Abstract
Obstructive sleep apnea syndrome (OSA) is a prevalent disease caused by increased collapsibility of the upper airway. OSA induces oxidative stress, inflammation and endothelial dysfunction, with important clinical consequences such as neurocognitive alterations and cardiovascular diseases. Although it has been shown that bone marrow-derived stem cells play a protective and reparative function in several diseases involving inflammatory processes and endothelial dysfunction, the data currently available on the potential role of adult stem cells in OSA are scarce. The present review presents recent data on the potential role of bone marrow-derived mesenchymal stem cells (MSC) in OSA. The results obtained in animal models that realistically mimic the events characterizing this sleep breathing disorder strongly support the notion that MSC are mobilized in circulating blood and then activated to play an anti-inflammatory role in OSA.
Keywords: Obstructive sleep apnea, Bone marrow mesenchymal stem cells, Migration, Adhesion, Wound healing and inflammation
The obstructive sleep apnea syndrome
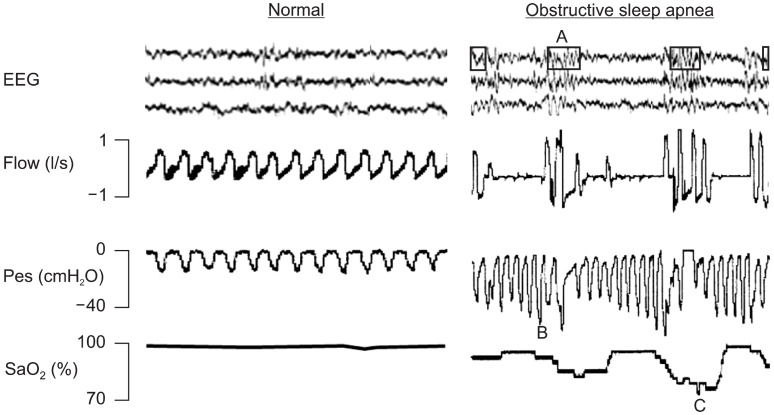
Obstructive sleep apnea (OSA) is a sleep breathing disorder that has been recognized as an important public health problem, as it affects approximately 4% of the adult population (1) and is becoming increasingly prevalent in children (2-7). This sleep disorder is characterized by increased upper airway collapsibility, which triggers recurrent partial or total collapses of the upper airway (8). Fig. 1 shows signals recorded during polysomnography, illustrating the physiological challenges experienced by patients with OSA during sleep. First, as illustrated by the breathing flow signal, the patient with OSA experiences recurrent apneas, each lasting a period of time corresponding to several breathing cycles. This pattern of interrupted breathing contrasts with the regular flow observed in a normal patient. Second, the figure shows that inspiratory efforts during apneas, as measured by esophageal pressure, are considerably greater than those of a healthy subject, because the patient tries to breath against a closed upper airway. The magnitude of these breathing efforts induces strong negative intrathoracic pressure swings, which affect the heart function. Third, the patient with OSA experiences recurrent arousals at the end of each apneic event, as illustrated by the electroencephalographic signals (in contrast with the regular sleep of a normal patient). Finally, the signal of arterial oxygen saturation indicates that, unlike healthy subjects, OSA patients experience severe intermittent hypoxia (Fig. 1).
Fig. 1. Signals of electoencephalography (EEG), breathing flow, esophageal pressure (Pes) and arterial oxygen saturation (SaO2) recorded during sleep in a normal patient (left) and in a patient with obstructive sleep apneas (OSA). A, B and C in the recordings of the OSA (right) patient indicate arousal, increased breathing efforts and hypoxia/reoxygenation events respectively. See text for explanation.
There is well established evidence that OSA has instantaneous, mid- and long-term consequences in severely diseased patients. Recurrent apneas induce sleep disruptions that prevent a patient from achieving normal sleep architecture, with the result that the patient suffers from excessive daytime sleepiness (9-11). This leads to increased workplace and traffic accidents (12, 13). Moreover,the oxidative stress and inflammation induced by inter
mittent hypoxia can result in neurocognitive dysfunction, hypertension, heart failure, cardiac arrhythmias, sudden death, cerebrovascular disease and ischemia (14, 15).
It is known that the release of bone marrow-derived stem cells to the peripheral circulation system is a physiological response that decreases inflammation and repairs damaged tissues in several diseases (16-20). Few data are available, however, on the role played by the different types of adult stem cells in OSA. There is evidence that endothelial progenitor cells contribute to endothelial dysfunction repair in OSA patients (21). Moreover, recent data in a mouse model has shown that very small embryonic-like stem cells are released from the bone marrow into the circulation in response to intermittent hypoxia mimicking OSA (22). In this review we present recent data obtained in animals, which suggest that bone marrow-derived mesenchymal stem cells (MSC) play a role in the physiological response to the challenges characterizing OSA.
Non-invasive animal model of obstructive sleep apnea
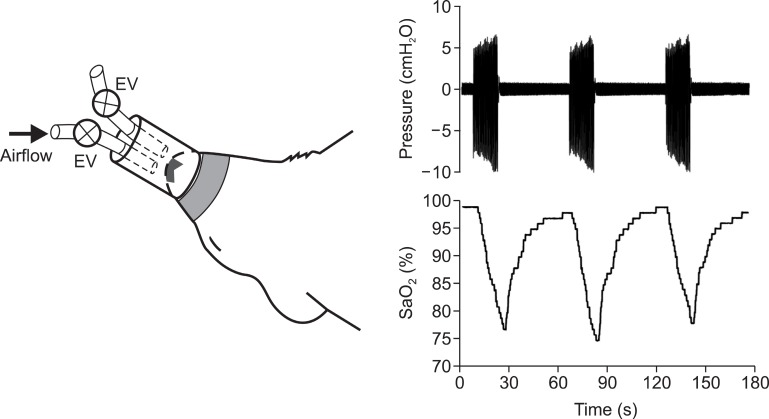
The only data currently available on MSC in OSA have been obtained by means of an animal model (23). This rat model, shown in Fig. 2, was able to induce realistic recurrent airway obstructions by means of a nasal mask specially designed to fit the snout of an anesthetized rat. The mask was connected to two tubes, each with a digitally controlled electrovalve. To allow normal rat breathing, both the electrovalves were kept open and rebreathing was avoided by passing a constant flow of room air through the mask. The two valves were closed to apply airway obstructions. A regular time pattern, consisting of 15 s of apneas followed by 45 s of normal breathing, was used to mimic the recurrent upper airway obstructions characterizing OSA. The application of this experimental setting made it possible to induce arterial oxygen desaturations of a duration and magnitude very close to those observed in patients with severe OSA. Indeed, as shown in Fig. 2, arterial oxygen saturation fluctuated between ≈75% nadir and normal values ≈98% at a rate of 60 events/h. The application of this pattern of recurrent airway obstructions for a continuous period of 5 h realistically simulated the events experienced by an OSA patient over the course of a night.
Fig. 2. (Left) Diagram of nasal mask designed to noninvasively apply recurrent obstructive apneas in rats. The mask was connected to onetube open to the atmosphere and to another tube connected to an airflow source to prevent rebreathing. Two electronically synchronized electrovalves (EV) allowed the application of airway obstruction (bothvalves closed) or spontaneous breathing (both valves open). (Right) Pressure inside the nasal mask and arterial blood oxygen saturation (SaO2) recorded during obstructive apneas. Reproduced from references 23 and 24 with permission.
Recurrent obstructive apneas release MSC into peripheral circulation
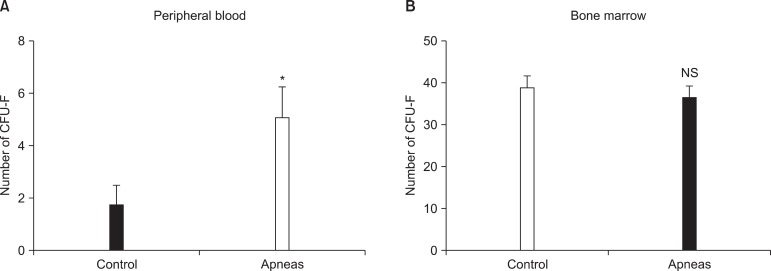
In a first study using the OSA rat model described in Fig. 2, it was found that the non-invasive application of recurrent airway obstructions induced early release of MSC into the circulating blood (23). This result was obtained by comparing the results obtained from two groups of rats, one subjected to the recurrent pattern of obstructive apneas (Fig. 2) and a control group of normally breathing rats. Accordingly, the difference between the two groups was the application of recurrent obstructive apneas. After 5 h of apneas (or control), the animals were sacrificed and MSC from the blood and femur bone marrow were isolated and cultured for 9 days to count the total number of colony-forming unit fibroblasts (CFU-F). As shown in Fig. 3, the results indicated that the number of CFU-F in circulating blood was significantly higher in the rats subjected to recurrent obstructive apneas than in the
Fig. 3. Mesenchymal stem cells in peripheral blood (A) and in bone marrow (B) of the rats subjected to recurrent obstructive apneas and in controls. Number of colony-forming units (CFU-F) of mesenchymal stem cells obtained from 1.25×106 plated cells. Reproduced fromreference 23 with permission. *p<0.05.
controls. Consequently, this study indicated that the application of a pattern of airway obstructions mimicking the breathing patterns of patients with OSA induce an early release of MSC into circulating blood.
Recurrent obstructive apneas increase MSC mobility
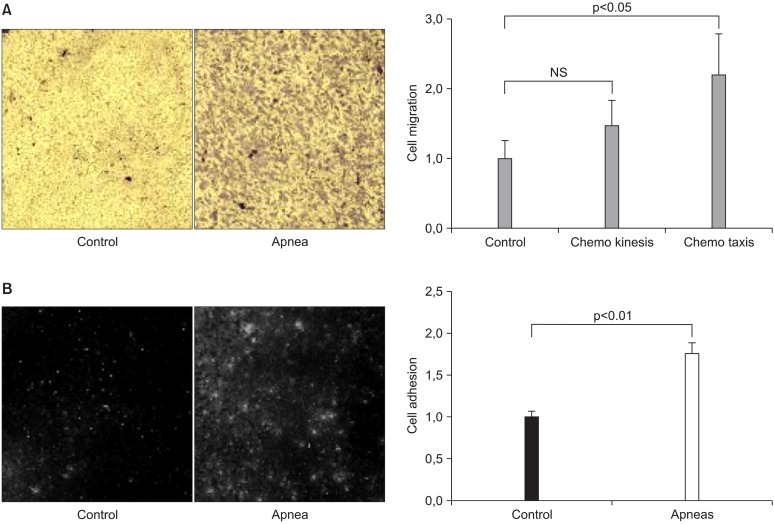
Another recent study using the same animal model of OSA (Fig. 2) have shown that MSC are activated by the application of recurrent airway obstructions similar to those experienced by patients with this sleep breathing disorder (24). The sera of control rats and others subjected to apneas (15 s each, 60 apneas per hour, for 5 hours) were collected and used in three in vitro experiments with rat bone marrow-derived MSC. One of these experiments sought to assess whether the serum of apneic rats (as compared with control serum) increased the chemokinesis and chemotaxis of MSC via a transwell setting. MSC were cultured on the upper side of the transwell membrane. Three transwell measurements were performed by placing the serum of apneic or control rats in the upper and lower compartments of the transwell: 1) control serum in both the upper and lower compartment of the transwell (CS/CS);2) apneic serum in both the upper and lower compartment of the transwell (AS/AS); and 3) control serum (CS) and apneic serum (AS) in the upper and lower transwell compartment,respectively (CS/AS). After 8 h of incubation the transwell membranes were processed to calculate the number of MSC that migrated through the membrane. A normalized chemokinesis index was computed as the number of cells counted in AS/AS transwells divided by the number of cells counted in CS/CS transwells. Similarly, a normalized chemotaxis index was computed by dividing the number of cells counted in CS/AS transwells by the number of cells counted in CS/CS transwells. This study indicated that cells cultured in apneic serum exhibited a tendency to increased chemokinesis and significant chemotaxis (Fig. 4), indicating that application of a pattern of airway obstructions similar to those experienced by patients OSA increases the motility of MSC.
Fig. 4. (A) Examples of a field of view of transwell membrane showing stained mesenchymal stem cells (MSC) that migrated to the lower membrane side of the transwell when the lower compartment contained control serum (CONTROL) and serum from rats subjected to recurrent obstructive apneas (APNEA). Serum of control rats was placed in the lower transwell compartment in both cases. Normalized indices for the migration of MSC (chemotaxis and chemokinesis) induced by the serum of control and apneic rats are represented on the right side of the figure. Data are mean±SEM. NS: non-significant (p<0.05). (B) Examples of fields of view of the fluorescence-labelled MSC adhered to cultured endothelial cells when incubated in control serum (CONTROL) or in serum from rats subjected to recurrent obstructive apneas (APNEA). The normalized indices for the adhesion of MSC to endothelial cells in control rat serum and in serum from rats subjected to recurrent obstructive apneas are represented on the right panel. Data are mean±SEM. Reproduced from reference 24 with permission.
Recurrent obstructive apneas increase adhesion of MSC onto the endothelium
Another interesting finding resulting from the previously described experimental setting was that MSC cultured in apneic serum exhibits increased adhesion onto endothelial cells (24). Bone marrow-derived MSC were fluorescent-labeled and incubated overnight in the serum of rats subjected to obstructive apneas and controls. Subsequently, the MSC were incubated on a monolayer of rat aortic endothelial cells for 6 h and the number of remaining MSC adhered to the endothelial cells were counted. A normalized adhesion index was computed by dividing the number of cells counted in apneic serum and in control serum. As shown in Fig. 4, MSC adhesion to the endo
thelium was greater when these cells were incubated in the serum of rats subjected to recurrent obstructive apneas mimicking OSA.
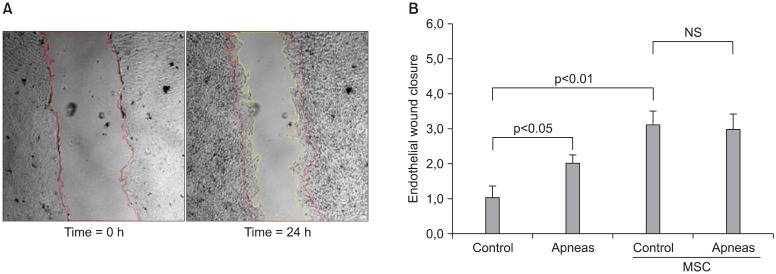
Recurrent obstructive apneas enhance endothelium wound healing
Another interesting result obtained from the rat model of OSA was that MSC enhanced endothelial repair, as indicated by a wound healing assay (24). A confluent monolayer of aortic endothelial cells was scratch-wounded and the endothelial culture medium was replaced by serum from rats subjected to recurrent obstructions and control animals, with or without preconditioning with bone marrow-derived MSC. A multiwell plate was placed on the motorized stage of a microscope equipped with a CCD camera and a microscope incubator, with the cells maintained at 37℃ , 5% CO2 and 100% humidity throughout the experiment. The endothelial wound healing process was assessed by automatically recording images of each well every 10 min, from the scratch-wounded time point up to 24 h of sera incubation. An endothelial wound closure index was computed by comparing the wound areas’ initial and final images, normalized to the mean increase in control serum. As shown in Fig. 5, endothelial wound healing was significantly higher when the injured monolayer was cultured in apneic serum compared with control serum. This figure also shows that preconditioning both control and apneic sera with MSC resulted in an increase in the endothelial wound closure index. These data dem-
Fig. 5. (A) Images from a representative example of an endothelial wound healing test, showing the wound at the beginning of the experiment and after 24 h the culture medium was replaced by apneic rat serum. Red and yellow lines indicate the initial and final wound borders. (B) Normalized index for wound closure when the endothelial cells were cultured in medium consisting of serum of control rats and of rats subjected to recurrent obstructive apneas, with or without preconditioning with mesenchymal stem cells (MSC). Data are mean±SEM.Reproduced from reference 24 with permission.
onstrate demonstrate that the application of recurrent upper airway obstructions with a pattern similar to those experienced by OSA patients enhanced the repair of endothelial wound healing, particularly in the presence of MSC.
Intravenous infusion of MSC reduces inflammation triggered by recurrent obstructive apneas
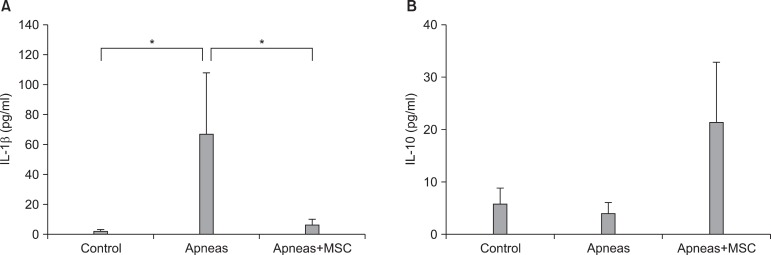
Whereas the above-mentioned works provided data in support of the notion that recurrent obstructive apneas mobilize and activate MSC, the last paper included in this review provides further experimental evidence that MSC contribute to the anti-inflammatory response induced by recurrent apneas (25). Using the same experimental setting shown in Fig. 2, the authors investigated three groups of animals. In one group, the rats were subjected to recurrent obstructive apneas (60 per hour, lasting 15 seconds each) for 5 hours. In another group, the rats were subjected to the same challenge but received an intravenous infusion of bone marrow-derived MSC before the application of apneas. A third group of animals, not subjected to apneas, was used as control. After the 5 h of recurrent upper airway obstructions (or control), a blood sample was collected to assess the concentration of pro- and anti-inflammatory cytokines (IL-1β and IL-10, respectively), by ELISA. As seen in Fig. 6, the blood serum concentration of IL-1β was significantly higher in rats subjected to recurrent apneas (compared with controls), indicating that recurrent apneas triggered an early inflammatory process.
Fig. 6. Concentration of pro-inflammatory (IL-1β ; A) and anti-inflammatory (IL-10; B) cytokines in serum from control rats (CONTROL), from rats subjected to recurrent obstructive apneas (APNEAS), and from rats subjected to apneas and receiving an intravenous injection of mesenchymal stem cells (APNEAS MSC). Data are mean±SEM. Reproduced from reference 25 with permission. *p< 0.05.
Interestingly, in the group of apneic rats subjected to MSC injection, IL-1β was significantly reduced. No significant difference was found between the control group and the group of apneic animals injected with MSC. Although not statistically significant, the apneic rats injected with MSC exhibited an increase in the anti-inflammatory cytokine IL-10. Accordingly, this study strongly suggests that MSC act as an anti-inflammatory agent that counteracts the inflammatory process triggered by recurrent obstructive apneas.
Conclusion
Although there are few data on the role of bone marrow-derived MSC in OSA, the information currently available from a well-controlled animal model indicates that these adult stem cells could play a protective anti-inflammatory role by counterbalancing the main OSA challenges (intermittent hypoxia and increased inspiratory efforts). However, further studies, particularly in chronic animal models (26) and in patients, are required to substantiate this notion. This research could deepen our understanding of OSA and open up potential new treatment approaches based on the protective and reparative role played by adult stem cells.
Potential conflict of interest
The authors have no conflicting financial interest.
References
- 1.Durán J, Esnaola S, Rubio R, Iztueta A. Obstructive sleepapnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am JRespir Crit Care Med. 2001;163:685–689. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 2.Montgomery-Downs HE, O'Brien LM, Holbrook CR, Gozal D. Snoring and sleep-disordered breathing in young children:subjective and objective correlates. Sleep. 2004;27:87–94. doi: 10.1093/sleep/27.1.87. [DOI] [PubMed] [Google Scholar]
- 3.Spruyt K, O'Brien LM, Macmillan Coxon AP, Cluydts R, Verleye G, Ferri R. Multidimensional scaling of pediatric sleep breathing problems and bio-behavioral correlates. Sleep Med. 2006;7:269–280. doi: 10.1016/j.sleep.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 4.Khalyfa A, Serpero LD, Kheirandish-Gozal L, Capdevila OS, Gozal D. TNF-α gene polymorphisms and excessive daytime sleepiness in pediatric obstructive sleep apnea. J Pediatr. 2011;158:77–82. doi: 10.1016/j.jpeds.2010.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim J, Lee S, Bhattacharjee R, Khalyfa A, Kheirandish-Gozal L, Gozal D. Leukocyte telomere length and plasma catestatin and myeloid-related protein 8/14 concentrationsin children with obstructive sleep apnea. Chest. 2010;138:91–99. doi: 10.1378/chest.09-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Brien LM, Ivanenko A, Crabtree VM, Holbrook CR, Bruner JL, Klaus CJ, Gozal D. Sleep disturbances in children with attention deficit hyperactivity disorder. Pediatr Res. 2003;54:237–243. doi: 10.1203/01.PDR.0000072333.11711.9A. [DOI] [PubMed] [Google Scholar]
- 7.Schlaud M, Urschitz MS, Urschitz-Duprat PM, Poets CF. The German study on sleep-disordered breathing in primary school children: epidemiological approach, representa-tiveness of study sample, and preliminary screening results. Paediatr Perinat Epidemiol. 2004;18:431–440. doi: 10.1111/j.1365-3016.2004.00589.x. [DOI] [PubMed] [Google Scholar]
- 8.Farré R, Rotger M, Montserrat JM, Calero G, Navajas D. Collapsible upper airway segment to study the obstructive sleep apnea/hypopnea syndrome in rats. Respir Physiol Neurobiol. 2003;136:199–209. doi: 10.1016/s1569-9048(03)00082-x. [DOI] [PubMed] [Google Scholar]
- 9.Lam JC, Sharma SK, Lam B. Obstructive sleep apnoea: definitions, epidemiology & natural history. Indian J Med Res. 2010;131:165–170. [PubMed] [Google Scholar]
- 10.Ursavaş A, Karadag M, Oral AY, Demirdogen E, Oral HB, Ege E. Association between serum neopterin, obesity and daytime sleepiness in patients with obstructive sleep apnea. Respir Med. 2008;102:1193–1197. doi: 10.1016/j.rmed.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Q, Liu ZH, Luo Q, Zhao ZH, Zhang HL, Wang Y. Effects of continuous positive airway pressure on blood pressure and daytime sleepiness in obstructive sleep apnea patients with coronary heart diseases under optimal medications. Sleep Breath. 2011 doi: 10.1007/s11325-011-0498-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Pagel JF. The burden of obstructive sleep apnea and associated excessive sleepiness. J Fam Pract. 2008;57(8 Suppl):S3–8. [PubMed] [Google Scholar]
- 13.Bîrleanu LA, Rusu G, Mihaescu T. Obstructive sleep apnea(OSA) syndrome and traffic accidents. Rev Med Chir Soc Med Nat Iasi. 2010;114:700–706. [PubMed] [Google Scholar]
- 14.Caples SM, Garcia-Touchard A, Somers VK. Sleep-disordered breathing and cardiovascular risk. Sleep. 2007;30:291–303. doi: 10.1093/sleep/30.3.291. [DOI] [PubMed] [Google Scholar]
- 15.Campana L, Eckert DJ, Patel SR, Malhotra A. Pathophysiology& genetics of obstructive sleep apnoea. Indian J Med Res. 2010;131:176–187. [PMC free article] [PubMed] [Google Scholar]
- 16.Giordano A, Galderisi U, Marino IR. From the laboratorybench to the patient's bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007;211:27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- 17.Xu J, Gonzalez ET, Iyer SS, Mac V, Mora AL, Sutliff RL, Reed A, Brigham KL, Kelly P, Rojas M. Use of senescence-accelerated mouse model in bleomycin-induced lung injury suggests that bone marrow-derived cells can alterthe outcome of lung injury in aged mice. J Gerontol A Biol Sci Med Sci. 2009;64:731–739. doi: 10.1093/gerona/glp040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo J, Lin GS, Bao CY, Hu ZM, Hu MY. Anti-inflammationrole for mesenchymal stem cells transplantation in myocardial infarction. Inflammation. 2007;30:97–104. doi: 10.1007/s10753-007-9025-3. [DOI] [PubMed] [Google Scholar]
- 19.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 20.Tyndall A, Walker UA, Cope A, Dazzi F, De Bari C, Fibbe W, Guiducci S, Jones S, Jorgensen C, Le Blanc K, Luyten F, McGonagle D, Martin I, Bocelli-Tyndall C, Pennesi G, Pistoia V, Pitzalis C, Uccelli A, Wulffraat N, Feldmann M. Immunomodulatory properties of mesenchymal stem cells:a review based on an interdisciplinary meeting held at the Kennedy Institute of Rheumatology Division, London, UK,31 October 2005. Arthritis Res Ther. 2007;9:301. doi: 10.1186/ar2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jelic S, Padeletti M, Kawut SM, Higgins C, Canfield SM, Onat D, Colombo PC, Basner RC, Factor P, LeJemtel TH. Inflammation, oxidative stress, and repair capacity of thevascular endothelium in obstructive sleep apnea. Circulation. 2008;117:2270–2278. doi: 10.1161/CIRCULATIONAHA.107.741512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gharib SA, Dayyat EA, Khalyfa A, Kim J, Clair HB, Kucia M, Gozal D. Intermittent hypoxia mobilizes bone marrow-derived very small embryonic-like stem cells and activates developmental transcriptional programs in mice. Sleep. 2010;33:1439–1446. doi: 10.1093/sleep/33.11.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carreras A, Almendros I, Acerbi I, Montserrat JM, Navajas D, Farré R. Obstructive apneas induce early release of mesenchymal stem cells into circulating blood. Sleep. 2009;32:117–119. [PMC free article] [PubMed] [Google Scholar]
- 24.Carreras A, Rojas M, Tsapikouni T, Montserrat JM, Navajas D, Farré R. Obstructive apneas induce early activation of mesenchymal stem cells and enhancement of endothelial wound healing. Respir Res. 2010;11:91. doi: 10.1186/1465-9921-11-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carreras A, Almendros I, Montserrat JM, Navajas D, Farré R. Mesenchymal stem cells reduce inflammation in a rat model of obstructive sleep apnea. Respir Physiol Neurobiol. 2010;172:210–212. doi: 10.1016/j.resp.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 26.Farré R, Nácher M, Serrano-Mollar A, Gáldiz JB, Alvarez FJ, Navajas D, Montserrat JM. Rat model of chronic recurrent airway obstructions to study the sleep apnea syndrome. Sleep. 2007;30:930–933. doi: 10.1093/sleep/30.7.930. [DOI] [PMC free article] [PubMed] [Google Scholar]