Abstract
Background and Objectives:
The incidence of human autologous transplanted skeletal myoblast (SkM) cell death in ischemic myocardium was higher in the first few days after cell therapy. We proposed that human SkM treated by human stromal cell-derived factor (SDF-1α) protein or tranfected by SDF-1α, precondition them against oxidative or anoxic injury.
Methods and Results:
The purification of human SkM (80∼90%) culture was assessed for desmin and CXCR4 expression using immunostaining and flow cytometry respectively. Cells were transfected to overexpress SDF-1α or treated with rSDF-1α (10∼200 ng/ml, 1∼4 h) were either exposed to anoxia or treated with 100μM H2O2 for different time periods (1∼6 h anoxia) (1∼3 h H2O2). Optimized conditions for transfection of SDF-1α gene into human SkM were achieved, using FuGeneTM6/phSDF-1α(3:2 v/w, 4 h transfection) with 125μ M ZnCl2 (p< 0.001), up to 7 days post-transfection as compared with transfected SkM without ZnCl2 and non-transfected control cells. Transfection efficiency was assessed by immunostaining, ELISA, western blots and PCR. LDH analysis showed significant decrease in release of LDH after exposure to 6 h anoxia or 100μ M H2O2 for 2 h as compared with the normal un-treated or un-transfected SkM (p< 0.001). In western blots assay, SDF-1α over-expressing human SkM or treated with rSDF-1α induced marked expression of total Akt (1.2-fold) and phospho-Akt (2.7-fold), Bcl2 (1.6-fold) and VEGF (5.8-fold) after exposure to 6 h anoxia as compared with human SkM controls.
Conclusions:
The preconditioning of donor transplanted human SkM with SDF-1α increased cell survival and promoted cytoprotective effect against oxidative or anoxic injury that may be an innovative approach for clinical application.
Keywords: Apoptosis, Skeletal myoblast, SDF-1α , Anoxia, H2O2, Cytokine
Introduction
Recently, heart cell therapy for cellular myocardial regeneration and gene therapy for angiogenesis have shown promise in cardiovascular therapeutics (1, 2). Despite encouraging results from experimental animal studies and human clinical trials, progress in both of these approaches has been hampered by some inherent problems. Clinical experience using autologous skeletal myoblasts has revealed difficulty in obtaining, in elderly patients, sufficient numbers of cells and inducing their expansion ex vivo (3, 4).
A high mortality rate of injected myoblast cells 70% to 80% during the first 3 days following transplantation in myocardial infarction (MI), caused by an inflammatory process (5, 6). Cell death immediately before and during injection because of mechanical/technical stress may play a role (7). Since a significant number of transplanted cells may undergo necrotic or apoptotic cell death after transplantation (8), 25% cell survival one day after transplantation, 93% of SkMs were lost in 2 days after transplantation (9), and also, 1% cell graft survival in clinical application (4).
Many problems seem to be unresolved before a clinical application. Some ideas and suggestions regarding how to best improve the results of cellular cardiomyoplasty alone or with simultaneous therapeutic gene transfer were previously assessed in experimental trials (2). Preconditioning of SkM started from pre-injection of the anterior tibialis muscle with bupivacaine or xylazine (pharmacological stressor) to activate muscle satellite cells before muscle biopsy used for cell extraction (10, 11). Another beneficial approach might be culturing the cells in a biodegradable 3-dimensional scaffold (12, 13), or seeding SkMs on highly porous polyurethane membrane; (14, 15), usually referred to as tissue engineering (16). Transplanted cells mixed with fibronectin (17) to promote cell-cell and cell-ECM interactions. Also, pretreatment of an infarcted region of the heart with angiogenic mediators such as VEGF 121 (18), FGF (19), SDF-1α (20) and granulocyte-monocyte colony-stimulating factor (GM-CSF) protein (21) before cell transplantation to avoid the deleterious effects of inflammation on cell survival (8) and also to avoid an “ischemic interval” that may adversely affect cell survival and the consequent functional benefits of cellular cardiomyopathy (19).
Many methods used for increasing the survival of SkM after transplantation such as repeated SkMs transplantation (22) or pharmacologically preconditioning SkMs in vitro by Diazoxide (10) or mesenchymal stem cells by Trimetazidine (23) before transplantation. The survival of grafted cells were improved by irreversible caspase inhibition using AcYVADcmk (24), heat shock treatment (8), activation of the Akt-kinase pathway (25) and anti-lymphocyte function antigen-1 (LFA-1) (6). Gene therapy using phSDF-1α gene that modulate Akt-kinase signal pathway (2) in preconditioning of skeletal myoblasts may be an attractive and better alternative, and might especially be relevant for use in the elderly in whom the availability of SkM transplantation is limited.
The present study was to explore the use of synthetic non-viral cationic vector (FuGeneTM6) with addition of zinc chloride in human skeletal myoblast in primary culture in order to optimise transfection efficiency. We also hypothesize that human SkM treated by rhSDF-1α protein or transient SDF-1α overexpression of human SkM, precondition them against oxidative or anoxic injury.
Materials and Methods
Human Skeletal myoblasts and hSDF-1α plasmid and transfections
Human SkM were purchased from Zen-Bio Inc, USA.Cells were cultured in Dulbecco’ s modified Eagle’ s medium (DMEM, Gibco BRL) and identified by anti-desmin immunostaining prior to use in all experimental studies. Cell viability assessed with a modified MTT reduction test as previously described (26). Briefly, the number of viable cells was evaluated using a modified 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide (MTT) reduction test was used in order to assay mitochondrial function which is an index of cell viability. Results are expressed as the percentage of untreated control values.
We used an expression vector containing hSDF-1αgene (pORF-hSDF-1α) purchased from InvivoGen, USA. Preparation of plasmid was performed per supplier’ s instructions. Briefly, the disk of lyophilized GT100 E. coli bacteria transformed by pORF-hSDF-1α was dissolved in E.coli FastMediaTM agar medium containing ampicillin. The hSDF-1α gene, was cut out by using SgrA I and Nhe I enzymes, followed by Simian virus 40 late polyadenylation (SV40 pAn) and a minimal E.coli origin of replication pMB1-Ori that necessary for the transcription process. The expression vector had ampicillin resistance gene that allowed the selection of bacteria carrying the pORF plasmid. Plasmid DNA-hSDF-1α was purified with Maxprep protocol (Qiagen) as per supplier’ s instructions. The purified plasmid hSDF-1α was stored at −20℃ until used for transfection of SkM.
Human SkM at 80∼90% confluence were transfected with pDNA-hSDF-1α using commercially available cationic vector FuGeneTM6 (Roche). For this vector, transfection condition was optimized at various DNA: vector(v/w) ratios, in presence of 0∼1000μM ZnCl2 (Merck, Germany) at 37℃ or room temperature. Firstly, ZnCl2 cytotoxicity was evaluated. For this purpose, confluent human SkMs were incubated for 2∼4 hours with 0, 30, 60, 125, 250, 500, 750, 1000μM ZnCl2 in DMEM with 10% FCS. Every two days, the conditioned medium was replaced by fresh medium (i.e: DMEM with 10% FCS). The cell number and viability were assessed using the MTT test. Non-toxic concentrations of ZnCl2 were added to the transfection mixture containing FuGeneTM6/phSDF-1α in basal DMEM for 2, 3 and 4 hours at 37℃ (see below). As maximum transfection efficiency with cell viability was achieved using FuGeneTM6/phSDF-1α (v/w) 3:2 ratio in basal DMEM containing 125μM ZnCl2 (for 4 h at 37℃), this transfection conditions were used throughout the study.
Flow cytometry
The human SkMs were scraped in buffer and tested for estimation the receptor density for the human chemokine SDF-1α on cell surfaces by flow cytometry, using biotinylated rhSDF-1α (10 μL of 1 mg/mL/106cells, Fluorokine, R& D system) as per manufacture’ s instruction. Fluorescence was detected by flow cytometry (FACS Calibur, BD).
ELISA for hSDF-1α
The amount of hSDF-1α secreted by the transfected SkM in vitro was assessed by hSDF-1α ELISA kit (R& D systems) per supplier’ s instructions. For in vitro hSDF-1α expression, conditioned medium and cell lysate from transfected and non-transfected SkM were collected at regular time intervals after transfection and used for ELISA. The results were expressed as amount of SDF-1α/mg protein.
PCR for gene expression
Isolation of total RNA (1μg) from the different groups of hSkM, and their subsequent first strand cDNA synthesis were performed using RNeasy mini kit (Qiagen) and Omniscript Reverse Transcriptase kit (Qiagen) respectively as per manufacture’ s instruction. The following Primers sequences were used for PCR; hSDF-1α (270 bp), Forward 5’ -CATGAACGCCAAGGTCGTG-3’ , Reverse 5’ -TCCAGGTACTCCTGAATCC-3’ ; human GAPDH (738 bp), Forward, 5’ -GAGCCACATCGCTCAGACAC-3’ ; Reverse, 5’ -CATGTAGTTGAGGTCAATGAAGG-3’ .
Immunohistochemical studies
Immunostaining was performed as earlier described (2). The primary antibodies included monoclonal anti-desmin (1:50, Sigma), mouse anti-hSDF-1α (10μg/ml, R& D systems) and anti-CXCR4 (10μg/ml, Axxora). The detection system was based on immunoperoxidase secondary detection system (Chemicon) followed by nuclear counterstaining using fluorescence-labeled secondary antibodies with Alexa Fluor 594 goat anti-mouse IgG (H+ L) (2 mg/ml) highly cross absorbed (Molecular Probe, USA).
Preconditioning of human SkM with hSDF-1α before anoxia and oxidative stress
The human SkM (3.75×104 cells/cm2) were grown on Petri dishes, either were incubated with different concentration of rhSDF-1α (10∼200 ng/ml, R& D system) for 1∼4 hours or transfected with phSDF-1α as above.Human SkM either transfected or treated with rhSDF-1α was exposed to 1∼6 hours anoxic injury in Hypoxic/Anoxic workstations Device (Don Whitley Scientific Inc). Also, these cells were exposed to different concentration of H2O2 (up to 100μM, Sigma) for 1∼3 hours. All experiments were performed in serum- and glucose-free DMEM. At the end of the experiment, hSkM and their supernatants were removed for molecular studies to assess cellular injury from oxidative stress and cytoprotective effects of preconditioning.
Lactate dehydrogenase assay
Lactate dehydrogenase (LDH) is a soluble cytosolic enzyme that is released into the culture medium following loss of membrane integrity resulting from either apoptosis or necrosis. LDH activity, therefore, can be used as an indicator of cell membrane integrity and serves as a general means to assess cytotoxicity resulting from chemical compounds or environmental toxic factors. LDH leakage was measured in the cell-conditioned medium sample using an LDH Assay kit (Diagnostic Chemicals Ltd) or LDH Cytotoxicity Assay Kit (Promega) according to the instructions of the manufacturer.
Western immunoblotting
Protein samples were fractionated by 12% SDS-polyacrylamide gel electrophoresis (ISC BioExpress) and electro-transferred onto a Nitrocellulose membrane. The membrane was blocked for 1 hour with 1 TBS Blocking buffer (Cell Signaling Technology) and 5% nonfat dry milk, followed by incubation with gentle shaking at 4oC with anti-hSDF-1α (1:200, R& D System), Akt and phospho-Akt (1:1000, Cell Signaling Technology), Bcl2(1:200, Santa Cruz Biotechnology) and rabbit polyclonal VEGF-147 (1:200 Santa Cruz Biotechnology) diluted in blocking buffer. The membrane was washed 3 times for 5 minutes each with TBS Blocking buffer and 0.1% TweenⓇ (TBS/T). The primary antibody reaction was detected by incubating for 1 hour with horseradish peroxidase (HRP)-conjugated secondary antibody (1:2000) and HRP-conjugated anti-biotin antibody (1:1000, Cell Signaling Technology) diluted in TBS/T with 5% nonfat dry milk. The membrane was washed and developed using LumiGLOⓇ Peroxide reagent and exposed to X-ray for detection the expressing bands.
Statistical analysis
Statistical analysis was performed with Statview 5.0 software. All values were expressed as means±standard errors using the Student t-test, a one-way ANOVA or a two-way factorial ANOVA for repeated measures. p< 0.05 was considered significant.
Results
Characterization of hSkM and have higher percentage of CXCR4
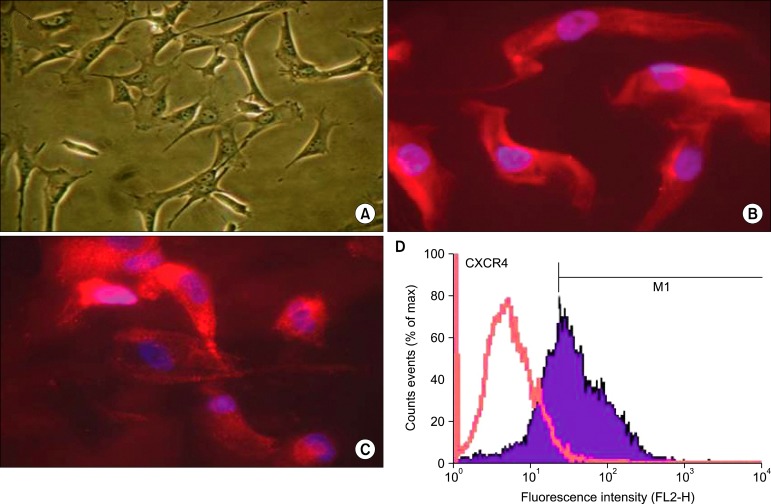
Human SkM were characterized by their typical cytoskeleton, their peculiar wave-like alignment that radiates from a common origin, and their ability to form myotubes under low-serum concentration cell culture conditions.High purity of human SkMs > 90% was further confirmed by desmin expression (Fig. 1A, B). These cells have CXCR4 receptor on their surfaces identified by CXCR4 immuno-stained antibodies (Fig. 1C) and the percentage of CXCR4 density was >65% (Fig. 1D).
Fig. 1. Characterization of human skeletal myoblasts in vitro: Skeletal myoblast (A) was identified histologically by anti-desmin staining (B, red) with nucleus DAPI (blue). SkM possess CXCR4 that was identified by immunostaining anti CXCR4 (C, red) with nucleus DAPI(blue) and also CXCR4 antigen (D, blue wave) was detected by flow cytometry.
Preconditioning (PC) of human SkM with rhSDF-1α protein
The cumulative effect of insufficient nutrients, decreased oxygen availability, and lack of growth and survival factors make it harsh for cells to survive in ischemic myocardium. We stimulate these parameters in vitro by exposing hSkM to culture conditions without glucose and serum, and subjected those to anoxic for different time periods(2∼6 hours) or oxidative stress like H2O2 with different concentrations (100∼300μM) and times (1∼3 hours). Unlike the rod-shaped morphology of a normal myocyte, hypercontracted morphology (round or irregular shaped) was observed in hSkM exposed to certain condition of anoxia or H2O2. (data not shown)
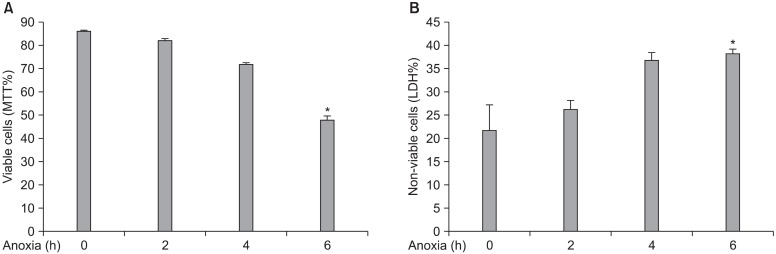
After exposure of human SkM to 1∼6 h anoxia, human SkM is an ischemic cell resistant and is sensitively affected after 6 hours exposures to anoxia. Cell viability % was assessed by MTT reduction test and LDH cytotoxicity Assay. LDH release as a marker of cellular injury was
highly increased in time dependent manner. Cell viability(MTT % reduction test) was decreased up to 50% viable lived cells (Fig. 2A) in parallel to LDH increased up to 35 % non-viable cells on exposure to 6 hours anoxia (Fig.2B).
Fig. 2. Human skeletal myoblasts were tolerating anoxic injury for 1∼6 hour. The condition medium of hSkMs was collected for LDH released and cells were used for MTT test. Cell viability % was assessed to evaluate % of viable cells (A) and also parallel LDH leakage to evaluate % of non-viable cells (B). *p< 0.001 vs. anoxic stress and controls in (A), *p=0.03 vs. 2 h anoxic stress only in (B).
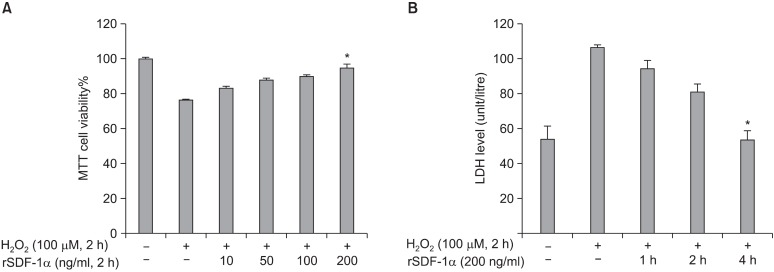
MTT cell viability was decreased up to 75% viable lived cells during exposure to 100μM H2O2 without preconditioning rhSDF-1α. This cell viability was improved up to 95% with cell preconditioned by rhSDF-1α in dose-dependent manner (the maximum dose is 200 ng/ml) (Fig. 3A). Moreover, LDH levels were decreased in cells preconditioned in presence of 200 ng/ml SDF-1α exposed to H2O2 in time-dependent manner. The maximum decrease of LDH levels were found in hSkM preconditioned by 200 ng/ml rhSDF-1α for 4 hours in compared with lower times exposure. In this optimal condition, the amount of LDH level released in conditioned medium was equal to the control level (Fig. 3B).
Fig. 3. Preconditioning of human SkM with rhSDF-1α before exposure to H2O2. hSkM cells (2.5× 104) were incubated with different concentration of rhSDF-1α (10 to 200 ng/ml) for 2 hours, then washed and incubated with 100μM H2O2 in free-serum DMEM for another 2 hours. Each point was performed in duplicate. The percentage of viable cells was assessed by MTT reduction test (A). Preconditioned treated cell with 200 ng/ml of rhSDF-1α for 1∼4 hours were assessed by measure of LDH released level in conditioned medium (B). The results were expressed as mean±SD. *p<0.05 (optimized condition, 200 ng/ml rSDF-1a) as in dose concentration manner in (A), and also with 4 h in time dependent in (B) vs. H2O2 stress only.
Optimization of human SkM with phSDF-1α gene delivery
According to our previous studies on rat SkM transfection (2), the efficiency of phSDF-1α transfection into human SkM was markedly influenced by vector/plasmid ratio and optimal efficiency was obtained with FuGeneTM6/phSDF-1α (v/w, 3:2). Short-term ZnCl2 exposure dis
played dose-dependent cytotoxicity on human SkM. However, cell viability observed with concentration <125μM ZnCl2 was significantly higher than with higher concentrations of > 250μM (p< 0.0001). Similarly, ZnCl2 concentration > 125μM also caused abrupt reduction in hSDF-1α expression. Consequently, we chose 125μM ZnCl2 to optimize transfection procedures with FuGeneTM6. We observed that cell viability significantly changed in presence or absence of 125μM ZnCl2 (p<0.05). Optimal transfection of human SkM was obtained with FuGeneTM6/phSDF-1α at 3:2 (v/w) for 4 h in presence of 125μM ZnCl2 (data not shown).
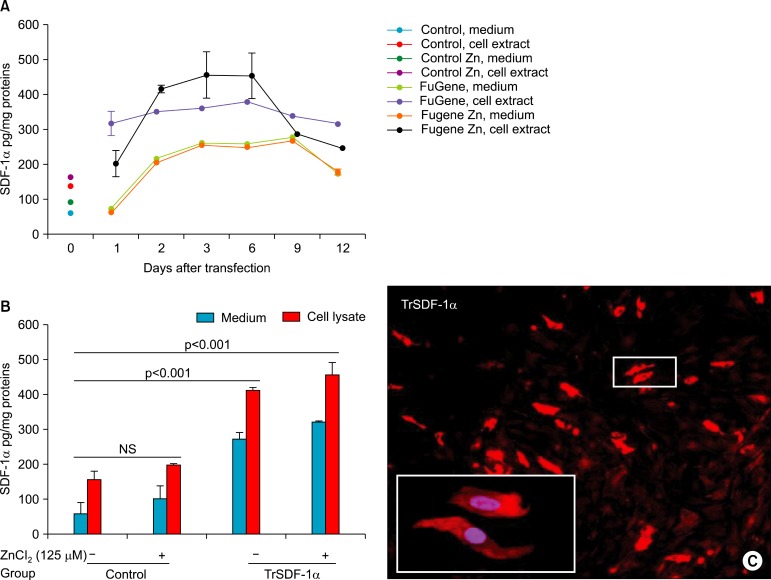
Human SkM transfection in presence of 125μM of ZnCl2, ELISA revealed that peak level hSDF-1α expression was observed at day-4 after transfection (455.4±48.8 pg/mg protein in cell lysate and 318.5±3.5 pg/mg protein in supernatant) versus hSkM transfection in absence of 125μM of ZnCl2 (411.2±6.1 pg/mg protein in cell lysate and 270.9±21.8 pg/mg protein in supernatant) followed by a gradual decline until 12 days of observation in vitro (Fig. 4A, B). The non-transfected SkM secreted negligible amounts of hSDF-1α (60.0±29.1 pg/mg protein in supernatant and 150.4±27.4 pg/mg protein in cell lysate).
Fig. 4. Assessment of transfection efficiency with SDF-1α in human SkM. The highest transfection and expression of SDF-1α in presence of 125μM ZnCl2 were achieved with FuGeneTM6. ELISA for SDF-1α level was monitored for 12 days (pg of hSDF-1α/mg of total protein)in the supernatant and cell lysate (A-B) from SkM transfected with FuGeneTM6/phSDF-1α in the presence and absence of ZnCl2. Immunostaining of SkM for SDF-1α overexpression in vitro after transfection with FuGeneTM6/phSDF-1α at day 4 (C).
The number of hSDF-1α positive SkM was markedly increased in presence of 125μM of ZnCl2 (Fig. 4C) as compared with the absence of ZnCl2 (p=0.007) at day 4 after transfection.
Cell survival molecules and growth factors in human SkM overexpressing hSDF-1α
RT-PCR showed that hSDF-1α expression in SkM
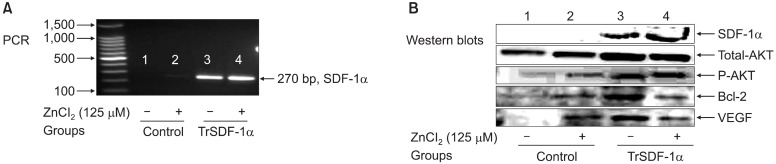
transfected in presence of ZnCl2 was significantly higher as compared with ones transfected in the absence of ZnCl2(p<0.001) using non-transfected SkM as control (p<0.01) (Fig. 5A). Also, in parallel to PCR, western blot bands of SDF-1α expression produced in cell lysate in transfected cells and this band was more prominent with ZnCl2. Also,marked overexpression in VEGF (7.8-fold), total Akt (1.8-fold), phospho-Akt (2.5-fold) and Bcl2 (2.9-fold) were observed in hSkM transfected by SDF-1α as compared with control hSkM (Fig. 5B).
Fig. 5. Assessment of human SkM overexpressing SDF-1α at day 4 post-transfection: PCR for SDF-1α bands (A), Western blot density bands of SDF-1α, total Akt, phospho-Akt, Bcl2 and VEGF (B) were shown in all different conditions controls versus transfected SkM in presence or absence of 125μM ZnCl2 from lane-1 to -4.
Cytoprotective effect of preconditioning (PC) in optimized human SkM with SDF-1α
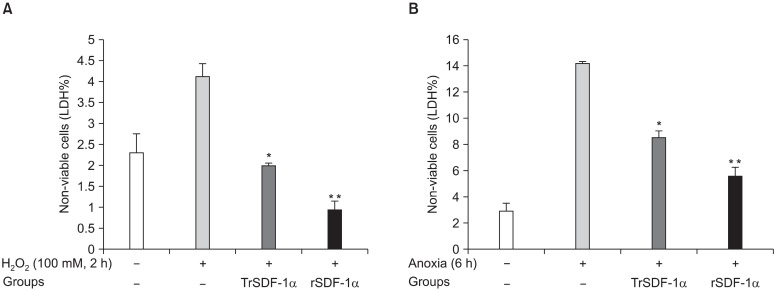
Six hours exposure of cells to anoxia or 100μM H2O2 for 2 hours were used as point that cells started pathological ischemic cascades. Also, hSkM preconditioned with rhSDF-1α (200 ng/ml rhSDF-1α, 4 hour incubation) were compared with hSkM overexpressed SDF-1α (transfected by phSDF-1α/FuGeneTM6, 4 hours incubation).This two conditions were exposed to 6 h anoxia or 2 hours with 100μM H2O2. LDH release was significantly higher upon exposure of cells to 100μM H2O2 for 2 hours in non-transfected SKM (control) compared with phSDF-1αtransfected SkM (p=0.01) or preconditioning with rhSDF(p=0.001) (Fig. 6A). Similarly, LDH release was significantly higher upon exposure of cells to 6 hours anoxia in non-transfected SKM (control) compared with phSDF-1α transfected SkM (p=0.01) or preconditioning with rhSDF (p=0.001) (Fig. 6B).
Fig. 6. Standardized comparison of optimized human SKM overexpressing SDF-1α with hSkM treated with rhSDF-1α before exposure to 100μM H2O2 (A) or 6 hour anoxia (B). LDH assay for non-viable cells % were obtained from quadruplicate wells. (*p<0.01, **p<0.001 vs. H2O2 or anoxic stress only).
Preconditioning of the hSkM with 200 ng/ml rhSDF-1α or transfected by SDF-1α gene for 4 hours could prevent the morphological changes of cell injury and hence was used throughout the study for preconditioning of cells. LDH leakage was significantly reduced in PC hSkM as compared with the non-PC hSkM when both of these were exposed to 100μM H2O2 or 6 hours anoxia (p<0.01). This cytoprotective effect of hSDF-1α to transplanted donor SkM could be useful before SkM transplantaion in
myocardial infarction.
Cell survival signaling molecules and growth factors in PC human SkM with SDF-1α
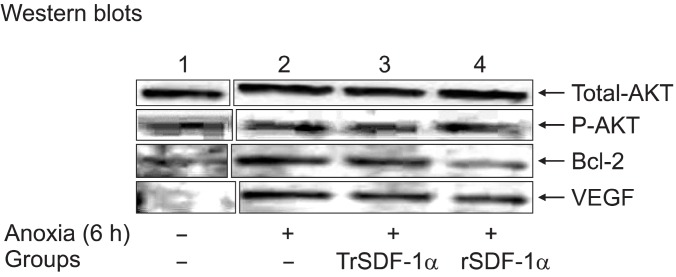
The changes in the gene expression of several candidate molecules were assessed by western blotting after exposure of hSkM to 6 hours anoxia in non-PC or PC with rhSDF-1α or transfected with phSDF-1α (overexpressed cells to hSDF-1α in Fig. 7. However, these parameters were also overexpressed during exposure of hSkM to zinc or 6 hours anoxia alone. Moreover, after exposure to 6 hours anoxia, upregulated expression of VEGF in PC hSkM with transfected by phSDF-1α (5.8-fold) and rhSDF (4.7-fold); total Akt in PC hSkM with transfected by phSDF-1α (1.2-fold) and rhSDF (1.4-fold) were observed as compared with non-PC hSkMs. This is supported by the significantly higher level of phospho-Akt expression in PC hSkM with transfected by phSDF-1α (2.7-fold) and rhSDF (2.5-fold) observed in cell subjected to anoxia as compared with non-PC hSkM. Also, little upregulated expression of Bcl2 in transfected by phSDF-1α (1.6-fold) and rhSDF (1.1-fold) were observed as compared with non-PC hSkMs, which reports the concomitant upregulation of VEGF and phospho-Akt occurred under ischemic conditions. This VEGF can initiate signaling pathways by binding to their cell surface receptor tyrosine kinases. We believe that in activation of these receptor tyrosine kinases is intracellular signaling along the phosphatidylinositol 3-kinase/Akt-dependent pathway.
Fig. 7. Cytoprotective effect of human SkM preconditioned with SDF-1α: Western blot density bands of total Akt, phospho-Akt,Bcl2 and VEGF were shown in human SkM controls, optimized human SkM overexpressing SDF-1α and hSkM treated with rhSDF-1α after 6 hours exposure to anoxia from lane-1 to 4.
Discussion
Gene therapy using skeletal myoblasts may be a new useful treatment for ischemic myocardium and might especially be relevant for use in the elderly patients in whom the availability of SkM transplantation is limited. Animal skeletal myoblasts were already successfully transfected with SDF-1α (2), VEGF (27), cardiotrophin-1 (28), TGF1β (29), hypoxia inducible factor-1α (30) and connexin-43 (31) leading to induce neovascularization and improve contractility of myocardium. Unfortunately, gene therapy using viral vectors in clinical trials led to immune and/or inflammatory reaction that limited their use. Moreover, the necrotic myocardium is resistant to direct gene transfer (32).
In order to overcome these limitations, indirect gene transfer via ex vivo genetic manipulation of donor cells using non-viral vector is a safe alternative and provides a tissue specific repository of the transgene product (26). The enhanced effect for gene transfer of skeletal myoblast with electroporation (33), hydrostatic pressure (hydroporation) (34) or ultrasound (sonoporation) (35) revealed higher transfection efficiencies despite cell or tissue damage. For this reason, in our previous studies (2, 26), we optimized non-viral cationic vectors such as FuGeneTM6 with ZnCl2 would be of great interest as a method for optimizing transfection conditions.
Apoptosis has been implicated as among the mechanisms of cell death in ischemic myocardium. Hence, developing strategy to alleviate apoptosis under ischemic stress is therefore of prime consequence in heart cell therapy. Indeed, possessing a vital role in cell bioenergetics and being key determinant of cell survival; mitochondria are the logical targets in pathological conditions to prevent cell apoptosis (36). Activation of mitochondrial pathways that promote cell survival is an endogenously occurring process of "ischemic preconditioning" as part of the homeostasis. Apparently, a brief exposure to ischemia opens the mitochondrial ATP-sensitive potassium (mitoK-ATP) channels and renders the heart more tolerant to subsequent lethal ischemic injury (36). Similar cytoprotective effects can be replicated by pharmacological agents that act on the mitoK-ATP channels such as Diazoxide (mitoK-ATP channel opener) has been widely demonstrated to suppress cell apoptosis and promote cell survival (10). Indeed, pharmacological now parallels ischemic preconditioning as among the most potent interventions to prevent apoptosis. Our group has already shown that cardiac protection from mitoK-ATP channels is dependent on Akt translocation from cytosol to mitochondria. Also, our groups have reported Diazoxide-activated PKC isoform translocation to mitochondria promoted phosphorylation-dependent activation of the channels (10). The protective effects afforded by Diazoxide against apoptosis can be extrapolated to maximize cell graft viability in infracted heart (10, 36). Our observed a prodigious improvement in PC SkM survival as compared with the non-PC SkMs under oxidant stress in vitro.
In the previous studies, up to 1 billion cells have been injected during animal and human studies to compensate for the donor SkM loss post-transplantation. Propagation of SkMs to achieve this large number is time consuming and has economic and logistic implications in clinical settings. In the physiological context, dead cells debris contributes to the donor cell-specific humoral and local inflammatory responses at the site of cell graft (3). Prevention of the donor cell apoptosis will reduce the intensity of these reactions and create a favorable environment for the transplanted cells.
The cytoprotective effects of hSDF-1α resulted in elevated expression of phospho-Akt and various growth factors that favored survival signaling as activation of Akt and its downstream molecules are known for cytoprotection (2). Therefore, it is logical to suggest that the upregulated levels of phospho-Akt and its downstream molecules in PC hSkM were at least in part responsible for the cytoprotective affects. Moreover, the cardioprotective effect of FGF-2 administration to the heart by perfusion in ischemia reperfusion injury to myocardium has also been reported (19). Also, our groups reported a role of Diazoxide preconditioning-induced FGF-2 and HGF stimulation of Akt / phosphatidylinositol 3-kinase signaling pathway in SkMs for their improved survival under oxidative stress (10, 36).
Ex vivo endothelial progenitor cells (EPC) or mesenchymal stem cells (MSC) priming with recombinant SDF-1α proteins before transplantation in hind-limb ischemia (37) or myocardial ischemia (38) considered an effective therapeutic strategy to improve the recruitment of injected cells in ischemic tissues. This novel, cell-based therapeutic approach has the potential in minimizing the adverse effects of ischaemia on cell death and cardiac remodelling. The induction of SDF-1α with hypoxia in vitro and ischemic preconditioning in vivo (39) suggests that this chemokine is important for myocardial protection, against ischemia/reperfusion-induced damage, through either a paracrine or an autocrine mechanism. SDF-1α - CXCR4 induced cardioprotection is associated with MAPK and AKT activation and decreased apoptosis (36, 40).
Finally, we propose that preconditioning of hSkMs promoted cell survival under oxidative stress by release of paracrine factors such as VEGF that will bind to their receptor tyrosine kinases to activate the phosphatidylinositol 3-kinase/Akt pathway. SDF-1α binds to CXCR4 receptor and modulates several biological functions through signal transduction pathways. These include increased cell growth, proliferation, cell survival and anti-apoptotic, emigration and transcriptional activation. SDF-1α is a coparticipant in angiogenesis that is regulated at the receptor level by VEGF and FGF-2. Cell survival signalling by Akt/Bcl2 led to enhanced cell survival under anoxic conditions in vitro.
In conclusion, this present study demonstrated that preconditioning was extremely effective to promote hSkM survival under oxidative stress in vitro. The major finding are: (i) preconditioning protected SkMs against oxidative stress via stimulation of the cell survival signaling mediators; (ii) preconditioning induced the cells to release paracrine growth factor such as VEGF; (iii) preconditioning mimetics improved SkM tolerance to oxidative stress, which is associated with a high level expression of paracrine factors from PC hSkMs, could be useful after transplantation in heart to promote angiomyogenesis.
Potential conflict of interest
The authors have no conflicting financial interest.
References
- 1.Bourahla B, Shafy A, Meilhac O, Elmadbouh I, Michel JB, Chachques JC. Mesothelial cells vs. skeletal myoblasts for myocardial infarction. Asian Cardiovasc Thorac Ann. 2010;18:153–160. doi: 10.1177/0218492310361793. [DOI] [PubMed] [Google Scholar]
- 2.Elmadbouh I, Haider HKh, Jiang S, Idris NM, Lu G, Ashraf M. Ex vivo delivered stromal cell-derived factor-1alpha promotes stem cell homing and induces angiomyogenesis in theinfarcted myocardium. J Mol Cell Cardiol. 2007;42:792–803. doi: 10.1016/j.yjmcc.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chachques JC. Cellular cardiac regenerative therapy inwhich patients? Expert Rev Cardiovasc Ther. 2009;7:911–919. doi: 10.1586/erc.09.84. [DOI] [PubMed] [Google Scholar]
- 4.Pagani FD, DerSimonian H, Zawadzka A, Wetzel K, Edge AS, Jacoby DB, Dinsmore JH, Wright S, Aretz TH, Eisen HJ, Aaronson KD. Autologous skeletal myoblasts trans-planted to ischemia-damaged myocardium in humans. Histological analysis of cell survival and differentiation. J Am Coll Cardiol. 2003;41:879–888. doi: 10.1016/s0735-1097(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 5.Beauchamp JR, Morgan JE, Pagel CN, Partridge TA. Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as themyogenic source. J Cell Biol. 1999;144:1113–1122. doi: 10.1083/jcb.144.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guérette B, Skuk D, Célestin F, Huard C, Tardif F, Asselin I, Roy B, Goulet M, Roy R, Entman M, Tremblay JP. Prevention by anti-LFA-1 of acute myoblast death following transplantation. J Immunol. 1997;159:2522–2531. [PubMed] [Google Scholar]
- 7.Müller-Ehmsen J, Peterson KL, Kedes L, Whittaker P, Dow JS, Long TI, Laird PW, Kloner RA. Rebuilding a damaged heart: long-term survival of transplanted neonatal rat cardiomyocytes after myocardial infarction and effect on cardiac function. Circulation. 2002;105:1720–1726. doi: 10.1161/01.cir.0000013782.76324.92. [DOI] [PubMed] [Google Scholar]
- 8.Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: graft celldeath and anti-death strategies. J Mol Cell Cardiol. 2001;33:907–921. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 9.Qu Z, Balkir L, van Deutekom JC, Robbins PD, Pruchnic R, Huard J. Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol. 1998;142:1257–1267. doi: 10.1083/jcb.142.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niagara MI, Haider HKh, Jiang S, Ashraf M. Pharmacologically preconditioned skeletal myoblasts are resistant to oxidative stress and promote angiomyogenesis via release of paracrine factors in the infarcted heart. Circ Res. 2007;100:545–555. doi: 10.1161/01.RES.0000258460.41160.ef. [DOI] [PubMed] [Google Scholar]
- 11.Pouzet B, Vilquin JT, Hagège AA, Scorsin M, Messas E, Fiszman M, Schwartz K, Menaschè P. Intramyocardial transplantation of autologous myoblasts: can tissue processingbe optimized? Circulation. 2000;102(19 Suppl 3):III210–III215. doi: 10.1161/01.cir.102.suppl_3.iii-210. [DOI] [PubMed] [Google Scholar]
- 12.Schussler O, Chachques JC, Mesana TG, Suuronen EJ, Lecarpentier Y, Ruel M. 3-dimensional structures to enhance cell therapy and engineer contractile tissue. Asian Cardiovasc Thorac Ann. 2010;18:188–198. doi: 10.1177/0218492310361531. [DOI] [PubMed] [Google Scholar]
- 13.Vacanti JP, Langer R. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet. 1999;354(Suppl1):SI32–34. doi: 10.1016/s0140-6736(99)90247-7. [DOI] [PubMed] [Google Scholar]
- 14.Spadaccio C, Chachques E, Chello M, Covino E, Chachques JC, Genovese J. Predifferentiated adult stem cells and matrices for cardiac cell therapy. Asian Cardiovasc Thorac Ann. 2010;18:79–87. doi: 10.1177/0218492309355836. [DOI] [PubMed] [Google Scholar]
- 15.Siepe M, Giraud MN, Liljensten E, Nydegger U, Menasche P, Carrel T, Tevaearai HT. Construction of skeletal myoblast-based polyurethane scaffolds for myocardial repair. Artif Organs. 2007;31:425–433. doi: 10.1111/j.1525-1594.2007.00385.x. [DOI] [PubMed] [Google Scholar]
- 16.Chachques JC, Jegaden O, Mesana T, Glock Y, Grandjean PA. Carpentier AF; French Cardiomyoplasty Investigators. Cardiac bioassist: results of the French multicenter cardiomyoplasty study. Asian Cardiovasc Thorac Ann. 2009;17:573–580. doi: 10.1177/0218492309349371. [DOI] [PubMed] [Google Scholar]
- 17.Elmadbouh I, Chen Y, Louedec L, Silberman S, Pouzet B, Meilhac O, Michel JB. Mesothelial cell transplantation inthe infarct scar induces neovascularization and improves heart function. Cardiovasc Res. 2005;68:307–317. doi: 10.1016/j.cardiores.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Retuerto MA, Schalch P, Patejunas G, Carbray J, Liu N, Esser K, Crystal RG, Rosengart TK. Angiogenic pretreatment improves the efficacy of cellular cardiomyoplasty performed with fetal cardiomyocyte implantation. J Thorac Cardiovasc Surg. 2004;127:1041–1049. doi: 10.1016/j.jtcvs.2003.09.049. [DOI] [PubMed] [Google Scholar]
- 19.Sakakibara Y, Nishimura K, Tambara K, Yamamoto M, Lu F, Tabata Y, Komeda M. Prevascularization with gelatin microspheres containing basic fibroblast growth factor enhances the benefits of cardiomyocyte transplantation. J Thorac Cardiovasc Surg. 2002;124:50–56. doi: 10.1067/mtc.2002.121293. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell-derived factor-1 effects onex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 21.Woo YJ, Grand TJ, Berry MF, Atluri P, Moise MA, Hsu VM, Cohen J, Fisher O, Burdick J, Taylor M, Zentko S, Liao G, Smith M, Kolakowski S, Jayasankar V, Gardner TJ, Sweeney HL. Stromal cell-derived factor and granulocyte-monocyte colony-stimulating factor form a combined neovasculogenic therapy for ischemic cardiomyopathy. J Thorac Cardiovasc Surg. 2005;130:321–329. doi: 10.1016/j.jtcvs.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 22.Premaratne GU, Tambara K, Fujita M, Lin X, Kanemitsu N, Tomita S, Sakaguchi G, Nakajima H, Ikeda T, Komeda M. Repeated implantation is a more effective cell deliverymethod in skeletal myoblast transplantation for rat myocardial infarction. Circ J. 2006;70:1184–1189. doi: 10.1253/circj.70.1184. [DOI] [PubMed] [Google Scholar]
- 23.Wisel S, Khan M, Kuppusamy ML, Mohan IK, Chacko SM, Rivera BK, Sun BC, Hideg K, Kuppusamy P. Pharmacological preconditioning of mesenchymal stem cells with trimetazidine(1-[2,3,4-trimethoxybenzyl]piperazine) protects hypoxic cells against oxidative stress and enhances recovery of myocardial function in infarcted heart through Bcl-2 expression. J Pharmacol Exp Ther. 2009;329:543–550. doi: 10.1124/jpet.109.150839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schierle GS, Hansson O, Leist M, Nicotera P, Widner H, Brundin P. Caspase inhibition reduces apoptosis and increases survival of nigral transplants. Nat Med. 1999;5:97–100. doi: 10.1038/4785. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki K, Smolenski RT, Jayakumar J, Murtuza B, Brand NJ, Yacoub MH. Heat shock treatment enhances graft cell survival in skeletal myoblast transplantation to the heart. Circulation. 2000;102(19 Suppl 3):III216–III221. doi: 10.1161/01.cir.102.suppl_3.iii-216. [DOI] [PubMed] [Google Scholar]
- 26.Elmadbouh I, Rossignol P, Meilhac O, Vranckx R, Pichon C, Pouzet B, Midoux P, Michel JB. Optimization of in vitro vascular cell transfection with non-viral vectors for in vivo applications. J Gene Med. 2004;6:1112–1124. doi: 10.1002/jgm.604. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki K, Smolenski B, Jayakumar RT, Murtuza IA, Brand N, Yacoub Y. Cell transplantation for the treatment of acute myocardial infarction using vascular endothelial growth factor-expressing skeletal myoblasts. Circulation. 2001;104((12 Suppl 1)):I207–212. doi: 10.1161/hc37t1.094524. [DOI] [PubMed] [Google Scholar]
- 28.Toh R, Kawashima S, Kawai M, Sakoda T, Ueyama T, Satomi-Kobayashi S, Hirayama S, Yokoyama M. Transplantation of cardiotrophin-1-expressing myoblasts to the leftventricular wall alleviates the transition from compensatory hypertrophy to congestive heart failure in Dahl salt-sensitive hypertensive rats. J Am Coll Cardiol. 2004;43:2337–2347. doi: 10.1016/j.jacc.2004.02.048. [DOI] [PubMed] [Google Scholar]
- 29.Koh GY, Kim SJ, Klug MG, Park K, Soonpaa MH, Field LJ. Targeted expression of transforming growth factor-beta1 in intracardiac grafts promotes vascular endothelial cell DNA synthesis. J Clin Invest. 1995;95:114–121. doi: 10.1172/JCI117627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haider KH, Kim HW, Ashraf M. Hypoxia-inducible factor-1alpha in stem cell preconditioning: mechanistic role of hypoxia-related micro-RNAs. J Thorac Cardiovasc Surg. 2009;138:257. doi: 10.1016/j.jtcvs.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki K, Brand NJ, Allen S, Khan MA, Farrell AO, Murtuza B, Oakley RE, Yacoub MH. Overexpression of connexin43 in skeletal myoblasts: Relevance to cell transplantation to the heart. J Thorac Cardiovasc Surg. 2001;122:759–766. doi: 10.1067/mtc.2001.116210. [DOI] [PubMed] [Google Scholar]
- 32.Taylor DA. Cellular cardiomyoplasty with autologous skeletal myoblasts for ischemic heart disease and heart failure. Curr Control Trials Cardiovasc Med. 2001;2:208–210. doi: 10.1186/cvm-2-5-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shafy A, Lavergne T, Latremouille C, Cortes-Morichetti M, Carpentier Carpentier, Chachques JC. Association of electrostimulation with cell transplantation in ischemic heart disease. J Thorac Cardiovasc Surg. 2009;138:994–1001. doi: 10.1016/j.jtcvs.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 34.Danialou G, Comtois AS, Matecki S, Nalbantoglu J, Karpati G, Gilbert R, Geoffroy P, Gilligan S, Tanguay JF, Petrof BJ. Optimization of regional intraarterial naked DNA-mediated transgene delivery to skeletal muscles in a large animal model. Mol Ther. 2005;11:257–266. doi: 10.1016/j.ymthe.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 35.Lu QL, Liang HD, Partridge T, Blomley MJ. Microbubble ultrasound improves the efficiency of gene transduction inskeletal muscle in vivo with reduced tissue damage. GeneTher. 2003;10:396–405. doi: 10.1038/sj.gt.3301913. [DOI] [PubMed] [Google Scholar]
- 36.Haider HKh, Ashraf M. Preconditioning and stem cellsurvival. J Cardiovasc Transl Res. 2010;3:89–102. doi: 10.1007/s12265-009-9161-2. [DOI] [PubMed] [Google Scholar]
- 37.Zemani F, Silvestre JS, Fauvel-Lafeve F, Bruel A, Vilar J, Bieche I, Laurendeau I, Galy-Fauroux I, Fischer AM, Boisson-Vidal C. Ex vivo priming of endothelial progenitor cells with SDF-1 before transplantation could increase their proangiogenic potential. Arterioscler Thromb Vasc Biol. 2008;28:644–650. doi: 10.1161/ATVBAHA.107.160044. [DOI] [PubMed] [Google Scholar]
- 38.Pasha Z, Wang Y, Sheikh R, Zhang D, Zhao T, Ashraf M. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res. 2008;77:134–142. doi: 10.1093/cvr/cvm025. [DOI] [PubMed] [Google Scholar]
- 39.Tang YL, Zhu W, Cheng M, Chen L, Zhang J, Sun T, Kishore R, Phillips MI, Losordo DW, Qin G. Hypoxic preconditioning enhances the benefit of cardiac progenitor celltherapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res. 2009;104:1209–1216. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu X, Dai S, Wu WJ, Tan Tan, Zhu X, Mu J, Guo Y, Bolli R, Rokosh G. Stromal cell derived factor-1 alpha confers protection against myocardial ischemia/reperfusion injury:role of the cardiac stromal cell derived factor-1 alpha CXCR4 axis. Circulation. 2007;116:654–663. doi: 10.1161/CIRCULATIONAHA.106.672451. [DOI] [PMC free article] [PubMed] [Google Scholar]