounds as mitogens towards mouse lymphocytes. As judged by labeling with [methyl-3H]thymidine, lipid X is mitogenic, and it mimics the properties of lipopolysaccharide and lipid A. The following evidence suggests that lipid X exerts its effects by a route similar to that of lipopolysaccharide: (i) lymphocytes from C3H/HeJ mice, which are unresponsive to lipopolysaccharide, are also not stimulated by lipid X; (ii) polymyxin B abrogates lymphocyte stimulation by lipid X; and (iii) lipid X induces the proliferation and maturation of lymphocytes to antibody-producing plaque-forming cells. Selective removal of the ester-linked hydroxymyristate moiety at position 3 totally abolishes mitogenic activity. Other phospholipids, such as phosphatidic acid, CDP-diglyceride, phosphatidylcholine, and lysophosphatidylcholine, have no activity as mitogens. If lipid X and lipid A induce by common mechanism(s) B-lymphocyte proliferation, then it follows from structural comparison that the reducing-end subunit of lipid A is the minimal structural requirement for this activity. Because the structure of lipid X is completely defined, biochemical and pharmacological dissection of B-cell activation by lipopolysaccharide should now be possible.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Melchers F., Galanos C., Lüderitz O. The mitogenic effect of lipopolysaccharide on bone marrow-derived mouse lymphocytes. Lipid A as the mitogenic part of the molecule. J Exp Med. 1973 Apr 1;137(4):943–953. doi: 10.1084/jem.137.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiller J. M., Skidmore B. J., Morrison D. C., Weigle W. O. Relationship of the structure of bacterial lipopolysaccharides to its function in mitogenesis and adjuvanticity. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2129–2133. doi: 10.1073/pnas.70.7.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Glomset J. A. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res. 1968 Mar;9(2):155–167. [PubMed] [Google Scholar]
- Hirata F., Axelrod J. Phospholipid methylation and biological signal transmission. Science. 1980 Sep 5;209(4461):1082–1090. doi: 10.1126/science.6157192. [DOI] [PubMed] [Google Scholar]
- Jacobs M. D., Morrison D. C. Dissociation between mitogenicity and immunogenicity of TNP-lipopolysaccharide, a T-independent antigen. J Exp Med. 1975 Jun 1;141(6):1453–1458. doi: 10.1084/jem.141.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee A. I., Schlesinger M. J. Fatty acid acylation of eucaryotic cell membrane proteins. Biochim Biophys Acta. 1982 Nov 30;694(3):279–289. doi: 10.1016/0304-4157(82)90008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura T., Mizushima S. Separation by density gradient centrifugation of two types of membranes from spheroplast membrane of Escherichia coli K12. Biochim Biophys Acta. 1968 Jan 3;150(1):159–161. doi: 10.1016/0005-2736(68)90020-5. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Nakae T. The outer membrane of Gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- Nishijima M., Raetz C. R. Characterization of two membrane-associated glycolipids from an Escherichia coli mutant deficient in phosphatidylglycerol. J Biol Chem. 1981 Oct 25;256(20):10690–10696. [PubMed] [Google Scholar]
- Nishijima M., Raetz C. R. Membrane lipid biogenesis in Escherichia coli: identification of genetic loci for phosphatidylglycerophosphate synthetase and construction of mutants lacking phosphatidylglycerol. J Biol Chem. 1979 Aug 25;254(16):7837–7844. [PubMed] [Google Scholar]
- Nishijima M., Sa-Eki T., Tamori Y., Doi O., Nojima S. Synthesis of acyl phosphatidylglycerol from phosphatidylglycerol in Escherichia coli K-12. Evidence for the participation of detergent-resistant phospholipase A and heat-labile membrane-bound factor(s). Biochim Biophys Acta. 1978 Jan 27;528(1):107–118. [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Qureshi N., Takayama K., Ribi E. Purification and structural determination of nontoxic lipid A obtained from the lipopolysaccharide of Salmonella typhimurium. J Biol Chem. 1982 Oct 10;257(19):11808–11815. [PubMed] [Google Scholar]
- Retzinger G. S., Meredith S. C., Takayama K., Hunter R. L., Kézdy F. J. The role of surface in the biological activities of trehalose 6,6'-dimycolate. Surface properties and development of a model system. J Biol Chem. 1981 Aug 10;256(15):8208–8216. [PubMed] [Google Scholar]
- Skidmore B. J., Chiller J. M., Morrison D. C., Weigle W. O. Immunologic properties of bacterial lipopolysaccharide (LPS): correlation between the mitogenic, adjuvant, and immunogenic activities. J Immunol. 1975 Feb;114(2 Pt 2):770–775. [PubMed] [Google Scholar]
- Sultzer B. M. Genetic control of leucocyte responses to endotoxin. Nature. 1968 Sep 21;219(5160):1253–1254. doi: 10.1038/2191253a0. [DOI] [PubMed] [Google Scholar]
- Sultzer B. M., Nilsson B. S. PPD tuberculin--a B-cell mitogen. Nat New Biol. 1972 Dec 13;240(102):198–200. doi: 10.1038/newbio240198a0. [DOI] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Mascagni P., Nashed M. A., Anderson L., Raetz C. R. Fatty acyl derivatives of glucosamine 1-phosphate in Escherichia coli and their relation to lipid A. Complete structure of A diacyl GlcN-1-P found in a phosphatidylglycerol-deficient mutant. J Biol Chem. 1983 Jun 25;258(12):7379–7385. [PubMed] [Google Scholar]
- Volk W. A., Galanos C., Lüderitz O. The occurrence of 4-amino-4-deoxy-L-arabinose as a constituent in Salmonella lipopolysaccharide preparations. Eur J Biochem. 1970 Dec;17(2):223–229. doi: 10.1111/j.1432-1033.1970.tb01157.x. [DOI] [PubMed] [Google Scholar]
- Watson J. Cyclic nucleotides as intracellular mediators of B cell activation. Transplant Rev. 1975;23:223–249. doi: 10.1111/j.1600-065x.1975.tb00161.x. [DOI] [PubMed] [Google Scholar]
- Watson J., Riblet R. Genetic control of responses to bacterial lipopolysaccharides in mice. I. Evidence for a single gene that influences mitogenic and immunogenic respones to lipopolysaccharides. J Exp Med. 1974 Nov 1;140(5):1147–1161. doi: 10.1084/jem.140.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Riblet R. Genetic control of responses to bacterial lipopolysaccharides in mice. II. A gene that influences a membrane component involved in the activation of bone marrow-derived lymphocytes by lipipolysaccharides. J Immunol. 1975 May;114(5):1462–1468. [PubMed] [Google Scholar]
- Wollenweber H. W., Broady K. W., Lüderitz O., Rietschel E. T. The chemical structure of lipid A. Demonstration of amide-linked 3-acyloxyacyl residues in Salmonella minnesota Re lipopolysaccharide. Eur J Biochem. 1982 May;124(1):191–198. doi: 10.1111/j.1432-1033.1982.tb05924.x. [DOI] [PubMed] [Google Scholar]
- Zilberstein A., Snider M. D., Porter M., Lodish H. F. Mutants of vesicular stomatitis virus blocked at different stages in maturation of the viral glycoprotein. Cell. 1980 Sep;21(2):417–427. doi: 10.1016/0092-8674(80)90478-x. [DOI] [PubMed] [Google Scholar]