Abstract
Pluripotent stem cells, such as embryonic stem (ES) cells, can differentiate into all cell types. So, these cells can be a biological resource for regenerative medicine. However, ES cells known as standard pluripotent cells have problem to be used for cell therapy because of ethical issue of the origin and immune response on the graft. Hence, recently reprogrammed pluripotent cells have been suggested as an alternative source for regenerative medicine. Somatic cells can acquire the ES cell-like pluripotency by transferring somatic cell nuclei into oocytes, by cell fusion with pluripotent cells. Retroviral-mediated introduction of four factors, Oct4, Sox2, Klf4 and c-Myc can successfully reprogram somatic cells into ES cell-like pluripotent stem cells, known as induced pluripotent stem (iPS) cells. These cells closely resemble ES cells in gene expression pattern, cell biologic and phenotypic characteristics. However, to reach the eventual goal of clinical application, it is necessary to overcome the major drawbacks such as low reprogramming efficiency and genomic alterations due to viral integration. In this review, we discuss the current reprogramming techniques and mechanisms of nuclear reprogramming induced by transcription factor transduction.
Keywords: Pluripotency, Embryonic stem cell, Induced pluripotent stem cell, Somatic cell nuclear transfer, Cell fusion hybrid
Introduction
Stem cells are undifferentiated cells capable of proliferation for an expanded period of time, dividing to generate daughter cells in a process called self-renewal, and may differentiate into a diverse range of specialized cell types. Stem cells can be classified based on their differentiation potential. Totipotent stem cells can give rise to an entire, viable organism as well as to cells of the three germ layers (ectoderm, mesoderm and endoderm). Only the fertilized embryos and blastomeres up to the 8 cell stage are considered to be totipotent cells. Pluripotent stem cells can give rise to every type of cell derived from the three germ layers, but not to a live organism without help of trophoplast cells. Embryonic stem (ES) cells derived from the ICM of pre-implantation blastocyst stage embryos can be propagated in vitro, and these cells retain pluripotency. Mouse ES cells were first derived by Evans and Kaufman in 1981(1). In 1998, the Thomson group derived human ES cells from human blastocyst and developed cell culture conditions for these cells (2). Multipotent stem cells can produce a limited number of cells and differentiate into multiple cell lineages, typically those of a closely related family of cells. For example, the neural stem cells can give rise to cells of the nervous system (e.g.neurons and glial cells) and the hematopoietic stem cell can give rise to cells of the hematopoietic system (e.g.mast cells, macrophages, erythrocytes and lymphocytes). Unipotent stem cells can be differentiated into only one cell type. The spermatogonial stem cells for example are unipotent.
So far, due to their plasticity and potentially unlimited capacity for self-renewal, human ES cell therapies have been proposed as a resource for regenerative medicine. However, there are two drawbacks to use human ES cells for therapeutic application. First, the process of isolating human ES cells requires destruction of human embryos. Second, the immune system of the patient recognizes ES
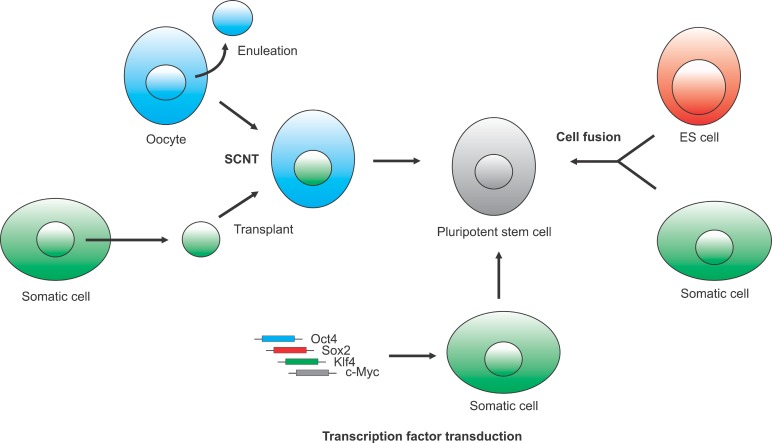
cell-derived cells and tissues as ‘ non-self’ , resulting in an immune rejection to the graft. To overcome these issues, several groups developed technology to derive ES cell-like pluripotent stem cells from differentiated somatic cells, so called “pluripotential reprogramming”. These cells reprogrammed from somatic cells show pluripotent characteristics, such as reactivation of pluripotency-related genes, inactivation of tissue-specific genes, differentiation potential to all three germ layers, and a specific epigenomic state corresponding to the pluripotent cells. Therefore, pluripotential reprogramming may allow us to use the easily accessible cells for treatment of disease without ethical issue. However, the mechanisms underlying cellular reprogramming are largely unknown. In this review, we discuss current strategies and mechanisms of generating reprogrammed pluripotent stem cells derived by somatic cell nuclear transfer, cell fusion and transduction of transcription factors (Fig. 1).
Fig. 1. Three different approaches to generate pluripotent stem cells from somatic cells. Pluripotent stem cells can be derived by somati ccell nuclear transfer, cell fusion and transduction of transcription factors.
Reprogrammed pluripotent stem cells
Somatic cell nuclear transfer
Basic technique of nuclear transfer (NT) involves transferring the nucleus of a diploid cell (full set of paired chromosomes) to an enucleated oocyte (Fig. 1). The reconstructed cells are stimulated to grow into embryos.Stimulation, or activation, is achieved by a transient increase in the intracellular-free calcium concentration induced either by electrical pulse or by chemical stimulation. The preimplantation stage embryos are then maintained in a sequential culture media and the developed embryos are transferred to a foster mother (3).
Nuclear transfer was first introduced in 1952 to study early embryonic development in frogs (4). In 1980’ s cloned cattle and sheep using embryonic cells as donor cells were generated. In 1997, the first successfully cloned animal by somatic cell nuclear transfer (SCNT) was reported in sheep by Wilmut and colleagues (5). They used mammary gland cells as donor cells that had been in G0 phase by serum starvation in culture. Schnieke et al. generated the first transgenic lamb, Polly, using NT technology (6). A year later, Wakayama’ s group cloned mice by direct injection of somatic cells into enucleated oocytes using piezo-driven manipulator (7). This instrument enabled the nucleus to be removed from the mouse oocyte and to be replaced with the nucleus of a somatic cell, which is more delicate procedures than that of the sheep. So far, various mammalian species have been successfully cloned by SCNT, including sheep, mice, cattle (8), goat (9), pig (10), cat (11), rabbit (12), mule (13), horse (14), rat (15), dog (16), and wolf (17). Unfortunately, SCNT shows very low success rates for full-term cloning. The efficiency of nuclear transfer depends on a number of important technical and biological variables such as oocyte quality, enucleation and cell transfer procedures, and oocyte activation.Moreover, cloned mice showed many abnormalities, including abnormal gene expression patterns (18), abnormal placentas (19), and early death (20). Wakayama and colleagues found that the efficiency of mouse cloning could be enhanced by up to five-fold through the addition of the histone deacetylation inhibitor trichostatin A (TSA) into the oocyte activation medium (21). Therefore, the successful reprogramming by SCNT must entail the correct epigenetic change by reprogramming factors of the oocyte, which induce all the epigenetic changes that follow downregulation of somatic genes and up-regulation of embryonic genes.
On the other hand, ES cells can be derived from NT blastocysts (22, 23), so called ntES cells. In contrast to the abnormalities seen in directly cloned live animals, ntES cells are transcriptionally and functionally indistinguishable from normal ES cells derived from fertilized blastocysts, presumably due to a selection of faithfully reprogrammed cells during culture. In spite of this remarkable progress, the application of the therapeutic cloning in primates remained questionable. Early attempts demonstrated that human SCNT embryos were unable to develop efficiently into blastocysts and mainly arrested at early cleavage stages (24). In addition, therapeutic cloning approach for reprogramming of human somatic cells into pluripotent ES cell-like cells poses ethical concerns since it involves the creation and subsequent destruction of viable oocytes and embryos with potential for full-term development. Furthermore, SCNT fails to produce phenotypically homogeneous clones because of the mitochondrial DNA remained in the enucleated oocytes, which can result in various cell function disorders and phenotype alteration in the resulting offspring. Thus, reprogramming by SCNT should be substituted by other safe technology such as fusion with ES cells or direct reprogramming by transduction of transcription factors.
Cell fusion-induced reprogramming
The reestablishment of new characteristics by cell fusion between two or more different cell types was suggested as early as in 1965. After this pioneering study, several groups have shown that pluripotent stem cells have an intrinsic capacity for pluripotential nuclear reprogramming of somatic cells by cell fusion (Fig. 1). Therefore, somatic cells can acquire a pluripotent state after being fused with pluripotent stem cells such as ES, embryonic germ (EG), and embryonal carcinoma (EC) cells (25-27). Tada and colleagues were the first to show pluripotential nuclear reprogramming of somatic cells by cell-cell fusion.They fused female EG cells, which are pluripotent cells derived from primordial germ cells, with thymocytes from adult mice (25). These fused tetraploid cells were pluripotent and could contribute to the all three germ layers in chimaeric embryos. Tada and colleagues also showed that somatic cells can acquire a pluripotency after being fused with ES cells (26). Interestingly, the methylation patterns of imprinted genes of somatic cells were different between EG- and ES cell-somatic hybrid cells. The methylation pattern of imprinted genes of somatic cells was not changed after fusion with ES cells (methylated on the maternal allele as in somatic cells), but changed after fusion with EG cells (not methylated on both alleles). These results suggest that EG cells contain an additional potential to induce methylation change of somatic cells.
In human, Cowan and colleagues extended this work by showing that nuclear reprogramming of human somatic cells can be achieved by fusion with human ES cells (28). The fusion hybrid cells present pluripotential characteristics,such as inactivation of tissue-specific genes, reactivation of pluripotent-related genes, differentiation property to three germ layers and a specific epigenetic state corresponding to the pluripotent human ES cells.Moreover, mouse pluripotent stem cells can reprogram nuclei of human somatic cells into pluripotent state. This result indicates that reprogramming factors can cross-act through another species (29). Hence, reprogrammed fusion hybrid cells have been used for study of reprogramming mechanism. In nuclear transfer, the ooplasm of an enucleated oocyte has the capacity to recondition or reset the epigenetic program of a somatic cell nucleus to the totipotent state. However, enucleated ES cells do not assume the ability to reprogram somatic cells (30). During nuclear reprogramming it is expected that the chromatin structure, which is believed to function in establishing cell-type-specific gene expression pattern, should be significantly modified by two major epigenetic events, DNA methylation and histone modification. Following hybridization of ES cells with thymocytes, the somatic cells undergo chromatin remodeling, which is induced by reprogramming factors reside in ES cells (31). Recently,Bhutani and colleagues demonstrated a new role of Activation-induced cytidine deaminase (AID, also known as AICDA) in DNA demethylation activity using cell fusioninduced reprogramming technology (32). They showed that OCT4 and NANOG promoter region of human fibroblasts were demethylated without replication and cell division after fusion with mouse ES cells, indicating that AID may function as an active DNA demethylase. However,the function of AID in reprogramming process is not clear, as we previously showed that when the reprogramming occurs without DNA replication and cell division, Oct4-GFP reactivation in somatic genome require passive DNA demethylation by pre-treatment of 5-Aza C, a DNA methyltransferase inhibitor (30). Therefore, AID may not be enough to induce complete pluripotential reprogramming.
Although the fusion hybrid cells show pluripotential characteristics, the fusion hybrid cells are not identical to the pluripotent fusion partner cells. The fusion hybrid cells can form chimera but not contribute to germline. Although fusion-induced reprogramming is very efficient(about 95%), but the resultant hybrid cells lack therapeutic potential because of their tetraploidy and the presence of exogenous genes from the pluripotent fusion partner cells.
Transcription factor transduction
In 2006, Takahashi and Yamanaka screened a combination of 24 pluripotency-associated candidate genes and found that retroviral transduction of four previously known transcription factors (Oct4, Sox2, Klf4 and c-Myc) can convert mouse embryonic fibroblast (MEFs) and tail tip fibroblasts into ES cell-like cells, which are almost indistinguishable from mouse ES cells in terms of morphology and differentiation potency (Fig. 1) (33). These induced pluripotent stem (iPS) cells can differentiate into all three germ layers and contribute to germline competent chimera like mouse ES cells. This finding was independently confirmed by the Jaenisch group (34). After
this breakthrough study, various iPS cells have been derived from a number of different species, including humans (35), rats (36), rhesus monkeys (37), pigs (38), rabbits (39), and sheep (40), by expression of the four (Oct4,Sox2, Klf4 and c-Myc) or six (Lin 28 and Nanog in addition to 4 factors) transcription factors. One of the major concerns about reprogramming by transduction of transcription factors has been the low reprogramming efficiency.Many groups have tried to increase the efficiency in deriving iPS cells from somatic cells by combining transcription factors and small chemical molecules that inhibit G9a histone methyltransferase, histone deacetylase, and MEK and GSK3 signaling (41-43). Thus, these studies demonstrate that fundamental transcriptional network governing pluripotency closely related with chromatin remodeling and signaling pathways.
The retroviral vector systems are widely used as a gene transfer tools for both clinical gene therapy and basic research because of their high transduction efficiency and well-known mechanism. The retroviral vector used in previous studies is a Moloney murine leukaemia virus(MMLV)-based retrovirus vector, and the transgenes were driven by the 5’ MMLV long terminal repeat (LTR) promoter, which usually is silenced in ES and EC cells.Indeed, reprogramming factors were silenced by DNA methylation after reprogrammed into iPS cells. However, the MMLV LTR promoter often became spontaneously reactivated and drove c-Myc expression on differentiation of the iPS cells, which subsequently cause tumor formation in iPS cell-derived chimeric mice (44). Moreover, the random insertion of viral genes into host genome caused severe genetic modification, such as activation or inactivation of host genes, resulting in tumor formation. Therefore, in reprogramming by retroviral vector system, several major challenges must be overcome, before it can be considered clinical tools. To be used in clinical trials, iPS cells have to be generated with a safe method. A number of strategies have been recently performed, including delivering reprogramming factors via the non-integrating adeno-or Sendai-virus, transient plasmid, protein and synthetic mRNA (Table 1).
Table 1.
Integration-free iPS generation methods
| Deliveries | Factors | Species | Efficiency | Reference |
|---|---|---|---|---|
|
| ||||
| Adenovirus | OKSM | Mouse | 0.0006~0.0018% | 44 |
| Sendai virus | OKSM | Human | 0.1% | 46 |
| Plasmid | OKSM | Mouse | 0.0001~0.001% | 45 |
| Single polycistronic plasmid | OKSM | Mouse | 0.1% | 47 |
| Purified recombinant protein from E. coli | OKSM+VPA | Mouse | 0.006% | 51 |
| Whole protein extracts from genetically engineered cell | OKSM | Human | 0.001% | 52 |
| Synthetic mRNA | OKSM | Human | ~1% | 53 |
Abbreviations: O, Oct4; K, Klf4; S, Sox2; M, c-Myc; VPA, valproic acid.
The first exogene-free iPS cells were generated from adult mouse hepatocytes (45) and from mouse embryonic fibroblasts (MEFs) (46) by adenoviral infection and plasmid transfection, respectively. These experiments provided the proof of principle that transient expression of the four reprogramming factors is indeed sufficient to induce pluripotency in somatic cells. Somatic cells have also been reprogrammed into iPS cells with Sendai virus (47) and single polycistronic vectors (48). However, reprogramming efficiency by non-integrating vector systems is much lower than that by retroviral vector system. To avoid this drawback, several groups have developed integration-dependent gene delivery vectors with incorporated loxP sites that can be subsequently excised from the host genome by transient expression of Cre recombinase (49). Each of the floxed reprogramming factor gene was independently integrated into different sites within the host genome, but activation of Cre recombinase resulted in multiple excisions of transgenes, which potentially lead to genome rearrangement and genomic instability. Townes group recently generated iPS cells using a single polycistron encoding the 2A sequence-linked reprogramming factors in a self-inactivating (SIN) lentiviral vector with a loxP site in the truncated 5’ and 3’ LTR (50). However, exogenous sequences still harbor in these iPS cells. Another approach to generate exogene-free iPS cells is to use the piggyBac transposons, mobile genetic materials that can be introduced into and removed from the host genome by transient expression of transposase (51). However, it also remains unclear whether the expression of transposase induces nonspecific genomic alteration in iPS cells.
In 2009, two groups reported that DNA-free iPS (protein-based iPS, so called piPS) cells have been derived from mouse and human fibroblasts by delivering four reprogramming factors as recombinant proteins (52, 53). The two methods differ in several important respects.Sheng Ding’ s method use proteins that were expressed in E. coli inclusion body, which was then solubilized, refolded and purified. However, this method required additional treatment of histone deacetylase (HDAC) inhibitor, valproic acid (VPA), to facilitate chromatin remodeling. The mouse piPS cells fulfilled the criteria for pluripotent stem cells including chimera formation and germline transmission. On the other hand, Kim’ s group used whole protein extracts from genetically engineered HEK293 cells that expressed high levels of the four reprogramming factors. They generated human iPS cells without use of small molecules. In both methods, repeated transduction of protein to somatic cells was needed for the successful generation of iPS cells, which were free of nucleic acid delivery. Although the reprogramming efficiency is extremely low (0.001∼0.006%), the expression system of reprogramming factors in E. coli and mammalian cells facilitates the large-scale production of recombinant protein and quality control of the established iPS cell in future.
In late 2010, a more efficient (∼1%) and safe way to produce integration-free iPS cells induced by the introduction of synthetic mRNAs encoding the reprogramming factors into somatic cells were validated by the Rossi group (54). These synthetic mRNAs transfected into human somatic cells are translated in the cytoplasm and does not cause permanent genetic change. The introduction of synthetic mRNA as a means to manipulate protein expression in target cells was first used in gain-of-function experiments to dissect early embryogenesis of the Xenopus laevis. However, synthetic mRNA led to cytotoxicity due to the interferon-mediated innate immune response in human. It is known that exogenous single-stranded RNA (ssRNA) activates antiviral defenses in mammalian cells through interferon- and NF- K B-dependent pathways. In addition, these synthetic mRNAs have some drawbacks, such as low translation efficiency, instability of the delivered mRNA. To induce reprogramming using mRNA, Rossi and colleagues modified the original technology to solve these problems. First, in an attempt to further reduce innate immune responses to transfected RNA, they modified ribonucleoside bases of synthetic mRNA. Complete substitution of either 5-methylcytidine (5mC) for cytidine or pseudouridine for uridine in transcripts markedly improved viability and increased ectopic protein expression. Second, media supplementation with a recombinant B18R protein, which is a Vaccinia virus decoy receptor for type I interferon, reduced cytotoxicity of the RNA.The combination of these approaches ultimately led to successful RNA-mediated reprogramming of target cells.Although mRNA-based reprogramming is technically complex, this method eliminate the risk of genome alteration by exogenous sequences, which is a great advance for future clinical application.
Conclusion
Pluripotential reprogramming indicates that somatic cells could be reprogrammed into ES cell-like pluripotent cells by somatic cell nuclear transfer, cell fusion-induced reprogramming, transcription factor transduction, and modifying cell culture condition. Since Yamanaka group’ s first report of iPS cell generation in mouse by the retroviral transduction of Oct4, Sox2, Klf4 and c-Myc, this pioneering study stunned the stem cell society because of the great clinical potential of these iPS cells. Prior to this breakthrough study, nuclear reprogramming may constitute an attractive alternative to reprogramming somatic cells by SCNT and cell fusion. However, these two approaches for clinical application have several problems, including technical difficulty, ethical concern, polyploidy of resulting cells, and non-autologous gene expression.Therefore, iPS cell derivation is ethically and technically more feasible than SCNT or cell fusion. In order to use iPS cells as an efficient research tool and a useful technology for clinical application, suitable techniques to deliver reprogramming factor into a cell and efficient methods to identify the faithfully reprogrammed cells are crucial. Hence, researchers have developed efficient and safe approaches to generate iPS cells without transgene reactivation,viral integration and genetic alterations. To this end, several numbers of strategies were recently developed:using the non-integrating adeno- and sendai-virus,plasmid vector, recombinant protein, or synthetic mRNA delivery. So far, most studies concerning iPS cells have focused on how to reprogram somatic cells efficiently and safely. This approach will be also the future direction for iPS cells and reprogramming study.
Acknowledgments
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (Grant 20100008528).
Potential conflict of interest
The authors have no conflicting financial interest.
References
- 1.Evans MJ, Kaufman MH. Establishment in culture of pluripotentialcells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 2.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem celllines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 3.Fulka J, Jr, Loi P, Fulka H, Ptak G, Nagai T. Nucleus transferin mammals: noninvasive approaches for the preparationof cytoplasts. Trends Biotechnol. 2004;22:279–283. doi: 10.1016/j.tibtech.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Briggs R, King TJ. Transplantation of living nuclei fromblastula cells into enucleated frogs' eggs. Proc Natl Acad Sci USA. 1952;38:455–463. doi: 10.1073/pnas.38.5.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammaliancells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 6.Schnieke AE, Kind AJ, Ritchie WA, Mycock K, Scott AR, Ritchie M, Wilmut I, Colman A, Campbell KH. Human factorIX transgenic sheep produced by transfer of nuclei fromtransfected fetal fibroblasts. Science. 1997;278:2130–2133. doi: 10.1126/science.278.5346.2130. [DOI] [PubMed] [Google Scholar]
- 7.Wakayama T, Perry AC, Zuccotti M, Johnson KR, Yanagimachi R. Full-term development of mice from enucleatedoocytes injected with cumulus cell nuclei. Nature. 1998;394:369–374. doi: 10.1038/28615. [DOI] [PubMed] [Google Scholar]
- 8.Kato Y, Tani T, Sotomaru Y, Kurokawa K, Kato J, Doguchi H, Yasue H, Tsunoda Y. Eight calves cloned from somaticcells of a single adult. Science. 1998;282:2095–2098. doi: 10.1126/science.282.5396.2095. [DOI] [PubMed] [Google Scholar]
- 9.Baguisi A, Behboodi E, Melican DT, Pollock JS, Destrempes MM, Cammuso C, Williams JL, Nims SD, Porter CA, Midura P, Palacios MJ, Ayres SL, Denniston RS, Hayes ML, Ziomek CA, Meade HM, Godke RA, Gavin WG, Overström EW, Echelard Y. Production of goats by somaticcell nuclear transfer. Nat Biotechnol. 1999;17:456–461. doi: 10.1038/8632. [DOI] [PubMed] [Google Scholar]
- 10.Betthauser J, Forsberg E, Augenstein M, Childs L, Eilertsen K, Enos J, Forsythe T, Golueke P, Jurgella G, Koppang R, Lesmeister T, Mallon K, Mell G, Misica P, Pace M, Pfister-Genskow M, Strelchenko N, Voelker G, Watt S, Thompson S, Bishop M. Production of cloned pigs from invitro systems. Nat Biotechnol. 2000;18:1055–1059. doi: 10.1038/80242. [DOI] [PubMed] [Google Scholar]
- 11.Shin T, Kraemer D, Pryor J, Liu L, Rugila J, Howe L, Buck S, Murphy K, Lyons L, Westhusin M. A cat cloned by nucleartransplantation. Nature. 2002;415:859. doi: 10.1038/nature723. [DOI] [PubMed] [Google Scholar]
- 12.Chesné P, Adenot PG, Viglietta C, Baratte M, Boulanger L, Renard JP. Cloned rabbits produced by nuclear transferfrom adult somatic cells. Nat Biotechnol. 2002;20:366–369. doi: 10.1038/nbt0402-366. [DOI] [PubMed] [Google Scholar]
- 13.Woods GL, White KL, Vanderwall DK, Li GP, Aston KI, Bunch TD, Meerdo LN, Pate BJ. A mule cloned from fetalcells by nuclear transfer. Science. 2003;301:1063. doi: 10.1126/science.1086743. [DOI] [PubMed] [Google Scholar]
- 14.Galli C, Lagutina I, Crotti G, Colleoni S, Turini P, Ponderato N, Duchi R, Lazzari G. Pregnancy: a clonedhorse born to its dam twin. Nature. 2003;424:635. doi: 10.1038/424635a. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Q, Renard JP, Le Friec G, Brochard V, Beaujean N, Cherifi Y, Fraichard A, Cozzi J. Generation of fertile clonedrats by regulating oocyte activation. Science. 2003;302:1179. doi: 10.1126/science.1088313. [DOI] [PubMed] [Google Scholar]
- 16.Lee BC, Kim MK, Jang G, Oh HJ, Yuda F, Kim HJ, Hossein MS, Kim JJ, Kang SK, Schatten G, Hwang WS. Dogs cloned from adult somatic cells. Nature. 2005;436:641. doi: 10.1038/436641a. [DOI] [PubMed] [Google Scholar]
- 17.Kim MK, Jang G, Oh HJ, Yuda F, Kim HJ, Hwang WS, Hossein MS, Kim JJ, Shin NS, Kang SK, Lee BC. Endangered wolves cloned from adult somatic cells. Cloning Stem Cells. 2007;9:130–137. doi: 10.1089/clo.2006.0034. [DOI] [PubMed] [Google Scholar]
- 18.Bortvin A, Eggan K, Skaletsky H, Akutsu H, Berry DL, Yanagimachi R, Page DC, Jaenisch R. Incomplete reactivationof Oct4-related genes in mouse embryos clonedfrom somatic nuclei. Development. 2003;130:1673–1680. doi: 10.1242/dev.00366. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka S, Oda M, Toyoshima Y, Wakayama T, Tanaka M, Yoshida N, Hattori N, Ohgane J, Yanagimachi R, Shiota K. Placentomegaly in cloned mouse concepti caused by expansion of the spongiotrophoblast layer. Biol Reprod. 2001;65:1813–1821. doi: 10.1095/biolreprod65.6.1813. [DOI] [PubMed] [Google Scholar]
- 20.Ogonuki N, Inoue K, Yamamoto Y, Noguchi Y, Tanemura K, Suzuki O, Nakayama H, Doi K, Ohtomo Y, Satoh M, Nishida A, Ogura A. Early death of mice cloned from somatic cells. Nat Genet. 2002;30:253–254. doi: 10.1038/ng841. [DOI] [PubMed] [Google Scholar]
- 21.Kishigami S, Mizutani E, Ohta H, Hikichi T, Thuan NV, Wakayama S, Bui HT, Wakayama T. Significant improvementof mouse cloning technique by treatment with trichostatin A after somatic nuclear transfer. Biochem Biophys Res Commun. 2006;340:183–189. doi: 10.1016/j.bbrc.2005.11.164. [DOI] [PubMed] [Google Scholar]
- 22.Munsie MJ, Michalska AE, O|Brien CM, Trounson AO, Pera MF, Mountford PS. Isolation of pluripotent embryonic stemcells from reprogrammed adult mouse somatic cell nuclei. Curr Biol. 2000;10:989–992. doi: 10.1016/s0960-9822(00)00648-5. [DOI] [PubMed] [Google Scholar]
- 23.Wakayama T, Tabar V, Rodriguez I, Perry AC, Studer L, Mombaerts P. Differentiation of embryonic stem cell linesgenerated from adult somatic cells by nuclear transfer. Science. 2001;292:740–743. doi: 10.1126/science.1059399. [DOI] [PubMed] [Google Scholar]
- 24.Stojkovic M, Stojkovic P, Leary C, Hall VJ, Armstrong L, Herbert M, Nesbitt M, Lako M, Murdoch A. Derivation ofa human blastocyst after heterologous nuclear transfer toonated oocytes. Reprod Biomed Online. 2005;11:226–231. doi: 10.1016/s1472-6483(10)60962-5. [DOI] [PubMed] [Google Scholar]
- 25.Tada M, Tada T, Lefebvre L, Barton SC, Surani MA. Embryonic germ cells induce epigenetic reprogramming ofsomatic nucleus in hybrid cells. EMBO J. 1997;16:6510–6520. doi: 10.1093/emboj/16.21.6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tada M, Takahama Y, Abe K, Nakatsuji N, Tada T. Nuclear reprogramming of somatic cells by in vitro hybridization with ES cells. Curr Biol. 2001;11:1553–1558. doi: 10.1016/s0960-9822(01)00459-6. [DOI] [PubMed] [Google Scholar]
- 27.Do JT, Han DW, Gentile L, Sobek-Klocke I, Stehling M, Schöler HR. Enhanced reprogramming of Xist by induced upregulation of Tsix and Dnmt3a. Stem Cells. 2008;26:2821–2831. doi: 10.1634/stemcells.2008-0482. [DOI] [PubMed] [Google Scholar]
- 28.Cowan CA, Atienza J, Melton DA, Eggan K. Nuclear reprogrammingof somatic cells after fusion with human embryonic stem cells. Science. 2005;309:1369–1373. doi: 10.1126/science.1116447. [DOI] [PubMed] [Google Scholar]
- 29.Flasza M, Shering AF, Smith K, Andrews PW, Talley P, Johnson PA. Reprogramming in inter-species embryonal carcinoma-somatic cell hybrids induces expression of pluripotency and differentiation markers. Cloning Stem Cells. 2003;5:339–354. doi: 10.1089/153623003772032844. [DOI] [PubMed] [Google Scholar]
- 30.Do JT, Schöler HR. Nuclei of embryonic stem cells reprogram somatic cells. Stem Cells. 2004;22:941–949. doi: 10.1634/stemcells.22-6-941. [DOI] [PubMed] [Google Scholar]
- 31.Kimura H, Tada M, Nakatsuji N, Tada T. Histone code modifications on pluripotential nuclei of reprogrammed somatic cells. Mol Cell Biol. 2004;24:5710–5720. doi: 10.1128/MCB.24.13.5710-5720.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhutani N, Brady JJ, Damian M, Sacco A, Corbel SY, Blau HM. Reprogramming towards pluripotency requires AIDdependent DNA demethylation. Nature. 2010;463:1042–1047. doi: 10.1038/nature08752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 34.Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 36.Chang MY, Kim D, Kim CH, Kang HC, Yang E, Moon JI, Ko S, Park J, Park KS, Lee KA, Hwang DY, Chung Y, Lanza R, Kim KS. Direct reprogramming of rat neural precursor cells and fibroblasts into pluripotent stem cells. PLoS One. 2010;5:e9838. doi: 10.1371/journal.pone.0009838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu H, Zhu F, Yong J, Zhang P, Hou P, Li H, Jiang W, Cai J, Liu M, Cui K, Qu X, Xiang T, Lu D, Chi X, Gao G, Ji W, Ding M, Deng H. Generation of induced pluripotent stem cells from adult rhesus monkey fibroblasts. Cell Stem Cell. 2008;3:587–590. doi: 10.1016/j.stem.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 38.West FD, Terlouw SL, Kwon DJ, Mumaw JL, Dhara SK, Hasneen K, Dobrinsky JR, Stice SL. Porcine induced pluripotent stem cells produce chimeric offspring. Stem Cells Dev. 2010;19:1211–1220. doi: 10.1089/scd.2009.0458. [DOI] [PubMed] [Google Scholar]
- 39.Honda A, Hirose M, Hatori M, Matoba S, Miyoshi H, Inoue K, Ogura A. Generation of induced pluripotent stem cellsin rabbits: potential experimental models for human regenerative medicine. J Biol Chem. 2010;285:31362–31369. doi: 10.1074/jbc.M110.150540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y, Cang M, Lee AS, Zhang K, Liu D. Reprogramming of sheep fibroblasts into pluripotency under a drug-inducible expression of mouse-derived defined factors. PLoS One. 2011;6:e15947. doi: 10.1371/journal.pone.0015947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huangfu D, Maehr R, Guo W, Eijkelenboom A, Snitow M, Chen AE, Melton DA. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008;26:795–797. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi Y, Desponts C, Do JT, Hahm HS, Schöler HR, Ding S. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell. 2008;3:568–574. doi: 10.1016/j.stem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Silva J, Barrandon O, Nichols J, Kawaguchi J, Theunissen TW, Smith A. Promotion of reprogramming to ground statepluri potency by signal inhibition. PLoS Biol. 2008;6:e253. doi: 10.1371/journal.pbio.0060253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K, Chiba T, Yamanaka S. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321:699–702. doi: 10.1126/science.1154884. [DOI] [PubMed] [Google Scholar]
- 45.Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chatterjee S, Chaklader M, Basak P, Das P, Das M, Pereira JA, Dutta RK, Chaudhuri S, Law S. An animal model of chronic aplastic bone marrow failure following pesticide exposure in mice. Int J Stem Cell. 2010;3:54–62. doi: 10.15283/ijsc.2010.3.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seki T, Yuasa S, Oda M, Egashira T, Yae K, Kusumoto D, Nakata H, Tohyama S, Hashimoto H, Kodaira M, Okada Y, Seimiya H, Fusaki N, Hasegawa M, Fukuda K. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell. 2010;7:11–14. doi: 10.1016/j.stem.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez F, Barragan Monasterio M, Tiscornia G, Montserrat Pulido N, Vassena R, Batlle Morera L, Rodriguez Piza I, Izpisua Belmonte JC. Generation of mouse-induced pluripotent stem cells by transient expression of a single nonviral polycistronic vector. Proc Natl Acad Sci USA. 2009;106:8918–8922. doi: 10.1073/pnas.0901471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soldner F, Hockemeyer D, Beard C, Gao Q, Bell GW, Cook EG, Hargus G, Blak A, Cooper O, Mitalipova M, Isacson O, Jaenisch R. Parkinson|s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136:964–977. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang CW, Lai YS, Pawlik KM, Liu K, Sun CW, Li C, Schoeb TR, Townes TM. Polycistronic lentiviral vector for "hit and run" reprogramming of adult skin fibroblasts to induced pluripotent stem cells. Stem Cells. 2009;27:1042–1049. doi: 10.1002/stem.39. [DOI] [PubMed] [Google Scholar]
- 51.Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, Hämäläinen R, Cowling R, Wang W, Liu P, Gertsenstein M, Kaji K, Sung HK, Nagy A. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, Kim KS. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, Trauger S, Bien G, Yao S, Zhu Y, Siuzdak G, Schöler HR, Duan L, Ding S. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, Daley GQ, Brack AS, Collins JJ, Cowan C, Schlaeger TM, Rossi DJ. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]