Abstract
Background and Objectives:
AD-MSCs (adipose-derived mesenchymal stem cells), as an easy obtainable population of multipotent cells, have been successfully applied in many diseases in animals. Having very similar properties and morphology to these collected from bone marrow, are very attractive object for regenerative medicine.
Conclusions:
Ovine subcutaneous fat from many regions of the body is rich source of stem cells, which could be used in locomotive system disorders experimental therapy on sheep models.
Keywords: AD-MSC, Ovis, Immunophenotype, Morphometry, SEM
Introduction
The beginning of 21th century brought the discovery of multilineage potential cells contained in adipose tissue (1). The number of researchers being exploring mesenchymal stem cells’ (MSC) capabilities is still increasing. It is almost obvious that this type of tissue has great regenerate potential (2, 3). Substantial part of scientists has successfully proved, and even improved the beneficial influence of stem cells applications in orthopedics diseases, mainly in animals. Canine and equine cases are well known. In some countries, this form of therapy is already commercially available. However, targets list is still being extended for successive species, in order to find best animal models of diseases’ treatment (4). Sheep were being used as a suitable model for locomotive disorders treatment in large animals. At present researchers are mainly focused on taking advantage of MSCs isolated from bone marrow (BM) combined with various forms of implants (5). It is unclear if the fat tissue, as one of the stem cell source, also could be exploited for these kinds of purposes (6, 7). In this study in order to better characterize the future regenerative potential and possible applications of ovine MSCs in regenerative medicine and implantology, ovine white adipose tissue was analyzed. Samples were collected from four different regions of the body to compare quantity, morphology, behavior and phenotype of cells with regards to regenerative purposes.
Materials and Methods
Isolation and culture process
Two male sheep (blackbelly barbados/somalian sheep hybrid x local wild sheep) were euthanatized due to standard slaughter process (8). Animals were fed only with hay with addition of oats. Immediately after death, subcutaneous fat tissue samples (5 grams) were collected into tubes with sterile Hank’s salt/antibiotic solution (Sigma) and transported to laboratory. Samples were taken from four different areas: back, perineum, sternum and tail base. Part of the material was fixed with formalin and prepared for histological examination, and part was washed few times in solution mentioned above and placed in digestion buffer, composed of 0,2% collagenase/0,25% trypsin dissolved in HBSS (Sigma). Digestion process proceeded in humidified incubator, with 5%CO2, 37℃, for about forty minutes. Tubes with samples were shaked every five minutes during digestion. Next, tubes were centrifuged at 1,200 g for 10 minutes. After that, remaining undigested tissues were transferred to new buffer and digested again. Supernatants were discarded, and nucleated cells pellets, with some visible red blood cells (RBCs), were dissolved in DMEM/F12:Ham mixture with 10% FBS and and 1% antibiotic/antimycotic solutions (Sigma) and plated, every region separately, in T-25 culture flasks. Digestions were made three times. Culture vessels were properly signed and placed in 5%CO2, 37℃, 100% humidity conditions for culture. After cells attached to surface, about 24 hours after, they were washed with HBSS with 2%FBS to remove RBCs and covered with fresh nutrient. Medium was changed two times a week, cultures were observed every day by inverted contrast-phase microscope. After adherent cells reached about 90% confluency, they were detached with EDTA/trypsin solution (Sigma), counted in Thoma counting chamber and plated at 5×104 cells/cm2 in six well plate, with high glucose DMEM/10% FBS/1% antibiotic. Cells were passaged regularly before achieving full confluency. 3×106 cells from every region and donor were suspended in cold serum free freezing medium, frozen and stored in liquid nitrogen, using MrFrosty freezing box (Nalgene) placed in standard -20o freezer. Week after that, some part of frozen cells were thawed, counted for viability and plated in six-well plate to check resistance for freezing assay. Cultured cells were maintained for six passages, and than, all of them were frozen and stored in liquid nitrogen for future researches.
Morphological analysis
To examine cells’ appearance and measurements, morphometrical analysis by means of light microscopy were conducted. Some part of cells were also detached and plated on sterile glass coverslips, with scanning electron microscope destination. Immediately after adhesion (1∼2 hours after plating) and one day after, they were washed three times with phosphate buffer (pH 7.4) and fixed with 2,5% glutaraldehyde in phosphate buffer (pH 7.4) with DMEM at 1:1 ratio, for 30 minutes in 37℃. Afterwards, coverslips were washed three times again and placed in osmium tetroxide for 2 hours. Next, after triple washing in phosphate buffer (pH 7.4), cells were dried on air, sputtered with gold and observed in SEM. Additionally, some part of resected tissues were fixed in formalin, included in paraffin and prepared for histological analysis by standard H&E staining protocol. The measurements of the following parameters: cells’ length, cells’ width at the nucleus level, distance between cells on culture surface, nucleus diameter, rounded cells’ (detached) diameter were analysed by means of light inverted microscope. Width and length of microprocesses were measured at SEM obtained pictures, while lipid droplets diameter of mature adipocytes were measured with light microscope. Five randomly set fields with cells layer were photographed to measurement assay. Different magnifications were chosen, depending on analyzed structure (50× for distance between cells, 100×, 200× and 400× for remaining measurements). Obtained values were averaged and tested statistically with student T-test for comparison.
Molecular phenotyping
Due to ensuring that cultured cells belong to mesenchymal stem cells population, immunofluorescence stainings were performed. Before applying antibodies (human MSC panel, Abcam), human and ovine antigens’ aminoacid sequences were compared to check similarity (NCBI database, Bioedit software) between them. Cells from passage 2 were plated on 4-well culture coverslips and cultured 1∼2 days to about 70% confluency. After that, they were washed three times with PBS/2%FBS solution, fixed with ice cold 4% paraformaldehyde for 10 minutes, washed three times again in PBS/2%FBS, and incubated with primary antibodies (RbIgG antiCD29, MsIgG antiCD44, RbIgG antiCD45, MsIgG antiCD90 and MsIgG antiCD105) for 1 hour in humidified atmosphere in darkness. Next, coverslips were rinsed three times in washing solution and incubated with secondary, fluorochrome conjugated antibodies for 1 hour (antiRbIgG, antiMsIgG). Finally, culture wells were gently washed three times in PBS/2%FBS and filled with HBSS to examination. Fluorescence was examined by means of inverted fluorescent microscope (Zeiss) with camera included.
Results
Culture’s course and morphology
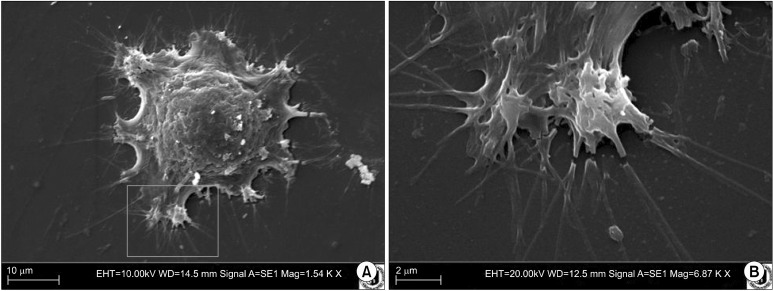
Number of cells obtained by digestion was relatively high, comparing to number of cells from equine fat, isolated with same methods by our team earlier. One day after isolation, cells had rounded shape, with excentrically located nucleus and low nuclear-cytoplasmic ratio. In majority of them, prominent lipid droplets were seen. Next day, cells were more fibroblast-like, there were more abundant and at least two times bigger than at the day before. Washing with Hanks/FBS solution almost completely cleared the culture environment from RBCs. On sixth day in culture, cells were spindle shaped, growing tightly by each other, and almost reaching full confluency. In this stage they were passaged. The time of adhering was about one hour after seeding. The number of prominent nucleoli in majority of cells amounts to five (Fig. 1). Cells from different regions and individuals acted practically identically, with the same population doubling times and the
Fig. 1. (A) Cell fixed during adhesion to surface. Ovine perineum region, scanning electron microscope, mag. 1,540×. (B) Close-up of region marked on previous figure, mag. 6,870×.
same morphology. Thawed cells revealed almost 100% viability and the same morphology and behavior than unfreezed ones.
Morphometry
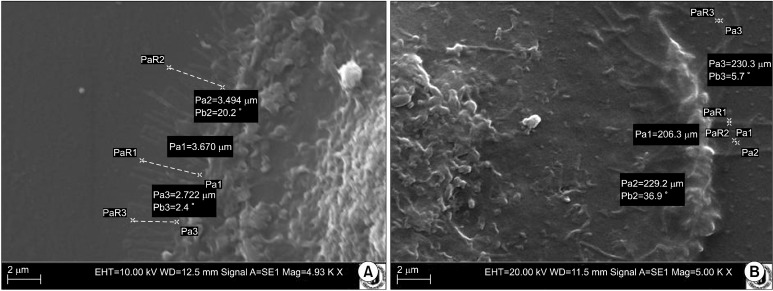
Cells from different individuals and areas were of similar sizes (Table 1, Fig. 2, 3). Although measured parameters averages values were different in particular locations, those differences were not statistically significant. Only lipid droplets diameter in adipocytes from sternum region was smaller than the in others areas, what was confirmed by T-test analysis (p<0,01).
Table 1.
Average results of morphometrical analysis
| Back | Perineum | Sternum | Tail base | |
|---|---|---|---|---|
|
| ||||
| Average length (μm) (LIM) | 125,71 | 130,68 | 126,52 | 109,34 |
| Average width on nucleus level (μm) (LIM) | 50,35 | 52,08 | 57,67 | 48,56 |
| Average nuclei diameter (μm) (LIM) | 23,72 | 21,42 | 21,9 | 22,85 |
| Average distance between cells in culture (μm) (LIM) | 61,31 | 67,29 | 61,61 | 63,05 |
| Average diameter of rounded, suspended cells (μm) (LIM) | 31,02 | 30,77 | 31,78 | 31,16 |
| Average length of micro processes (μm) (SEM) | 3,61 | 3,43 | 2,77 | 4,22 |
| Average width of micro processes (μm) (SEM) | 0,21 | 0,17 | 0,2 | 0,23 |
| Average diameter of lipid droplets in adipocytes (μm) (LM) | 72,12 | 73,54 | 66,07 | 71,70 |
Fig. 2. (A) Length of micro processes sprouted by cell during adhesion. Ovine perineum region, scanning electron microscope, mag. 4,930×. (B) Width of micro processes sprouted during adhesion. Ovine sternum region, scanning electron microscope, mag. 5,000×.
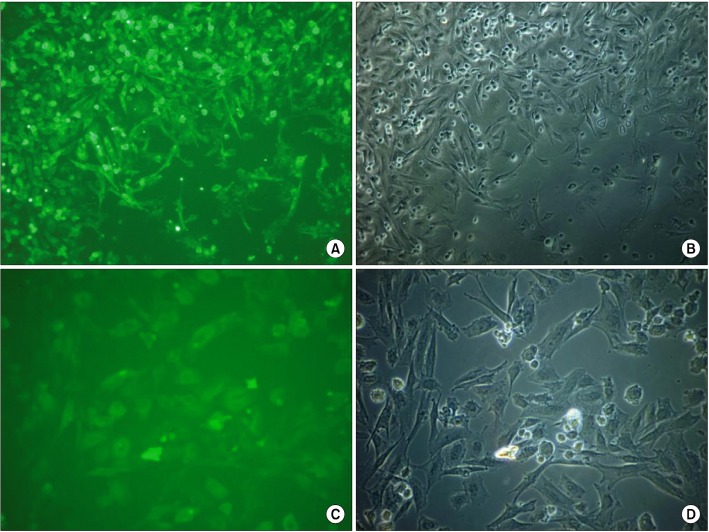
Fig. 3. (A) CD44 antigen on ovine MSCs from tail-base region. Phase-contrast microscope, mag. 100×. (B) Picture of same region as on the previous figure, but in phase-contrast microscope; mag. 100×. (C) CD105 on ovine MSCs from sternum region in fluorescence, mag. 200×. (D) Picture of same region as on the previous figure in phase contrast microscope, mag. 200×.
Immunofluorescence
Comparison of protein sequences from human and sheep revealed over 94% similarity between CD29 (integrin beta- 1) proteins and about 78% similarity between CD44 proteins (Table 2). Unfortunately, there were no ovine CD90 and CD105 sequences available in the database, so comparison of these proteins hasn’ t been done yet. Any-
Table 2.
Results of CD29 and CD44 antigens’ sequences from two hosts comparison; NCBI, BioEdit
| Sequence 1: | gi|166157504|ref|NP_001107242.1| integrin beta-1 precursor (Ovis aries) |
| Sequence 2: | gi|18042960|gb|AAH20057.1| Integrin, beta 1 (fibronectin receptor, beta polypeptide, antigen CD29 includes MDF2, MSK12) (Homo sapiens) |
| Identities: | 0,9473684 |
| Similarities: | 0,9473684 |
| Sequence 1: | gi|169655748|gb|ACA62735.1| CD44 (Ovis aries) |
| Sequence 2: | gi|158631979|gb|ABW75083.1| CD44 (Homo sapiens) |
| Identities: | 0,7804878 |
| Similarities: | 0,7804878 |
way, cells from every region and both individuals, examined under inverted fluorescent microscope, showed positive reactions with all msc-markers antibodies. Expression of CD90 was less pronounce, but still visible. No reaction with CD45 antibody was noticed. In sternum region, CD105 antigen-antibody reaction was the weakest, basing on subjective evaluation.
Discussion
At present, the knowledge concerning morphology of AD-MSC is limited. For this reason in this study, ovine stem cells were morphologically analysed in detailed manner. Stem cells isolated from fat tissue, growed in culture could be characterized by appearance and deposition pattern typical for mesenchymal tissue. Comparing to canine and equine adipose-derived stem cells, ovine had more numerous nucleoli and were relatively bigger. In the culture course, they adhered faster than other species cultured in our facility (horse and dog) (9). Appearance of MSCs from different regions of body confirmed their morphological similarity. Adipose-derived mesenchymal stem cells applications have become the one of the best therapeutic factor in locomotive disorders. Bioengineers working on biodegradable implants and cell biologists culturing stem cells have cooperated on field of regenerative medicine, and achieved many successes. Many combinations of MSC-implant seem to be effective. Although treatment with these methods is well checked in dogs and horses, there are still other species in which MSCs could be used. Some of researches have already been done in case of sheep (10), but only with bone marrow - derived MSCs. According to mentioned paper, ovine stem cells, isolated from fat tissue, had similar morphology and behavior to cells from BM, so they probably could be used in biomaterials testing and regeneration of cartilage. Their immunophenotype checked by immunofluorescent staining, agreed with International Society of Cellular Therapy criteria (11). CD29 (integrin 1-beta) is a particle that participate in cell adhesion. CD44 is a glycoprotein involved in intercellular connections, migrations and also adhesion (12). CD90 is a protein that function isn’t completely explored yet, probably it takes a part in cell-ECM interactions (13). Staining with antibodies against it didn’ t give effects equal with remaining markers. It could be caused by insufficient similarity between human and ovine CD90 aminoacids sequence. Endoglin (CD105), as a part of TGF beta receptor complex, plays a crucial role in differentiation and in creating the structure of growing tissue (14). Weaker expression in sternum-derived cells could be the clue that MSCs from this region have less differentiation potential, so isolation from this body fragment should be avoided. CD45, as a marker for hematopoietic cells, should not be present on MSCs, as it is expressed by other cell lineage (15). To be sure, it should be tested if cells from every site of the body posses same differentiation abilities, and it will be done in further researches. Anyway, this work confirms AD-MSC existence and similarity to BM-MSC, which enables possibilities of therapeutic applications.
Acknowledgments
We would like to thank for supporting us with materials to prof. Piotr Nowakowski from Institute of Animal Husbandry, of our University.
Potential conflict of interest
The authors have no conflicting financial interest.
References
- 1.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 2.Caterson EJ, Nesti LJ, Albert T, Danielson K, Tuan R. Application of mesenchymal stem cells in the regeneration of musculoskeletal tissues. Med Gen Med. 2001;E1 [PubMed] [Google Scholar]
- 3.Huang NF, Li S. Mesenchymal stem cells for vascular regeneration. Regen Med. 2008;3:877–892. doi: 10.2217/17460751.3.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steindler DA. Stem cells, regenerative medicine, and animal models of disease. ILAR J. 2007;48:323–338. doi: 10.1093/ilar.48.4.323. [DOI] [PubMed] [Google Scholar]
- 5.Fadel L. Morphologic characterization of mesenchymal stem cells from umbilical cord blood and adipose tissue collected through intraabdominal and uterine in sheep. Thesis, Faculdade de Medicina Veterinaria e Zootecnia. Univesidade de Sao Paulo; 2009. [Google Scholar]
- 6.Martínez-Lorenzo MJ, Royo-Cañas M, Alegre-Aguarón E, Desportes P, Castiella T, García-Alvarez F, Larrad L. Phenotype and chondrogenic differentiation of mesenchymal cells from adipose tissue of different species. J Orthop Res. 2009;27:1499–1507. doi: 10.1002/jor.20898. [DOI] [PubMed] [Google Scholar]
- 7.Niemeyer P, Fechner K, Milz S, Richter W, Suedkamp NP, Mehlhorn AT, Pearce P. Comparison of mesenchymal stem cells from bone marrow and adipose tissue for bone regeneration in a critical size defect of the sheep tibia and the influence of platelet-rich plasma. Biomaterials. 2010;31:3527–3579. doi: 10.1016/j.biomaterials.2010.01.085. [DOI] [PubMed] [Google Scholar]
- 8.Martyniuk E, Rzepecki R. Sheep husbandry in Poland - an outline. Cahiers Options Mediterraneennes. 1995;11:121–131. [Google Scholar]
- 9.Grzesiak J, Marycz K, Czogala J, Wrzeszcz K, Nicpoń J. Comparison of behavior, morphology and morphometry of equine and canine adipose derived mesenchymal stem cells in culture. International Journal of Morphology. 2011;29 in press. [Google Scholar]
- 10.Mrugala D, Bony C, Neves N, Caillot L, Fabre S, Moukoko D, Jorgensen C, Noël D. Phenotypic and functional characterisation of ovine mesenchymal stem cells: application to a cartilage defect model. Ann Rheum Dis. 2008;67:288–295. doi: 10.1136/ard.2007.076620. [DOI] [PubMed] [Google Scholar]
- 11.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 12.Goodison S, Urquidi V, Tarin D. CD44 cell adhesion molecules. Mol Pathol. 1999;52:189–196. doi: 10.1136/mp.52.4.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rege TA, Hagood JS. Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J. 2006;20:1045–1054. doi: 10.1096/fj.05-5460rev. [DOI] [PubMed] [Google Scholar]
- 14.Sanz-Rodriguez F, Guerrero-Esteo M, Botella LM, Banville D, Vary CP, Bernabéu C. Endoglin regulates cytoskeletal organization through binding to ZRP-1, a member of the Lim family of proteins. FASEB J. 2004;279:32858–32868. doi: 10.1074/jbc.M400843200. [DOI] [PubMed] [Google Scholar]
- 15.Tchilian EZ, Beverley PC. Altered CD45 expression and disease. Trends Immunol. 2006;27:146–153. doi: 10.1016/j.it.2006.01.001. [DOI] [PubMed] [Google Scholar]