Abstract
Background and Objectives:
The clinical benefits of stem cell therapy have been reported in patients with peripheral arterial occlusive disease. However, those studies had no standard reporting system to assess the outcomes, so we made a scoring system and assessed the outcomes of the limbs that underwent whole bone marrow stem cell (WBMSC) therapy.
Methods and Results:
Between July 4 and June 2009, 90 limbs of 67 patients with symptomatic thromboangiitis obliterans (TAO) were enrolled. Autologous whole bone marrow was implanted into the limb by intramuscular injections. The primary outcomes were defined by the clinical and angiographic improvement in all the limbs and the secondary outcomes were the clinical improvement and the amputation-free rates in the critical ischemic limbs (CILs). Clinical improvement and angiographic improvement was observed in 55.6% and 43.2% of all the limbs and in 50% and 50%of the CILs, respectively. The 1, 3 and 5-year amputation-free rates were 91.9%, 88.5% and 84.6% for all the limbs, respectively, and 83.9%, 77.5% and 70.4% for the CILs, respectively. A history of sympathectomy/sympathetic block was shown to be a negative prognostic factor for clinical improvement in all the limbs and in the CILs. In addition, a history of sympathetic block/sympathectomy and the smoking state were the major predictors of amputation for the CILs.
Conclusions:
This study indicated that autologous WMBSC therapy improves the clinical status and reduces amputation factors in the limbs with symptomatic TAO and a history of sympathetic block/sympathectomy and the smoking state are useful prognostic factors.
Keywords: Thromboangiitis obliterans, Buerger’ s disease, Stem cells, Neovascularization, Bone marrow
Introduction
Several studies have recently reported that cell therapy using stem cells induces the development of new collateral vessels and it improves the ischemic symptoms in patients with Thromboangiitis obliterans (TAO, Buerger’s disease) (1-4). However, there are several limitations to directly apply stem cell therapy into the clinical field. First, most of the previous studies have had a small number of subjects and they reported only the short-term results. Second, a standardized reporting system is lacking for assessing the outcomes. Third, the ideal source and types of stem cells and their amount and route of administration have not been established. Most of all, as a source of stem
cells, the majority of studies used autologous bone marrow- derived or peripheral blood-derived mononuclear cells (3, 5-8). However, the isolation of mononuclear cells from bone marrow or peripheral blood is highly complex and expensive and there is a potential danger of contamination. We have previously reported that autologous whole bone marrow stem cell (WBMSC) therapy is a simple, safe and effective strategy to induce angiogenesis in patients with TAO (1).
TAO is defined as a nonatherosclerotic, segmental inflammatory disease that commonly affects the small and medium sized arteries of the legs and/or arms (9). The symptoms of TAO are characterized by limb ischemia, ulcer or gangrene, and TAO occurs in young smokers before the age of 45 years. The exact cause remains unknown; however, it is known that the use of tobacco is strongly associated with the initiation and progression of disease (9-11). Even though the only definitive therapy for TAO is cessation of smoking, the presence of ischemic symptoms requires additional treatment modalities, including revascularization procedures. However, revascularization procedures such as a bypass operation or percutaneous transluminal angioplasty are frequently not feasible and they are often unsuccessful because of the diffuse segmental involvement and the distal nature of the disease (2).
In the present study, we have focused on assessing the long-term clinical outcomes, based on the clinically relevant findings, of autologous WBMSC therapy in patients with TAO. Moreover, a newly developed scoring system that is composed of ‘recanlization of run-off vessels’ , ‘ angiogenesis’ and ‘ arteriogenesis’ was applied to evaluate the angiographic outcome after WBMSC therapy (Table 1).
Table 1.
Assessment of the clinical & angiographic outcomes
| Assessment of the clinical outcome | |||||
|---|---|---|---|---|---|
|
| |||||
| Aggravation | No change | Improvement | |||
|
| |||||
| Claudication | Development of new ischemic foot lesion after WBMSC therapy | No statistically significant difference of the pain-free walking distance | Return to normal activity Statistically significant change of the pain-free walking distance | ||
| Critical ischemia | Ischemic rest pain | Development of new ischemic foot lesion after WBMSC therapy Any amputation | No change of ischemic rest pain | Relief of ischemic rest pain | |
| Tissue loss | Recurrence of a healed ischemic lesion Development of new ischemic foot lesion after complete wound healing Any amputation | No change of ischemic foot wound | Complete wound healing with or without planned debridement or amputation | ||
|
| |||||
| Assessment of the angiographic outcome | |||||
|
| |||||
| -2 | -1 | 0 | +1 | +2 | |
|
| |||||
| Recanalization of run-off vessels | ≥50% progression of occlusion in the previous lengths of the run-off vessels | <50% progression of occlusion in the previous length of the run-off vessels | No change of the overall extent of the run-off vessels | <50% segmental recanalization in the previous lengths of the run-off vessels | ≥50% segmental recanalization in the previous lengths of the run-off vessels |
| Angiogenesis | Any loss of firstorder collateral vessels | Any loss of second-order collateral vessels | No change of the overall extent of collateral vessels | Development of more than second-order collateral vessels | Development of first-order |
| Arteriogenesis | Decreased diameter and length of the pre-existing collateral vessels | Decreased diameter or length of the pre-existing collateral vessels | No change of the overall extent of collateral vessels | Increased diameter or length of the pre-existing collateral vessels | Increased diameter and length of the pre-existing collateral vessels |
The primary outcomes were defined by the clinical and angiographic improvement in all the limbs, and the secondary outcomes were determined by the clinical improvement and the amputation-free rates in the critical ischemic limbs (CILs). In addition, we analyzed the predisposing factors that affect the clinical improvement and amputation.
Materials and Methods
Patients population
Between July 2004 and June 2009, a total of 94 limbs (69 patients) with TAO and intermittent claudication, ischemic rest pain or tissue loss for a minimum of 2 weeks underwent WBMSC therapy, and these limbs were considered to not be suitable candidates for revascularization procedures. It was recommended that all the patients who were to undergo WBMSC therapy continue their medications. TAO was diagnosed by the clinical diagnostic criteria suggested by Shionoya (12). The exclusion criteria were a diagnosis of atherosclerotic peripheral arterial occlusive disease, signs of acute inflammation, a previous (less than 5 years) or current history of malignant diseases, and refusal or inability to give informed consent. The patients’ eligibility for WBMSC therapy was confirmed by the Samsung Vascular Disease Team, which was composed of cardiologists, interventional radiologists and vascular surgeons. This prospective study received institutional review board approval and we obtained written informed consent from all the patients for participation in the study.
Study protocol
The ankle-brachial index (ABI), the toe brachial index (TBI) and the digital subtraction angiography of the lower extremities were preoperatively examined. All the angiographies were performed at 3 days before and 6 months after WBMSC therapy and the same protocol was used that strictly fixed the force of contrast injection, the amount of contrast media and the position of the patients and the catheter tip. In addition, 75μg of recombinant human granulocyte colony stimulating factor (rhG-CSF, Cheil Jedang, Korea) was subcutaneously injected each day for 3 days before WBMSC therapy and the rhG-CSF dosage was adjusted daily to maintain a white blood cell count around 20,000 cells/ml. Peripheral blood samples were obtained daily for examining the count of the peripheral mononuclear cells and the levels of CD34 and CD133 molecules in the sera, which represented the potential endothelial progenitor cells (EPCs). Flow cytometric analysis was performed after staining the mononuclear cells with CD34-fluorescein isothiocyanate and CD133-phosphatidylethanolamine monoclonal antibodies (BD Pharmingen, San Diego, CA, USA).
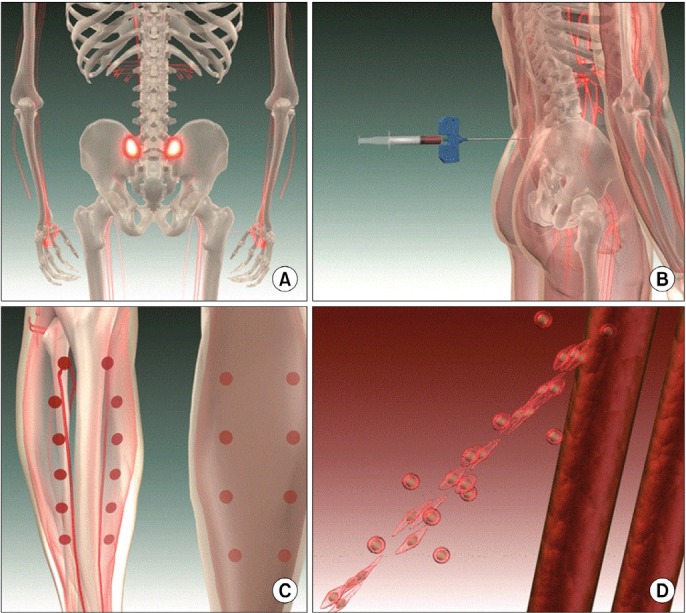
On the day of the therapy, the patients were placed in the prone position under general or spinal anesthesia, and then 20 ml of autologous whole bone marrow was aspirated from the posterior superior iliac spine, and this was next split into 1 ml aliquots using 2 mm syringes. Approximately 1 ml of whole bone marrow was implanted into the calf muscles and the muscles around the run-off vessels by multiple injections (about 20 times). The procedures were finished after placing a compression dressing on the injection sites (Fig. 1).
Fig. 1. Procedures of the whole bone marrow stem cell therapy. (A, B) Aspiration of autologous whole bone marrow (about 20 ml) from the posterior superior iliac spine. (C) Multiple injections (about 20 ml) of the whole bone marrow into the calf muscles and the muscles around the run-off vessels. (D) Note the development of new collateral vessels from pre-existing vascular structures.
We followed up the patients at 1, 3, 6 and 12 months and every 1 year thereafter. The status of the wound, the development of new ischemic foot lesion, amputation, the pain-free walking distance, the ABI and the TBI were examined at every visit. On July 2009, the final information for the clinical outcomes was confirmed by a direct telephone survey, which was performed for all the patients who underwent WBMSC therapy.
Study outcomes and definitions
In present study, the primary outcomes were defined as the overall clinical and angiographic improvement in all the limbs. The secondary outcomes were determined based on the clinical improvement and the amputation in the CILs.
The clinical and angiographic outcomes were divided into ‘aggravation’ , ‘ no change’ and ‘ improvement’ (Table 1). The clinical improvement was defined as complete relief of ischemic rest pain without the need for analgesics or complete wound healing with or without planned removal of the non-viable tissue in the CILs. The clinical outcome for intermittent claudication was determined as the significant changes of the pain-free walking distance at the last follow-up as compared to that at baseline. In contrast, the aggravation of the clinical outcome was represented by the development of new ischemic foot lesion or any amputation due to aggravation of the wound after WBMSC therapy (Table 1). The angiographic outcomes were assessed by three categories; 1) recanalization of the major run-off vessels, 2) angiogenesis (the development of new collateral vessels) and 3) arteriogenesis (the increased diameter or length of the pre-existing collateral vessels) (Table 1). Each category was scored from ‘ -2’ to ‘ 2’ according to the degree of neovascularization and this was reviewed by 1 experienced radiologist. The angiographic outcomes were also defined as ‘ aggravation’ , ‘ no change’ and ‘ improvement’ by the cut-off values ‘ 0’ and ‘ 1’ that showed the statistically significant change of the sum of each category from baseline.
Statistics
The statistically significant changes of the pain-free walking distance were evaluated by the Mann-Whitney test. To analyze the independent prognostic factors associated with the primary and secondary outcomes, we applied the Student t test for the continuous variables and the Chi-square test for the non-continuous variables. The logistic regression model was applied for multivariate analysis of the factors affecting the clinical improvement and amputation. The Wilcoxon two-sample test was performed to identify the correlation between the EPCs count and clinical improvement. The amputation-free rate was estimated by the Kaplan-Meier method. The 95% confidence intervals (CIs) were estimated for the differences. Statistical significance was assumed at a two sided p value <0.005. The statistical analysis and construction of the scatter plots were performed with SPSS 15.0 for Window (SPSS Inc, Chicago, IL). A Bonferroni correction, which was used to prevent type I error, was applied to analyze the predisposing factors for clinical improvement in the CILs. All the statistical analyses were performed by the Department of Biostatistics, Samsung Biomedical Research Institute.
Results
The patients’ characteristics and demographic data
From July 2004 to June 2009, a total of 94 limbs (69 patients) with TAO underwent WBMSC therapy. Among these patients, 1 patient (2 limbs) who underwent WBMSC therapy due to non-healing gangrenous lesions in both feet committed suicide after 6 months of WBMSC therapy and the final information of 1 patient (2 limbs) could not be obtained by telephone survey. Therefore, 90 limbs of 67 patients were included in this study.
The clinical and demographic data is shown in Table 2. All the patients (n= 67) were males and their mean age was 39.8± 7.9 years and the mean duration of follow-up was 29.3± 18.1 months (range: 2± 61 months). The duration of ischemic symptoms was generally more than 12 months for the limbs with intermittent claudication and it was between 1 and 6 months for the CIL. Approximately 11% of the patients in the claudication and CIL groups had a history of revascularization procedures. Previous histories of amputation and sympathetic block/sympathectomy were more prevalent in the CIL group that those in the claudication group (2.5% vs. 17.4% , 2.3% vs. 30.4% , respectively). Four limbs that had a history of diabetes mellitus at the time of WBMSC therapy did not have diabetes mellitus at the time of diagnosing TAO. More than half of the included study population stopped smoking before WBMSC therapy, but a quarter continued
Table 2.
The patients’ data and their demographic data for the limbs with intermittent claudication (n=44) and the limbs with critical ischemia (n=46)
| Intermittent claudication (n=44) | Critical ischemia (n=46) | |
|---|---|---|
|
| ||
| Mean age (years)±SDa (range) | 39.8±7.9 (25∼64) | 39.8±7.8 (20∼58) |
| Duration of follow-up (mean±SD) | 28.8±17.3 | 29.9±19.1 |
| <12 months | 7 (16%) | 14 (30%) |
| 12~36 months | 18 (41%) | 13 (28%) |
| ≥36 months | 19 (43%) | 19 (41%) |
| Symptom onset (mean±SD) | 46.0±49.6 months | 7.5±9.7 months |
| <1 month | 0 (0%) | 6 (13.0%) |
| 1~6 months | 11 (25.0%) | 24 (52.2%) |
| 6~12 months | 7 (15.9%) | 13 (28.3%) |
| ≥12 months | 26 (59.1%) | 3 (6.5%) |
| Symptoms of the upper extremities | 4 (9.1%) | 4 (8.7%) |
| History of revascuarization | 5 (11.4%) | 5 (10.9%) |
| Bypass surgery | 3 | 5 |
| PTAb | 1 | 0 |
| Thrombectomy | 1 | 0 |
| History of amputation | 2 (4.5%) | 8 (17.4%) |
| History of sympathetic block/sympathectomy | 1 (2.3%) | 14 (30.4%) |
| Cormorbidities | 12 (27.3%) | 14 (30.4%) |
| Hypertension | 3 | 1 |
| Diabetes mellitus | 1 | 3 |
| Coronary artery disease | 0 | 0 |
| Cerebrovascular disease | 0 | 0 |
| Chronic kidney disease | 0 | 0 |
| Hyperlipidemia | 9 | 10 |
| Smoking | ||
| Quit before WBMSCc therapy | 29 (65.9%) | 25 (54.3%) |
| Quit after WBMSC therapy | 4 (9.1%) | 2 (4.3%) |
| Restart during F/Ud period | 2 (4.5%) | 5 (10.9%) |
| Still smoking | 9 (20.5%) | 14 (30.4%) |
| Medications | ||
| None | 0 (0%) | 2 (4.3%) |
| Vasodilator | 34 (72.3%) | 34 (73.9%) |
| Antiplatelet | 7 (15.9%) | 9 (19.6%) |
| Vasodilator+Antipletelet | 2 (4.5%) | 1 (2.2%) |
| Vasodilator+Anticoagulant | 1 (2.3%) | 0 (0%) |
| ABIe (mean±SD) | 0.82±0.30 | 0.76±0.26 |
| TBIf (mean±SD) | 0.45±0.24 | 0.12±0.13 |
aSD: standard deviation; bPTA: percutaneous transluminal angioplasty; cWBMSC: whole bone marrow stem cell; dF/U: follow-up; eABI: ankle-brachial index; fTBI: toe-brachial index.
smoking through the follow-up period (Table 2). The mean ABI was similar in both groups (0.82 vs. 0.76, respectively), but the TBI in the CIL group was lower than that in the claudication group (0.45 vs. 0.12, respectively). It was considered that the relatively higher ABI, in contrast with the severe ischemic symptoms, could be attributed to the involvement of the distal small arteries below the ankle of the patients with TAO.
Procedures and safety issues
All the patients well tolerated the WBMSC therapy and no procedure-related complications were observed, such as bleeding, osteomyelitis, vascular damage and the reported adverse effects due to the thrombotic properties of rhGCSF. However, one patient of 40 years, who had no evidence of hypertension, diabetes, hyperlipidemia, hypercoagulability or autoimmune disease, suffered myocardial infarction 25 months after WBMSC therapy, but the exact cause of his myocardial infarction remains unknown.
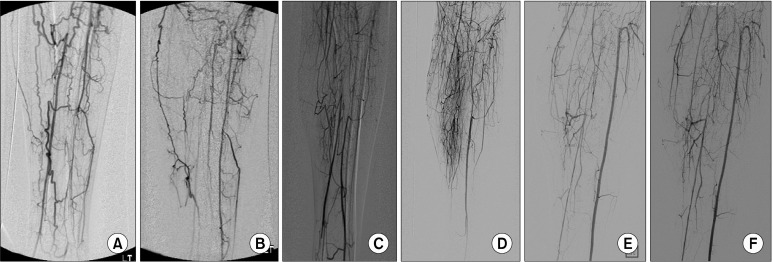
Pre- and postoperative angiographies of the lower extremities were obtained in 37 limbs (41.1% ) and no angiography- related complications occurred. The recanalization of the run-off vessels, the development of new collateral vessels (angiogenesis) and the increased diameter and/ or length of preexisting collateral vessels (arteriogenesis) were noted in 3 (8.1% ), 16 (43.2% ) and 15 (40.5% ) limbs, respectively (Fig. 2).
Fig. 2. Pre- and postoperative angiograms of the lower extremities. (A, C, E) The baseline digital subtraction angiographic studies. (B) Recanalization of the left anterior tibial artery. (D) Development of new collateral vessels (angiogenesis). (F) Note the increased diameter and length of the pre-existing collateral vessels (arteriogenesis).
Primary and secondary outcomes
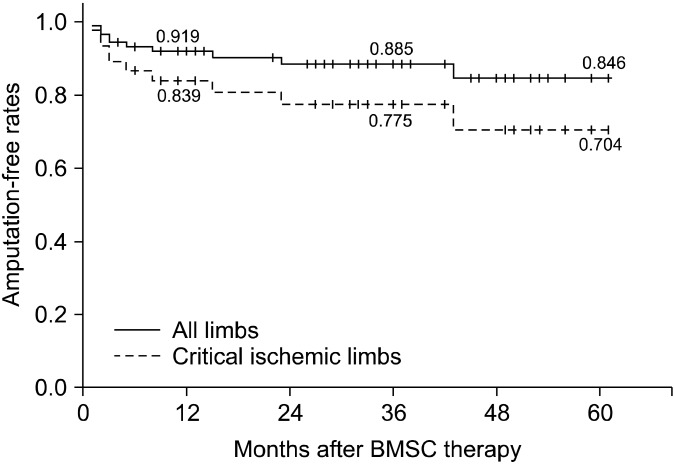
As the primary outcomes, clinical improvement occurred in 55.6% and angiographic improvement occurred in 43.2% of all the limbs (Fig. 3). However, there was no significant correlation between the clinical outcome and the angiographic outcome (p= 0.233). The cumulative 1-year, 3-year and 5-year amputation-free rates of all the limbs were 91.9% , 88.5% and 84.6% , respectively (Fig. 4). During the follow-up period, major amputation was performed on only 1 limb at 5 months after WBMSC therapy due to persistent non-healing ischemic foot ulcer.
Fig. 3. The cumulative amputation-free rates after autologous whole bone marrow stem cell therapy. The 1-, 3- and 5-year amputation- free rates of all the limbs were 91.9% , 88.5% and 84.6% , respectively. The 1-, 3- and 5-year amputation-free rates of the critical ischemic limbs were 83.9% , 77.5% and 70.4% , respectively.
Fig. 4. The pre- and postoperative photographs. (A) Non-healing ischemic ulceration on the left great toe. (B) Complete wound healing of the non-healing ulceration after 8 months of whole bone marrow stem cell therapy.
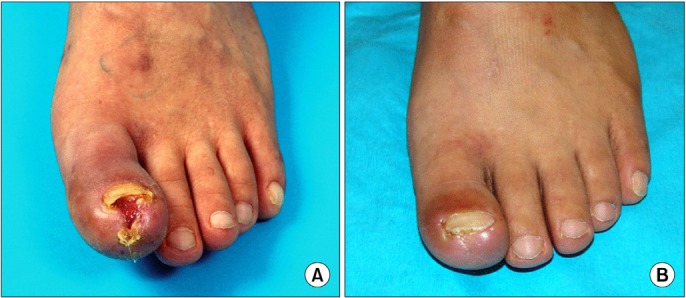
In the CIL group, clinical improvement and angiographic
improvement were observed in 50% and 50% , respectively. In addition, the cumulative 1-year, 3-year and 5-year amputation-free rates of the CIL group were 83.9% , 77.5% and 70.4% , respectively (Fig. 4).
Analysis of the prognostic factors for clinical improvement and amputation
For all the limbs (n=90 limbs), our multivariate analysis indicated that a period of more than 6 months after WBMSC therapy positively affected the clinical outcome (p=0.049, 95% CI: 1.001∼ 14.820, Hazard ratio: 3.852) and the patients with a history of sympathetic block/sympathectomy showed a worse clinical outcome than those patients with no history of sympathetic block/sympathectomy (p=0.048, 95% CI: 0.051∼0.0990, Hazard ratio: 0.224) (Table 3). In the CIL group (n= 46 limbs), a history of sympathetic block/sympathectomy had negative statistically significant effects on clinical improvement (p= 0.032, 95% CI: 0.007∼ 0.835, Hazard ratio: 0.074). The multivariate analysis demonstrated that a history of sympathetic block/ sympathectomy (p= 0.013, 95% CI: 2.172∼934.274, Hazard ratio: 45.052) and smoking at the time of
Table 3.
Univariate & multivariate analyses of the prognostic factors affecting clinical improvement in all limbs (n=90)
| Prognostic factors | No. | Univariate | Multivariate | ||
|---|---|---|---|---|---|
|
| |||||
| p value | Hazard ratio | 95% CIa | p value | ||
|
| |||||
| Age (mean±SDb) | 39.8±7.9 years | 0.899 | 1.000 | 0.940∼1.064 | 0.995 |
| Critical ischemic limbs | 46 (51%) | 0.278 | 0.931 | 0.353∼2.461 | 0.886 |
| Period after treatment ≥6 months | 77 (86%) | 0.179 | 3.852 | 1.001∼14.820 | 0.049 |
| History of revascularization | 10 (11%) | 0.329 | 0.530 | 0.121∼2.330 | 0.400 |
| History of amputation | 10 (11%) | 0.322 | 0.955 | 0.175∼5.219 | 0.957 |
| History of sympathetic block/sympathectomy | 15 (17%) | 0.013 | 0.224 | 0.051∼0.990 | 0.048 |
| Smoking at WBMSCc therapy | 29 (32%) | 0.157 | 0.425 | 0.159∼1.137 | 0.088 |
| Vasodilators (%) | 72 (80%) | 0.595 | 0.626 | 0.194∼2.026 | 0.434 |
aCI: confidence interval; bSD: standard deviation; cWBMSC: whole bone marrow stem cell.
WBMSC therapy (p= 0.015, 95% CI 1.753∼ 220.432, Hazard ratio 19.660) were the statistically significant major determinants to predict amputation in the CILs (Table 4).
Table 4.
Multivariate analysis of the prognostic factors affecting clinical improvement and amputation in critical ischemic limbs (n=46)
| Prognostic factors | Clinical improvement | Amputation | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Hazard ratio | 95% CIa | p value | Hazard ratio | 95% CI | p value | |
|
| ||||||
| Age | 0.932 | 0.822∼1.057 | 0.417 | 1.079 | 0.934∼1.245 | 0.302 |
| Symptom onset ≥6 months | 0.789 | 0.155∼4.014 | 1.000 | 0.224 | 0.020∼2.500 | 0.228 |
| Period after treatment ≥6 months | 2.700 | 0.298∼24.478 | 0.625 | 0.504 | 0.022∼11.644 | 0.668 |
| History of revascularization | 0.201 | 0.010∼3.896 | 0.450 | 8.007 | 0.376∼170.531 | 0.182 |
| History of amputation | 4.788 | 0.222∼103.168 | 0.505 | 0.030 | 0.001∼2.194 | 0.109 |
| History of sympathetic block/sympathectomy | 0.074 | 0.007∼0.835 | 0.032 | 45.052 | 2.172∼934.274 | 0.013 |
| Smoking at treatment | 0.702 | 0.142∼3.480 | 1.000 | 19.660 | 1.753∼220.432 | 0.015 |
| Vasodilators | 1.596 | 0.237∼10.747 | 1.000 | 1.997 | 0.097∼40.949 | 0.653 |
aCI: confidence interval.
Analysis of the EPCs count
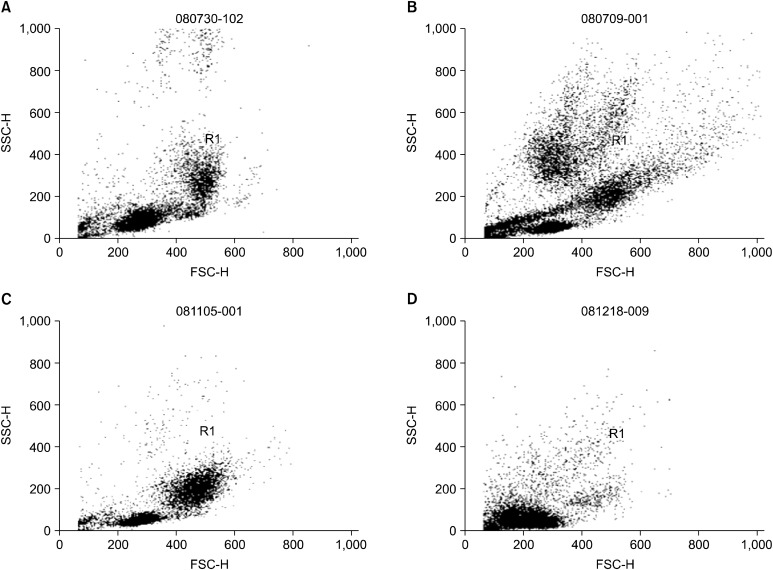
We analyzed the number of rhG-CSF-mobilized peripheral blood EPCs per milliliter (n= 44 limbs), including the number of MNCs, CD34 , CD133 and CD34 CD133 cells and the number of implanted bone marrow EPCs per milliliter (n=39 limbs) (Table 5). In addition, the peripheral blood and bone marrow mononuclear cell composition was analyzed by flow cytometric dot plot analysis according to the forward and side scatter (Fig. 5). After the injection of rhG-CSF, the cell numbers of lymphocytes were highest and the cell numbers of granulocytes were lowest on day 1 (Fig. 5B). The cell numbers of granulocytes were increased on day 1, but the cell numbers of monocytes were highest on day 2 (Fig. 5C, D). The mean number of implanted MNCs, CD34 , CD133 and CD34 CD133 cells were 4.46× 106 ± 1.02× 107/ ml, 1.62× 105± 2.53× 105, 1.16× 105± 1.35× 105 and 4.73× 104± 6.74× 104, respectively. However, the numbers of rhG-CSF-mobilized
Table 5.
Average number of peripheral blood-derived (n=44) and bone marrow-derived (n=39) mononuclear cells, CD34+, CD133+ and CD34+CD133+ cells and the correlation between the peripheral blood and bone marrow mononuclear cells, CD34+, CD133+ and CD34+CD133+ cell counts and the clinical improvement
| Total MNCsa (/ml) | CDb34+ (/ml) | CD133+ (/ml) | CD34+CD133+ (/ml) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Mean No. | p | Mean No. | p | Mean No. | p | Mean No. | p | ||
|
| |||||||||
| PBc | Day 0 | 2.71×106±1.13×106 | 0.247 | 2.23×105±2.57×105 | 0.620 | 1.22×105±1.61×105 | 0.943 | 6.19×104±1.63×105 | 0.143 |
| Day 1 | 3.19×106±1.77×106 | 0.688 | 3.34×105±3.43×105 | 0.509 | 2.13×105±2.42×105 | 0.464 | 8.38×104±9.19×104 | 0.150 | |
| Day 2 | 2.87×106±1.68×106 | 0.472 | 3.59×105±4.61×105 | 0.906 | 2.04×105±2.05×105 | 0.706 | 6.84×104±6.93×104 | 0.345 | |
| BMd | Day 3 | 4.46×106±1.02×107 | 0.090 | 1.62×105±2.53×105 | 0.167 | 1.16×105±1.35×105 | 0.142 | 4.73×104±6.74×104 | 0.225 |
aMNCs: mononuclear cells; bCD: cluster of differentiation; cPB: peripheral blood; dBM: bone marrow.
Fig. 5. Flow cytometric dot plot analysis of the peripheral blood and the bone marrow mononuclear cell composition after the injection of recombinant human granulocyte colony stimulating factor (rhG-CSF). (A) Baseline. (B) The peripheral blood mononuclear cell composition on day 1 after the injection of rhG-CSF. (C) The peripheral blood mononuclear cell composition on day 2. (D) The bone marrow mononuclear cell composition on day 3 after the injection of rhG-CSF.
peripheral blood and bone marrow EPCs had no significant correlation with clinical improvement (Table 5).
Discussion
All the previous clinical studies that used stem cells for treating peripheral arterial occlusive disease had no standardized reporting system to assess the outcomes, so we made up a new scoring system to assess the outcomes and we evaluated our results based on this new scoring system. Our present study indicates that autologous WBMSC therapy improved the clinical and angiographic statuses of the limbs with symptomatic TAO. To assess the long-term clinical and angiographic outcomes, we created an assessment system composed of ‘ aggravation’ , ‘ no change’ and ‘ improvement’ . The assessment of the long-term clinical outcome was based on the clinical status such as the change of the pain-free walking distance, relief from ischemic foot pain, complete wound healing, amputation and development of new ischemic foot lesion. To evaluate the angiographic outcome as was assessed at 6 months after WBMSC therapy, the angiographic findings were divided into 3 categories: recanalization of the run-off vessels, angiogenesis and arteriogenesis, and then each category was scored from ‘ -2’ to ‘ 2’ . In the present study, angiogenesis was defined as the sprouting of new capillaries from pre-existing vascular structures, and arteriognesis was defined as the growth of pre-existing vessels and their caliber (13). Changes of the run-off vessels were noted in 6 limbs (3 limbs with aggravation and 3 limbs with improvement). Our suggested mechanism for the occlusion and recanalization of major run-off vessels is that the occlusion might be the result of progression of TAO and the recanalization of the occluded arteries was the result of the healing process by the stem cells.
Several previous clinical studies have been performed using autologous bone marrow derived- or peripheral blood-derived stem cells in patients with peripheral arterial occlusive disease and they reported favorable results for the ischemic signs and symptoms. In 2002, Tateishi- Yuyama et al. (5) (the TACT study) conducted a randomized controlled study, and they demonstrated improvement in the transcutaneous oxygen pressure, resting pain and pain-free walking times during the 24 months of follow- up after the intramuscular injection of autologous bone marrow mononuclear cells in 25 patients with unilateral chronic limb ischemia. Lenk et al. (14) indicated that intra-arterial application of autologous circulating blood-derived progenitor cells improved the pain-free walking distance, the ABI and the transcutaneous oxygen pressure. Franz et al. (8) recently reported that for nine patients for whom limb amputation was recommend, 88.9% showed some level of improvement and 66.7% avoided amputation. In addition, as the long-term results after intramuscular implantation of bone marrow-derived mononuclear cells, Matoba et al. (15) assessed the 3-year safety and clinical outcomes of patients with chronic limbs ischemia (Fontaine stage III and IV, peripheral artery disease: n= 74 and TAO: n= 41) after they had undergone intramuscular implantation of bone marrow-derived mononuclear cells. They reported that the 3-year survival rate was 80% for the patients with atherosclerotic peripheral arterial disease and 100% for the patients with TAO, and the 3-year amputation-free rate was 60% for the patients with atherosclerotic peripheral artery disease and 91% for the patients with TAO. However, it is difficult to judge the efficacy of the stem cells therapy in these studies because they used different inclusion criteria and assessment methods, and they reported only the results for a small population.
In the present study, to assess the outcomes after WBMSC therapy, we enrolled the limbs that had only symptomatic TAO and we made a new assessment system for the outcomes. As a result, 55.6% of all the included limbs showed clinical improvement and 43.2% showed angiographic improvement. However, no statistically significant relations were found between the clinical and angiographic outcomes (p= 0.206). In addition, clinical improvement and angiographic improvement occurred in 50% and 50% of the CILs, respectively. In this study, the 1-year, 3-year and 5-year amputation-free rates were 91.8% , 88.3% and 84.5% for the all limbs and 83.7% , 77.2% and 70.2% in the CILs, respectively. Moreover, major amputation above the ankle joint was performed on only 1 limb during the 5-year follow-up period. Although we have no control date for conventional therapy in the TAO limbs, when considering the reported studies that the 5-year amputation rate was about 25% and the major amputation rate was about 11% for TAO limbs (16, 17), our results indicated that WBMSC therapy improved the clinical status and reduced the amputation rate for patients with TAO.
Matoba et al. (15) have reported that the severity of resting pain and repeated bypass surgery were the important prognostic factors for the amputation-free interval for patients with peripheral arterial disease by multivariate analysis. However, our statistical analysis revealed that a period more than 6 months after WBMSC therapy was a major determinant for clinical improvement. This finding means that a period of more than 6 months is needed to represent the clinical improvement of WBMSC therapy. A history of sympathetic block/sympathectomy was shown to be a significant prognostic factor for clinical improvement in all the limbs and in the CILs. In addition, it showed that a history of sympathetic block/sympathectomy and smoking at the time of WBMSC therapy were the most important predictors of amputation for CILs. Although the role of sympathectomy for preventing amputation or treating ischemic pain remains unclear (18-20), some physicians have employed it to reduce severe ischemic pain and to help wound healing. Moreover, smoking is a well known major determinant for the progression of the disease and amputation in patients with TAO: the complete discontinuation of any forms of smoking and tobacco use was the most important treatment option to halt disease progression and avoid further amputation in patients with TAO (18, 21, 22). For the CILs, our multivariate statistical results indicated that a previous history of severe ischemic pain and the smoking state were the major determinants for clinical improvement and amputation.
Most of the previous studies have used the angiographic outcome as well as the functional outcome to evaluate neovascularization after bone marrow-derived or peripheral blood-derived stem cell therapy, yet there have been no established methods for assessing neovascularization (3). The currently available angiographic studies have limitations to demonstrate angiogenesis or arteriogenesis, and especially for vessels measuring < 200μm in diameter (23). Our present study showed that the clinical improvement was better than the angiographic improvement, but there was no statistically significant difference between the clinical outcome and the angiographic outcome. There are two possibilities to explain why the clinical improvement was better than the angiographic improvement without statistically significant correlation. The first is that whole bone marrow might contain unknown cells and growth factors as well as stem cells that are required for neovascularization and they promote neovascularization that can not to be detected by angiography. The second is that the synergic effect of rhG-CSF-mobilization and implantation of WBMSC induces repair of the tissues that are impaired due to ischemic injury.
Moreover, most of the previous clinical studies on patients with limb ischemia have used isolated bone marrow- derived mononuclear cells or peripheral blood-derived mononuclear cells, so they could not demonstrate the ideal number of cells to obtain some benefit (5, 8, 14, 24). Kajiguchi et al. (25) reported that the number of transplanted mononuclear cells, CD34+ cells, CD133 cells and CD34 CD133 cells were not significantly different between the responders and non-responders after autologous progenitor cell transplantation in patients with critical limb ischemia. In addition, no correlation between the cell number and the clinical outcomes was demonstrated in the stem cell trials for patients with myocardial infarction (26, 27). We also tried to identity the definite numbers of different EPCs that are needed to obtain clinical improvement, but we failed to establish the numbers of the EPCs populations that are correlated with clinical improvement.
There were several limitations of our study. First of all, the major limitation was the absence of a control group to compare the outcomes after WBMSC therapy, yet it is not ethical to assign patients with symptomatic TAO to a control group. Second, all of the enrolled limbs had TAO, which is only one of many peripheral arterial occlusive diseases, so atherosclerotic peripheral occlusive diseases should be included in a future study. Third, any significant correlation between the numbers of different EPCs populations and the clinical outcome could not be found.
Acknowledgments
This study was supported by a grant of the Samsung Biomedical Research Institute (SBRI C-A7-405-2 & C-A7- 205-3), the Samsung Medical Center Clinical Research Development Grant Program (CRS-107-60-2 & CRS-107- 32-2) and the Seoul Research and Business Development Program (10548).
Potential conflict of interest
The authors have no conflicting financial interest.
References
- 1.Kim DI, Kim MJ, Joh JH, Shin SW, Do YS, Moon JY, Kim NR, Lim JE, Kim AK, Eo HS, Kim BS, Cho SW, Yang SH, Park CJ, Shim JS. Angiogenesis facilitated by autologous whole bone marrow stem cell transplantation for Buerger’ s disease. Stem Cells. 2006;24:1194–1200. doi: 10.1634/stemcells.2005-0349. [DOI] [PubMed] [Google Scholar]
- 2.Durdu S, Akar AR, Arat M, Sancak T, Eren NT, Ozyurda U. Autologous bone-marrow mononuclear cell implantation for patients with Rutherford grade II-III thromboangiitis obliterans. J Vasc Surg. 2006;44:732–739. doi: 10.1016/j.jvs.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 3.Ishida A, Ohya Y, Sakuda H, Ohshiro K, Higashiuesato Y, Nakaema M, Matsubara S, Yakabi S, Kakihana A, Ueda M, Miyagi C, Yamane N, Koja K, Komori K, Takishita S. Autologous peripheral blood mononuclear cell implantation for patients with peripheral arterial disease improves limb ischemia. Circ J. 2005;69:1260–1265. doi: 10.1253/circj.69.1260. [DOI] [PubMed] [Google Scholar]
- 4.Motukuru V, Suresh KR, Vivekanand V, Raj S, Girija KR. Therapeutic angiogenesis in Buerger’ s disease (thromboangiitis obliterans) patients with critical limb ischemia by autologous transplantation of bone marrow mononuclear cells. J Vasc Surg. 2008;48(6 Suppl):53S–60S. doi: 10.1016/j.jvs.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, Shimada K, Iwasaka T, Imaizumi T. Therapeutic Angiogenesis using Cell Transplantation (TACT) Study Investigators. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427–435. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 6.Van Tongeren RB, Hamming JF, Fibbe WE, Van Weel V, Frerichs SJ, Stiggelbout AM, Van Bockel JH, Lindeman JH. Intramuscular or combined intramuscular/ intra-arterial administration of bone marrow mononuclear cells: a clinical trial in patients with advanced limb ischemia. J Cardiovasc Surg (Torino) 2008;49:51–58. [PubMed] [Google Scholar]
- 7.Huang P, Li S, Han M, Xiao Z, Yang R, Han ZC. Autologous transplantation of granulocyte colony-stimulating factor- mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes Care. 2005;28:2155–2160. doi: 10.2337/diacare.28.9.2155. [DOI] [PubMed] [Google Scholar]
- 8.Franz RW, Parks A, Shah KJ, Hankins T, Hartman JF, Wright ML. Use of autologous bone marrow mononuclear cell implantation therapy as a limb salvage procedure in patients with severe peripheral arterial disease. J Vasc Surg. 2009;50:1378–1390. doi: 10.1016/j.jvs.2009.07.113. [DOI] [PubMed] [Google Scholar]
- 9.Olin JW. Thromboangiitis obliterans (Buerger’s disease). N Engl J Med. 2000;343:864–869. doi: 10.1056/NEJM200009213431207. [DOI] [PubMed] [Google Scholar]
- 10.Mills JL, Porter JM. Buerger’s disease (thromboangiitis obliterans) Ann Vasc Surg. 1991;5:570–572. doi: 10.1007/BF02015288. [DOI] [PubMed] [Google Scholar]
- 11.Papa M, Bass A, Adar R, Halperin Z, Schneiderman J, Becker CG, Brautbar H, Mozes E. Autoimmune mechanisms in thromboangiitis obliterans (Buerger’ s disease): the role of tobacco antigen and the major histocompatibility complex. Surgery. 1992;111:527–531. [PubMed] [Google Scholar]
- 12.Shionoya S. Diagnostic criteria of Buerger’ s disease. Int J Cardiol. 1998;66(Suppl 1):S243–245. doi: 10.1016/s0167-5273(98)00175-2. [DOI] [PubMed] [Google Scholar]
- 13.Lee KB, Kim DI. Clinical application of stem cells for therapeutic angiogenesis in patients with peripheral arterial disease. Int J Stem Cells. 2009;2:11–17. doi: 10.15283/ijsc.2009.2.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lenk K, Adams V, Lurz P, Erbs S, Linke A, Gielen S, Schmidt A, Scheinert D, Biamino G, Emmrich F, Schuler G, Hambrecht R. Therapeutical potential of blood-derived progenitor cells in patients with peripheral arterial occlusive disease and critical limb ischaemia. Eur Heart J. 2005;26:1903–1909. doi: 10.1093/eurheartj/ehi285. [DOI] [PubMed] [Google Scholar]
- 15.Matoba S, Tatsumi T, Murohara T, Imaizumi T, Katsuda Y, Ito M, Saito Y, Uemura S, Suzuki H, Fukumoto S, Yamamoto Y, Onodera R, Teramukai S, Fukushima M, Matsubara H. TACT Follow-up Study Investigators. Longterm clinical outcome after intramuscular implantation of bone marrow mononuclear cells (Therapeutic Angiogenesis by Cell Transplantation [TACT] trial) in patients with chronic limb ischemia. Am Heart J. 2008;156:1010–1018. doi: 10.1016/j.ahj.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 16.Cooper LT, Tse TS, Mikhail MA, McBane RD. Stanson AW, Ballman KV. Long-term survival and amputation risk in thromboangiitis obliterans (Buerger’ s disease). J Am Coll Cardiol. 2004;44:2410–2411. doi: 10.1016/j.jacc.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 17.Börner C, Heidrich H. Long-term follow-up of thromboangiitis obliterans. Vasa. 1998;27:80–86. [PubMed] [Google Scholar]
- 18.Olin JW, Young JR, Graor RA, Ruschhaupt WF, Bartholomew JR. The changing clinical spectrum of thromboangiitis obliterans (Buerger’ s disease). Circulation. 1990;82(5 Suppl):IV3–8. [PubMed] [Google Scholar]
- 19.Lau H, Cheng SW. Buerger’ s disease in Hong Kong: a review of 89 cases. Aust N Z J Surg. 1997;67:264–269. doi: 10.1111/j.1445-2197.1997.tb01960.x. [DOI] [PubMed] [Google Scholar]
- 20.Mills JL. Buerger’ s disease in the 21st century: diagnosis, clinical features, and therapy. Semin Vasc Surg. 2003;16:179–189. doi: 10.1016/s0895-7967(03)00023-1. [DOI] [PubMed] [Google Scholar]
- 21.Corelli F. Buerger’ s disease: cigarette smoker disease may always be cured by medical therapy alone. Uselessness of operative treatment. J Cardiovasc Surg (Torino) 1973;14:28–36. [PubMed] [Google Scholar]
- 22.Hooten WM, Bruns HK, Hays JT. Inpatient treatment of severe nicotine dependence in a patient with thromboangiitis obliterans (Buerger’ s disease). Mayo Clin Proc. 1998;73:529–532. doi: 10.4065/73.6.529. [DOI] [PubMed] [Google Scholar]
- 23.Takeshita S, Isshiki T, Mori H, Tanaka E, Eto K, Miyazawa Y, Tanaka A, Shinozaki Y, Hyodo K, Ando M, Kubota M, Tanioka K, Umetani K, Ochiai M, Sato T, Miyashita H. Use of synchrotron radiation microangiography to assess development of small collateral arteries in a rat model of hindlimb ischemia. Circulation. 1997;95:805–808. doi: 10.1161/01.cir.95.4.805. [DOI] [PubMed] [Google Scholar]
- 24.Miyamoto K, Nishigami K, Nagaya N, Akutsu K, Chiku M, Kamei M, Soma T, Miyata S, Higashi M, Tanaka R, Nakatani T, Nonogi H, Takeshita S. Unblinded pilot study of autologous transplantation of bone marrow mononuclear cells in patients with thromboangiitis obliterans. Circulation. 2006;114:2679–2684. doi: 10.1161/CIRCULATIONAHA.106.644203. [DOI] [PubMed] [Google Scholar]
- 25.Kajiguchi M, Kondo T, Izawa H, Kobayashi M, Yamamoto K, Shintani S, Numaguchi Y, Naoe T, Takamatsu J, Komori K, Murohara T. Safety and efficacy of autologous progenitor cell transplantation for therapeutic angiogenesis in patients with critical limb ischemia. Circ J. 2007;71:196–201. doi: 10.1253/circj.71.196. [DOI] [PubMed] [Google Scholar]
- 26.Assmus B, Schächinger V, Teupe C, Britten M, Lehmann R, Döbert N, Grünwald F, Aicher A, Urbich C, Martin H, Hoelzer D, Dimmeler S, Zeiher AM. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI). Circulation. 2002;106:3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 27.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]