Abstract
Ligands of transforming growth factor beta (TGF-β) family members have been implicated in the development and patho-physiological process of various organs. Embryonic stem cells (ESCs) are characterized by their ability to proliferate indefinitely and differentiated into all three germ layer cells, which are termed as pluripotency and self-renewal,respectively. For successful therapeutic application of ESCs, it is essential to understand the mechanisms underlying self-renewal and pluripotency, which involve complex networks among key factors including transcription factors, epigenetic control, microRNAs and signaling pathways. In this review, we discuss recent progress on the function of TGF beta family ligands and their canonical SMAD signaling in the maintenance of ESC’ s identity.
Keywords: TGF-beta, Nodal, Activin, Lefty, Embryonic stem cell, Pluripotency, Reprogramming
TGF beta family and pathway signaling
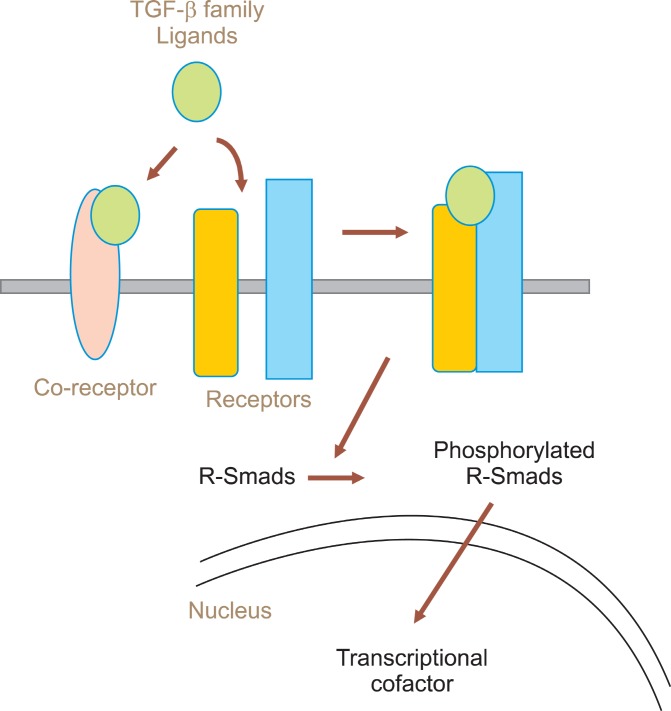
The TGF-β superfamily is consisted with more than 100 proteins including a multifunctional cytokine TGF-β, activin, nodal, inhibin, lefty, bone morphogenetic proteins(BMPs), one class of growth and differentiation factors (GDFs). Receptors of TGF-β family are classified as three different classes such as type I (TGFRI, also termed activin-like kinases (ALKs)), type II (TGFRII) and type III (TGFRIII) (1). Receptor type I and type II contains intracellular domain with serine threonine kinase activity (2-4). Receptors are activated by the binding of TGF-β family ligands and interact with the family of Smad protein to activate them by phosphorylation within the cytoplasm, and then translocate the phosphorylated Smad proteins into nucleus (Fig. 1). In the nucleus, Smad proteins function as transcriptional cofactors to activate target genes to determine the cell fate upon external stimulation. Depending upon their structure and on their function, Smad proteins are divided into three groups; receptorregulated “R-Smads” (Smad1, Smad2, Smad3, Smad5,
Fig. 1. Ligands and coreceptor molecules control the access of TGF-β family ligands to signaling receptors. Upon binding of ligands, receptor-Smad proteins are phosphorylated by serine-threonine kinase activity of the receptors.
Smad8), “Co-Smads”(Smad4), and inhibitory “I-Smads” (Smad6, Smad7) (5).
The TGF-β superfamily members can be divided into two distinct branches. Factors such as activin, nodal, myostatin, lefty and TGF-β are clustered in one family branch, and BMPs are grouped into the other branch (Table 1) (6-8).
Table 1.
Ligand-receptor-coreceptor-Smad relationships in the TGF-β and BMP branches of the TGF-β family
| Ligand | Receptor II | Receptor I | Coreceptor | Smad | |
|---|---|---|---|---|---|
|
| |||||
| TGF-β1, 2, 3 | TβR-II | TβR-I | Betaglucan | ||
| Activin-A, B, C | ActR-IIA,B | ALK4 | |||
| Myostatin | ActR-IIA,B | ALK4 | |||
| TGF-β | Nodal | ActR-IIA,B | ALK4,7 | Cripto | Smad2,3 with Smad4 |
| GDF-1,3 | ActR-IIA,B | ALK4,7 | Cruoti | ||
| Inhibin | ActR-IIA,B | - | Betaglycan | ||
| Lefty-1,2 | ActR-IIA,B | - | Cripto | ||
|
| |||||
| BMP-2,4 | BMPR-II, ActR-II | BMPR-IA,B | RMG-a,b,c | ||
| BMP-5,6,7 | BMPR-II, ActR-II | BMPR-1A, ALK2 | |||
| BMP | BMP-9,10 | BMPR-II, ActR-II | ALK1 | Endoglin | Smad1,5,8 with Smad4 |
| GDF-5,6,7 | BMPR-II, ActR-II | BMPR-1A,B | |||
| AMHY/MIS | AMHR-II | BMPR-1A, ALK2 | |||
The BMP ligands bind to type I receptor ALK2/3/6 and leads to phosphorylation of the transcription factors Smad1/5/8, which are subsequently translocated into nucleus. In the other branch, Activin/Nodal/TGF-β ligands activates type I receptors, ALK4/5/7, and phosphorylates Smad2/3 (9).
Among the TGF-β superfamily members, Lefty is the only inhibitor that is highly enriched in stem cells. Lefty locus contains two genes with the same transcriptional orientation in human, mice, and zebrafish. Human Lefty1 is identical to mice LeftyB and Lefty2 is identical to human LeftyA (10-13). LeftyB has 96% sequence identity with LeftyA and these two proteins differ only in 16 amino acids. However, LeftyB has only 82% sequence identity with Lefty1, which suggests that Lefty proteins has been evolved independently in mouse and humans after the duplication of a single Lefty gene (10).
TGF beta family pathway in the maintenance of pluripotency of embryonic stem cells
Members of TGF-β superfamily are enriched in stem cells, which suggests that these proteins are involved in the ES cell identity. The stemness of human and mouse ESCs can be maintained by co-culture with appropriate feed cells, like mouse embryonic fibroblasts (MEFs), which provide an essential cytokine, leukemia inhibitory factor (LIF), to maintain ESCs’ identity. BMP-4 is another cytokine provided by feeder cells to maintain the ESCs’ properties. It inhibits extracellular receptor kinase (ERK) and p38 mitogen-activated protein kinase (MAPK) pathway (14). BMP-4 also sustains self-renewal of mESCs in concert with LIF through the induction of the helix-loophelix protein Id (Inhibitor of differentiation) (15). Since BMPs are known to be a potent inhibitors of neural differentiation in vertebrate embryos (16), the BMP activity in ESCs may also be mediated by its inhibitory effects on neuronal differentiation of mESCs.
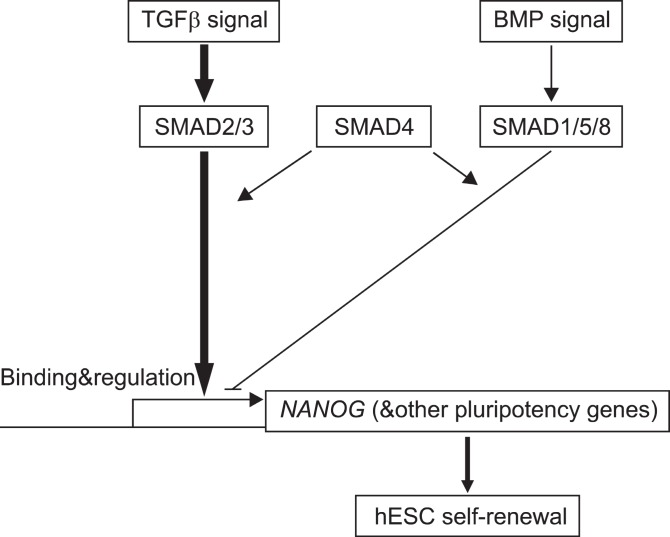
Nodal signals are also contributes to maintain the ES cell identity by the finding that Nodal-deficient mouse embryos exhibit an epiblast with very low levels of Oct-3/4 expression (17, 18). Moreover, nuclear localization of phosphorylated Smad2, which is induced by TGF-β, activin,or nodal signaling were observed in hESCs and it is decreased upon differentiation of the cells (19). Microarray analysis shows that activin supports the maintenance of pluripotency of hESCs possibly by inducing the expression of Oct4 and Nanog (20). Recently, it was reported that Actin/Nodal signaling maintains pluripotency of hESCs and mouse EpiSCs by controlling Nanog expression,which leads to block neuroectoderm differentiation of pluripotency cells (21). Furthermore, TGF-β/ activinmediated SMADs directly binds to the Nanog promoter in hESCs (Fig. 2) (22).
Fig. 2. Model of SMAD regulation of Nanog transcription in human ESCs (22).
Consistently, chemical inhibition of Smad2 phosphorylation with SB-431542 results in decreases of the expression of markers of undifferentiated ESCs (19, 23). These results suggest that Activin or Nodal produced by ESCs itself functions to promote the self-renewal of mESCs.
Lefty protein is another TGF-β family member that is highly expressed in human and mouse ESCs. Transcriptional expression of Lefty gene is regulated by Oct3/4, Sox2 and Klf4, which are core transcription factors in maintaining stemness of ESCs (24). Klf4 acts as a mediating factor that coorporates with Oct3/4 and Sox2 and occupy the proximal element of the Lefty1 promoter to activate the gene. Recently, it was shown that activation of canonical WNT signaling by inactivating GSK-3β leads to the high expression of Nodal, LeftyA and LeftyB in hESCs (25). Induction of Lefty expression by inhibiting WNT signaling requires ALKs4/5/7 (25). Inhibition of WNT pathway leads to the activation of Smad signaling (26). There are five potential binding sites for the Smad2/3 heterodimer in the promoter region of LeftyA and Lefty2 (25). Activation of Smad2/3 by the treatment of differentiating hESCs with Activin leads to the expression of Nodal, LeftyA, and LeftyB in hESCs (27). Consistently, inhibition of ALK4/5/7 by the kinase inhibitor, SB431542, which blocks activation of Smad2/3, downregulates the expression of LeftyA and B in the undifferentiated stem cells (25). These findings suggest that expression of Lefty in undifferentiated ESCs is mediated by ALK4/5/7- and Smad2/3- signaling pathway.
Taken together, TGF-β family signals play crucial roles in the maintenance of ESC’ s self-renewal and pluripotency both in human and mouse.
TGF beta family pathway during the differentiation of embryonic stem cells
TGF family members orchestrate diverse processes during embryogenesis. The canonical Smad signaling pathway involves a large numbers of Smad-interacting proteins, many of which are transcription factors. This signaling system establishes the basic differentiation plan of the embryo and drives morphogenetic processes in the embryo and fetus.
When the culture condition that maintain the undifferentiated state of ESCs are changed to induce differentiation of the cells, ESCs start to generate progeny that consists of derivatives of all three germ layers (28). For this reason, ESCs have been used as an in vitro experimental model to understand early stages of embryogenesis, which generates ectoderm, mesoderm and endoderm during gastrulation.
Differentiation of ESCs into ectoderm is often referred to as the default pathway, since neuroectoderm rapidly develops from ESCs in the culture media without serum.Recently, it was reported that endogenous retinoic acid (RA) production from vitamin A in the medium promotes development of neural progenitors from mESCs by suppressing Wnt-dependent Nodal signaling (29). In mESCs, BMPs inhibit the formation of neuroectoderm and induce its epidermal differentiation (30).
When BMP is added to mESCs in the presence of VEGF, it is able to induce hematopoietic differentiation of the cells in serum free culture (31). ER71, a number of the Ets transcription factor family, is a downstream target of BMPs and functions to regulate the generation of Flk+ blood and vessel progenitors (32). BMP signals initially induce mesoderm and later specify blood lineages via activation of the Cdx-Hox pathway and Wnt signaling (33).
BMP signals also promote the proliferation of mESCs derived endothelial cells by inducing the expression of Tie2 and Flk1, both of genes are known to promote endothelial-cell proliferation (34). In addition, TGF-β and activin inhibit the proliferation of endothelial cells by inducing the expression of p21 (35). These results suggest that TGF-β family signals play crucial roles during vascular development from mESCs. At the early state of ESC’ s differentiation, primitive streak-like populations are formed,which are further differentiated into endoderm by the sustained activation of Nodal/activin signals. Endoderm progenitors are generated by culturing ESCs in the presence of activin in serum-free conditions and subsequent culture of progenitors with BMP-4, activin, and fibroblast growth factor (FGF) produce highly enriched hepatic populations that expressing albumins (36). When activin and BMP-4 are added into ESCs’ differentiation culture media at various steps, Pdx1 (pancreatic and duodenal homeobox gene 1) positive cells are generated at a high efficacy (30%) (37).
During the formation of mesendoderm lineage, Smad2 mediates Activin/Nodal signaling in mESCs (38).
Taken together, these findings suggest that ectoderm is differentiated from human and mouse ESCs in the absence of TGF-β signal, while mesoderm and endoderm is differentiated by a combination of TGF-β family signals.
TGF beta family pathway during the reprogramming of differentiated cells
The fully differentiated somatic cells can reverse gene expression profile to that of a pluripotent cell through transgenic overexpression of a defined set of reprogramming factors-Oct4, Klf4, Sox2 and c-Myc (39). When TGF-β signaling pathway is blocked by the antibody mediated depletion of ligands or SB-431542 mediated inhibition of Alk-4, -5, -7, the reprogramming efficiency is significantly increased (40, 41). Moreover, a novel chemical, which inhibits a TGF-β receptor kinase, is capable of replacing the function of Sox2 during the reprogramming of mouse embryonic stem cells (MEFs). Ichida et al (2009) found that treatment of TGF-β signaling inhibitors to partial reprogrammed cells highly activated Id1, -2 and -3. This result suggest that TGF- β/Smad/BMP signaling plays an important role to activate pluripotency network during the process of reprogramming, possibly by regulating the efficiency of reprogrammed cells to enter the ground state of mESCs. However, TGF-β inhibition had no effect on human reprogramming, which is consistent with the fact that BMP activation in the absence of TGF-β/activin signaling promotes the differentiation of hESCs (22).
Conclusion
ESCs are useful sources for the therapeutic application of stem cells. To account for the unique properties of pluripotent ESCs or reprogrammed induced pluripotent stem cells, it will be necessary to understand the endogenous signaling network, which is connected with transcriptional regulatory mechanism responsible for maintaining ESC’ s identity. Unlike the conserved roles of core transcription factors like Nanog, Oct3/4, Sox2, and c-Myc etc to maintain pluripotency, mouse and human ESCs operate different internal mechanism to extrinsic signals. Among those systems, TGF-β family members have been recognized as crucial for both human and mouse ESC’ s cell fate determination. Therefore, understanding the effects and mechanisms of TGF-β family signals in human and mouse ESCs as well as reprogrammed iPS cells both in pluripotent state and in differentiation state is essential for applying ESCs to therapeutic cell therapy.
Acknowledgments
This work was supported by a grant of the Korea Healthcare Technology R & D Project, Ministry of Health, Welfare & Family Affairs, Republic of Korea (A091087-0911-0000200).
Potential Conflict of Interest
The authors have no conflicting financial interest.
References
- 1.Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- 2.Massagué J. Receptors for the TGF-beta family. Cell. 1992;69:1067–1070. doi: 10.1016/0092-8674(92)90627-o. [DOI] [PubMed] [Google Scholar]
- 3.Massagué J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 4.Massagué J, Chen YG. Controlling TGF-beta signaling. Genes Dev. 2000;14:627–644. [PubMed] [Google Scholar]
- 5.Dennler S, Goumans MJ, ten Dijke P. Transforming growth factor beta signal transduction. J Leukoc Biol. 2002;71:731–740. [PubMed] [Google Scholar]
- 6.Derynck R, Akhurst RJ. Differentiation plasticity regulated by TGF-beta family proteins in development and disease. Nat Cell Biol. 2007;9:1000–1004. doi: 10.1038/ncb434. [DOI] [PubMed] [Google Scholar]
- 7.Roberts AB, Wakefield LM. The two faces of transforming growth factor beta in carcinogenesis. Proc Natl Acad Sci U S A. 2003;100:8621–8623. doi: 10.1073/pnas.1633291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 9.Sakuma R, Ohnishi Yi Y, Meno C, Fujii H, Juan H, Takeuchi J, Ogura T, Li E, Miyazono K, Hamada H. Inhibition of Nodal signalling by Lefty mediated through interaction with common receptors and efficient diffusion. Genes Cells. 2002;7:401–412. doi: 10.1046/j.1365-2443.2002.00528.x. [DOI] [PubMed] [Google Scholar]
- 10.Yashiro K, Saijoh Y, Sakuma R, Tada M, Tomita N, Amano K, Matsuda Y, Monden M, Okada S, Hamada H. Distinct transcriptional regulation and phylogenetic divergence of human LEFTY genes. Genes Cells. 2000;5:343–357. doi: 10.1046/j.1365-2443.2000.00329.x. [DOI] [PubMed] [Google Scholar]
- 11.Adachi H, Saijoh Y, Mochida K, Ohishi S, Hashiguchi H, Hirao A, Hamada H. Determination of left/right asymmetric expression of nodal by a left side-specific enhancer with sequence similarity to a lefty-2 enhancer. Genes Dev. 1999;13:1589–1600. doi: 10.1101/gad.13.12.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saijoh Y, Adachi H, Mochida K, Ohishi S, Hirao A, Hamada H. Distinct transcriptional regulatory mechanisms underlie left-right asymmetric expression of lefty-1 and lefty-2. Genes Dev. 1999;13:259–269. doi: 10.1101/gad.13.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agathon A, Thisse B, Thisse C. Morpholino knock-down of antivin1 and antivin2 upregulates nodal signaling. Genesis. 2001;30:178–182. doi: 10.1002/gene.1059. [DOI] [PubMed] [Google Scholar]
- 14.Qi X, Li TG, Hao J, Hu J, Wang J, Simmons H, Miura S, Mishina Y, Zhao GQ. BMP4 supports self-renewal of embryonic stem cells by inhibiting mitogen-activated protein kinase pathways. Proc Natl Acad Sci U S A. 2004;101:6027–6032. doi: 10.1073/pnas.0401367101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 16.Wilson PA, Hemmati-Brivanlou A. Induction of epidermis and inhibition of neural fate by Bmp-4. Nature. 1995;376:331–333. doi: 10.1038/376331a0. [DOI] [PubMed] [Google Scholar]
- 17.Conlon FL, Lyons KM, Takaesu N, Barth KS, Kispert A, Herrmann B, Robertson EJ. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development. 1994;120:1919–1928. doi: 10.1242/dev.120.7.1919. [DOI] [PubMed] [Google Scholar]
- 18.Robertson EJ, Norris DP, Brennan J, Bikoff EK. Control of early anterior-posterior patterning in the mouse embryo by TGF-beta signalling. Philos Trans R Soc Lond B Biol Sci. 2003;358:1351–1357. doi: 10.1098/rstb.2003.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James D, Levine AJ, Besser D, Hemmati-Brivanlou A. TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development. 2005;132:1273–1282. doi: 10.1242/dev.01706. [DOI] [PubMed] [Google Scholar]
- 20.Xiao L, Yuan X, Sharkis SJ. Activin A maintains self-renewal and regulates fibroblast growth factor, Wnt, and bone morphogenic protein pathways in human embryonic stem cells. Stem Cells. 2006;24:1476–1486. doi: 10.1634/stemcells.2005-0299. [DOI] [PubMed] [Google Scholar]
- 21.Vallier L, Mendjan S, Brown S, Chng Z, Teo A, Smithers LE, Trotter MW, Cho CH, Martinez A, Rugg-Gunn P, Brons G, Pedersen RA. Activin/Nodal signalling maintains pluripotency by controlling Nanog expression. Development. 2009;136:1339–1349. doi: 10.1242/dev.033951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu RH, Sampsell-Barron TL, Gu F, Root S, Peck RM, Pan G, Yu J, Antosiewicz-Bourget J, Tian S, Stewart R, Thomson JA. NANOG is a direct target of TGFbeta/activin-mediated SMAD signaling in human ESCs. Cell Stem Cell. 2008;3:196–206. doi: 10.1016/j.stem.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci. 2005;118:4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- 24.Nakatake Y, Fukui N, Iwamatsu Y, Masui S, Takahashi K, Yagi R, Yagi K, Miyazaki J, Matoba R, Ko MS, Niwa H. Klf4 cooperates with Oct3/4 and Sox2 to activate the Lefty1 core promoter in embryonic stem cells. Mol Cell Biol. 2006;26:7772–7782. doi: 10.1128/MCB.00468-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Besser D. Expression of nodal, lefty-a, and lefty-B in undifferentiated human embryonic stem cells requires activation of Smad2/3. J Biol Chem. 2004;279:45076–45084. doi: 10.1074/jbc.M404979200. [DOI] [PubMed] [Google Scholar]
- 26.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saijoh Y, Adachi H, Sakuma R, Yeo CY, Yashiro K, Watanabe M, Hashiguchi H, Mochida K, Ohishi S, Kawabata M, Miyazono K, Whitman M, Hamada H. Left-right asymmetric expression of lefty2 and nodal is induced by a signaling pathway that includes the transcription factor FAST2. Mol Cell. 2000;5:35–47. doi: 10.1016/s1097-2765(00)80401-3. [DOI] [PubMed] [Google Scholar]
- 28.Smith AG. Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol. 2001;17:435–462. doi: 10.1146/annurev.cellbio.17.1.435. [DOI] [PubMed] [Google Scholar]
- 29.Engberg N, Kahn M, Petersen DR, Hansson M, Serup P. Retinoic acid synthesis promotes development of neural progenitors from mouse embryonic stem cells by suppressing endogenous, Wnt-dependent nodal signaling. Stem Cells. 2010;28:1498–1509. doi: 10.1002/stem.479. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe K, Kamiya D, Nishiyama A, Katayama T, Nozaki S, Kawasaki H, Watanabe Y, Mizuseki K, Sasai Y. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci. 2005;8:288–296. doi: 10.1038/nn1402. [DOI] [PubMed] [Google Scholar]
- 31.Park C, Afrikanova I, Chung YS, Zhang WJ, Arentson E, Fong Gh G, Rosendahl A, Choi K. A hierarchical order of factors in the generation of FLK1- and SCL-expressing hematopoietic and endothelial progenitors from embryonic stem cells. Development. 2004;131:2749–2762. doi: 10.1242/dev.01130. [DOI] [PubMed] [Google Scholar]
- 32.Lee D, Park C, Lee H, Lugus JJ, Kim SH, Arentson E, Chung YS, Gomez G, Kyba M, Lin S, Janknecht R, Lim DS, Choi K. ER71 acts downstream of BMP, Notch, and Wnt signaling in blood and vessel progenitor specification. Cell Stem Cell. 2008;2:497–507. doi: 10.1016/j.stem.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lengerke C, Schmitt S, Bowman TV, Jang IH, Maouche-Chretien L, McKinney-Freeman S, Davidson AJ, Hammerschmidt M, Rentzsch F, Green JB, Zon LI, Daley GQ. BMP and Wnt specify hematopoietic fate by activation of the Cdx-Hox pathway. Cell Stem Cell. 2008;2:72–82. doi: 10.1016/j.stem.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki Y, Montagne K, Nishihara A, Watabe T, Miyazono K. BMPs promote proliferation and migration of endothelial cells via stimulation of VEGF-A/VEGFR2 and angiopoietin-1/Tie2 signalling. J Biochem. 2008;143:199–206. doi: 10.1093/jb/mvm215. [DOI] [PubMed] [Google Scholar]
- 35.Watabe T, Nishihara A, Mishima K, Yamashita J, Shimizu K, Miyazawa K, Nishikawa S, Miyazono K. TGF-beta receptor kinase inhibitor enhances growth and integrity of embryonic stem cell-derived endothelial cells. J Cell Biol. 2003;163:1303–1311. doi: 10.1083/jcb.200305147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gouon-Evans V, Boussemart L, Gadue P, Nierhoff D, Koehler CI, Kubo A, Shafritz DA, Keller G. BMP-4 is required for hepatic specification of mouse embryonic stem cell-derived definitive endoderm. Nat Biotechnol. 2006;24:1402–1411. doi: 10.1038/nbt1258. [DOI] [PubMed] [Google Scholar]
- 37.Shiraki N, Yoshida T, Araki K, Umezawa A, Higuchi Y, Goto H, Kume K, Kume S. Guided differentiation of embryonic stem cells into Pdx1-expressing regional-specific definitive endoderm. Stem Cells. 2008;26:874–885. doi: 10.1634/stemcells.2007-0608. [DOI] [PubMed] [Google Scholar]
- 38.Weli SC, Fink T, Cetinkaya C, Prasad MS, Pennisi CP, Zachar V. Notch and hedgehog signaling cooperate to maintain self-renewal of human embryonic stem cells exposed to low oxygen concentration. Int J Stem Cell. 2010;3:129–137. doi: 10.15283/ijsc.2010.3.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 40.Ichida JK, Blanchard J, Lam K, Son EY, Chung JE, Egli D, Loh KM, Carter AC, Di Giorgio FP, Koszka K, Huangfu D, Akutsu H, Liu DR, Rubin LL, Eggan K. A small-molecule inhibitor of tgf-Beta signaling replaces sox2 in reprogramming by inducing nanog. Cell Stem Cell. 2009;5:491–503. doi: 10.1016/j.stem.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maherali N, Hochedlinger K. Tgfbeta signal inhibition cooperates in the induction of iPSCs and replaces Sox2 and cMyc. Curr Biol. 2009;19:1718–1723. doi: 10.1016/j.cub.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]