Abstract
The capacity of pluripotency and self-renewal of human embryonic stem (hES) cells may provide unlimited cell source for cell replacement therapy. In this manuscript we summarize hES differentiation protocols coculture with PA6 or MS5 stromal cells. After 7∼ 9 days of differentiation, neural rosettes were robustly appeared followed by coculture with sonic hedgehog over-expressing stromal cells efficiently generated dopaminergic neural precursor cells. Using this protocol, the majority of differentiated hES cells contained nestin positive cells (>95%) and after final differentiation a high percentage of TuJ1 positive neurons was tyrosine hydroxylase positive (>40%).
Keywords: Human embryonic stem cells, Dopamine neuron, Stromal cells
Introduction
Directed differentiation to a specific cell types from human embryonic stem (hES) cells represents the initial step for using these cells in developmental biology research as well as cell replacement therapies. Generation of dopamine (DA) neurons from hES cells is particular relevance as a renewable cell source for the treatment of Parkinson’ s disease. DA neuronal differentiation of hES cells is induced by stromal cell-derived inducing activity, has recently been demonstrated to be applicable for DA differentiation of hES cells (1-4). We describe a co-culture method in which hES cells sequentially and homogeneously differentiate into midbrainergic DA neuronal cells.
Materials and Methods
Culture medium and cell freezing
ITS+ AA medium: DMEM/F12 containing 3 mM D(+) glucose, 2 mM L-glutamine, 5 mg/L insulin, 50 mg/L transferrin, 30 nM sodium selenite, 28.5 mM sodium bicarbonate, 100 U penicillin/100μ g/ml streptomycin and 0.2 mM ascorbic acid.
Stock reagents: 10μ g/ ml basic fibroblast growth factor (× 500 stock), 800μ g/ ml polybrene (hexadimethrine bromide, × 100 stock), 1 mg/ ml blasticidin (× 100 stock), 250 μg/ ml poly-L-lysine (PLL, × 10 stock), 1 mg/ml laminin (× 100 stock), 15 mg/ ml poly-L-ornithine (PLO, × 1,000 stock), and 1 mg/ ml fibronectin (FN, × 1,000 stock).
Culture of stromal cells (PA6 or MS5)
Medium: α-MEM containing 20 mM sodium bicarbonate, 100 U penicillin/ 100μ g/ml streptomycin and 10% fetal bovine serum (stromal cell medium). Subculture when these stromal cells reached 80∼ 90% of confluence. For DA differentiation, plate the γ -irradiated (6,000 rad) stromal cells at 2× 104 cells/ cm2 on gelatin (0.1% )-coated plates.
Generation of sonic hedgehog (Shh) expressing retrovirus
Human sonic hedgehog N-terminal region was amplified with primers 5’ -CATATGCTGCTGCTGGCGAGAT- 3’ and 5’ -GTCGACTCAGCCTCCCGATTTGG-3’. The Shh PCR product was cloned into the pIRES-BsdEGFP-CL retroviral vector, which simultaneously expresses the antibiotic blasticidin and the EGFP fusion protein (ShhbeCB). The Shh-beCB plasmid was introduced into the 293gpg retrovirus packaging cell line (5) by transient transfection with Lipofectamine (Invitrogen). After 72 h, the supernatants were harvested.
Establishment of Shh over-expressing stromal cell line
The Shh-beCB retrovirus was infected to stromal cells with polybrene (8μ g/ ml) for overnight. Two days later, transduced stromal cells (Shh-PA6 or Shh-MS5) were selected by growing in the presence of 10μ g/ ml blasticidin (Invitrogen) for 1 week.
Dopaminergic differentiation from human embryonic stem cells
This protocol described here is based on Park et al. (2), with some modifications.
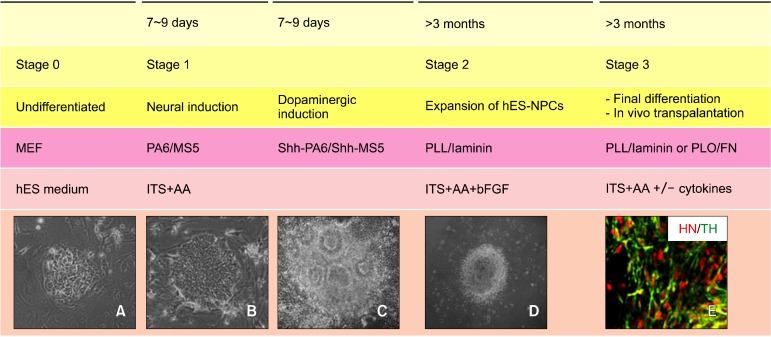
Stage 0: propagation of undifferentiated hES cells, Stage I: neural induction of hES cells on the stromal cells, Stage II: selection and expansion of neural precursor cells on
PLL/ laminin-coated plates, Stage III: terminal differentiation into DA neuronal cells (Fig. 1). Specific methods for the maintenance of each hES cell line should be followed according to the protocols provided by the cell line establishers.
Fig. 1. General scheme of dopaminergic differentiation from hES cells. (A) Undifferentiated hES cell colony. (B) hES colony on the γ- irradiated stromal cells. (C) Neural rossettes containing colony. (D) Neural precursor colony on PLL/laminin-coated dish. (E) TH positive cells colabelled with HN. ITS, insulin-transferrin-selenite medium; AA, ascorbic acid; Shh, sonic hedgehog; PLL, poly-L-lysine; PLO, poly-L-ornithine; FN, fibronectin; HN, human nuclei antigen; TH, tyrosine hydroxylase.
Stage I: neural induction of hES cells on the stromal cells
Prepare the γ -irradiated stromal cells at 2× 104 cells/cm2 on gelatin (0.1% )-coated dishes 1 day prior to starting the neural induction of hES cells with stromal cell medium. Wash the stromal cells with DMEM/ F12, and incubate in ITS+ AA medium. Split the hES cells into small pieces (50∼ 500 cells/cluster) using collagenase treatment and transfer hES colonies onto the stromal cell layer. Change the ITS AA medium every other day until > 80% of the cell colonies achieve a primitive neuroepithelial cell morphology (Fig. 1)B, such as neural rosettes (Fig. 1)C. Repeat the procedure of subculturing onto freshly prepared γ -irradiated Shh over-expressing stromal cells (shh-PA6/ Shh-MS5).
Stage II: selection and expansion of neural precursor cells on PLL/ laminin-coated plates
Plate the differentiated hES colonies on PLL/ laminin- coated dishes in ITS+ AA medium supplemented with 20 ng/ ml bFGF. Subculture onto freshly prepared PLL/ laminin-coated dishes every 7∼ 9 days two to four times. ITS+ AA+ bFGF medium should be changed every alternative day, and bFGF should be added every day. The differentiated neural clusters can be disrupted into single cells using Accutase treatment.
Stage III: terminal differentiation into DA neuronal cells
Inoculate the dissociated cells at 100,000 cells/ cm2 on PLL/Laminin-coated dishes (for maintenance and further propagation of the hES-neural precursor cells) or PLO/ FN-coated coverslips (for immunocytochemical phenotype determinations and functional analyses) with ITS+ AA+ bFGF medium. Terminal differentiation of hES-NP cells can be induced in the absence of bFGF but in the presence of brain-derived neurotrophic factor (BDNF, 20 ng/ ml), glial cell line-derived neurotrophic factor (GDNF, 20 ng/ ml), and dibutyryl cAMP (0.5 mM).
Characterization
Differentiation phenotypes can be assessed by immunological and RT-PCR analyses for markers specific for neurons (tubulin β -III, TuJ1; microtubule associated protein 2, MAP2), DA neurons (tyrosine hydroxylase, TH), astrocytes (glial fibrillary acidic protein; GFAP), and oligodendrocytes (CNPase), for example. In optimal culture conditions, up to 90% of cells are positive for the neuronal marker TuJ1, and 20∼ 80% of the TuJ1+ cells express the DA neuron marker tyrosine hydroxylase after 10∼ 15 days of differentiation (6).
Notes
Efficiency in the derivation of neural precursor cells and DA neurons is highly variable among hES cell lines. DA neurons have been efficiently derived from hES cell lines HSF6 (2), H1, H9 (1,3) and H7. Freshly prepared collagenase should be used.
Conclusions
The differentiation potential of hES cells assembled into small clusters tends to be greater, but cells are less viable if disrupted into clusters that are too small. Although 7∼ 9 days are adequate for subculture, this period can be varied depending on the state of the cultures, including cell viability of the feeder or hES cells as well as the passaging passaging number of hES cells. The optimal conditions for cluster size and cell density, as well as the appropriate interval for each cell passage, should be determined by each experimenter. Homogeneous nestin, a specific marker for neural precursor cells, positive neuroepithelial cells will be obtained after several subculturing.
Acknowledgments
This research was supported by a grant (SC-3140) from Stem Cell Research Center of the 21st Century Frontier Research Program, and KRF-2011-0008952 funded by the Ministry of Education, Science and Technology, Republic of Korea.
Potential conflict of interests
The authors have no conflicting financial interest.
References
- 1.Perrier AL, Tabar V, Barberi T, Rubio ME, Bruses J, Topf N, Harrison NL, Studer L. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci U S A. 2004;101:12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park CH, Minn YK, Lee JY, Choi DH, Chang MY, Shim JW, Ko JY, Koh HC, Kang MJ, Kang JS, Rhie DJ, Lee YS, Son H, Moon SY, Kim KS, Lee SH. In vitro and in vivo analyses of human embryonic stem cell-derived dopamine neurons. J Neurochem. 2005;92:1265–1276. doi: 10.1111/j.1471-4159.2004.03006.x. [DOI] [PubMed] [Google Scholar]
- 3.Ko JY, Park CH, Koh HC, Cho YH, Kyhm JH, Kim YS, Lee I, Lee YS, Lee SH. Human embryonic stem cell-derived neural precursors as a continuous, stable, and on-demand source for human dopamine neurons. J Neurochem. 2007;103:1417–1429. doi: 10.1111/j.1471-4159.2007.04898.x. [DOI] [PubMed] [Google Scholar]
- 4.Vazin T, Chen J, Lee CT, Amable R, Freed WJ. Assessment of stromal-derived inducing activity in the generation of dopaminergic neurons from human embryonic stem cells. Stem Cells. 2008;26:1517–1525. doi: 10.1634/stemcells.2008-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ory DS, Neugeboren BA, Mulligan RC. A stable human- derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc Natl Acad Sci U S A. 1996;93:11400–11406. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho MS, Lee YE, Kim JY, Chung S, Cho YH, Kim DS, Kang SM, Lee H, Kim JH, Leem JW, Oh SK, Choi YM, Hwang DY, Chang JW, Kim DW. Highly efficient and large-scale generation of functional dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci U S A. 2008;105:3392–3397. doi: 10.1073/pnas.0712359105. [DOI] [PMC free article] [PubMed] [Google Scholar]