Abstract
Background and Objectives:
Autosomal dominant polycystic kidney disease is a pathology mainly characterized by the progressive development and enlargement of cysts in each kidneys. Such as many adult epithelial tissue, renal tubule replaces damaged or death cells through the presence of stem/progenitor cells CD133+CD24+ Obviously, in ADPKD the repair of damages is insufficient to block the disease, but renal stem cells could have a role in the pathology. In this study we investigate the localization and the involvement of cells CD133+CD24+ in ADPKD progression.
Methods and Results:
Two normal kidneys and two ADPKD kidneys were examined. CD133 and CD24 expression was investigated by confocal microscopy and immunoblotting. Renal tissue and cells were analyzed. CD133 and CD24 have the same localization in ADPKD tissues and in normal kidneys: a subset of epithelial cells (PEC) of Bowman’ s capsule and luminal side of tubules. It is interesting that CD133+ CD24+ cells are statistically more represented in ADPKD tubules (p< 0.001) and in healthy glomeruli (p= 0.0016). Cysts express CD133 and CD24.
Conclusions:
Renal epithelial progenitors demonstrate to be involved in ADPKD pathogenesis but their role will have to be clarified and possibly managed to obtain improvement, or at least stabilization, of disease.
Keywords: Stem cells, CD133, kidney, ADPKD, Damage repair
Introduction
Autosomal Dominant Polycystic Kidney Disease (ADPKD) is a disease which can arise from mutations in either pkd1 (85%) or pkd2 (15%) with clinically indistinguishable manifestation (1). ADPKD affects over 1:1,000 of the world wide population and it causes 1:3,000 hospitalization. ADPKD is asymptomatic in about 50% of subjects with pkd genes mutated (2). It is a systemic disease associated with renal failure, intracranial arterial aneurysms, cardiac valvular defects, colonic diverticulosis and cyst formation in other organs such as liver, spleen and pancreas. Nevertheless ADPKD gives mainly renal manifestations: patients develop renal cysts which progressively lead to disruption of renal parenchyma, interstitial fibrosis, cellular infiltration and loss of functional nephrons. Renal degeneration finally progresses to end-stage kidney disease. APDKD accounts for up to 10% of all patients on renal replacement therapy (1).
Pkd-1 (chromosome 16) codes for a big transmembrane protein of 460 KDa involved in calcium permeation and in cell-cell/matrix interaction. Pkd-2 (chromosome 4) codes for a calcium channel of about 110 KDa. The polycystins form together a functional complex. In pathology, the alteration of this complex reduces Ca++ flow with absorption of water in tubules and cysts development (3, 4).
Previous studies have demonstrated a high rate of proliferation and apoptosis in the cysts of ADPKD patients (4). This unproductive attempt of tissue reparation suggests a likely involvement of renal progenitors.
During last years, many studies demonstrate the existence of a renopoietic system. Sagrinati et al. identified, in human adult kidneys, a subset of Parietal Epithelial Cells (PECs) in the Bowman’s capsule. PECs exhibit co-expression of stem cell markers CD24 and CD133 and absence of lineage-specific markers.
CD133 is a pentaspan transmembrane glycoprotein often expressed on adult stem cells, where it is thought to function in maintaining stem cell properties by suppressing differentiation and promoting proliferation. CD133 expression is also associated to several types of cancer (5-7). CD133 function is not clear but its presence on early and undifferentiated cells is suggestive of a growth factor receptor, and the presence of five tyrosine residues on the 50-aa cytoplasmic tail may indicate that the protein is phosphorylated in response to ligand binding and triggers a signal transduction (8).
CD24 is a sialoglycoprotein expressed on mature granulocytes (9), B and T lymphocyte subsets (10, 11), normal and cancer stem cells (12, 13). The protein is anchored via a glycosyl phosphatidylinositol to cell surface. It acts as adhesion molecule and is involved in cell migration and signal transduction.
CD24+ CD133+ PEC population, which could be purified from cultured capsulated glomeruli, revealed self-renewal potential and high cloning efficiency. Under appropriate culture conditions, individual clones of CD24+ CD133+ PEC could be induced to generate mature, functional, tubular cells with phenotypic features of proximal and/or distal tubules, osteogenic cells, adipocytes, and cells that exhibited phenotypic and functional features of neuronal cells (14).
A hierarchical distribution of renal progenitor cells was identified in adult human glomeruli. CD24+ CD133+ PECs are localized in the urinary pole and are in close contiguity with podocytes to the vascular stalk and with tubular renal cells to the other extremity. A transitional cell population displays features of either renal progenitors or podocytes and localizes between urinary pole and vascular pole. In the vascular stalk of the glomerulus, transitional cells are localized in close continuity with cells without progenitor markers but positive to podocyte markers. They will become committed podocytes. In the urinary pole, CD24+ CD133+ PECs will progressively become proximal tubular cells (15).
When CD24+ CD133+ PECs were isolated and injected into SCID mice with acute renal failure from glycerol-induced rhabdomyolysis, they induced regeneration of different portions of the nephron, reduced tissue necrosis and fibrosis and significantly improved renal function. No tumorigenic potential was observed (16). In a recent study, it was hypothesized that PECs were able to delay fibrotic response and inflammation process in chronic injury causes dysfunction of the tubular epithelial cells. This effect could allow to tissue regeneration rather than scar formation (17). Regenerative response in post-transplant acute tubular necrosis, underlying Delayed Graft Function (DGF), was characterized by an increase of proliferating PECs involved in repairing tubular damage (18).
ADPKD is characterized by expansion of cysts and tubular hyperplasia. So it is probable that PECs are involved in the pathophysiology of ADPKD through an attempt of unsuccessful tissue repair.
Materials and Methods
Antibodies
CD133 monoclonal (293C3) antibody for immunofluorescence was provided by Miltenyi Biotec (Miltenyi Biotec, Bergisch Gladbach, Germany). CD133 monoclonal (W6B9C1) antibody for Western blot was provided by Miltenyi Biotec (Miltenyi Biotec, Bergisch Gladbach, Germany). CD24 monoclonal (SN3) antibody was provided by Santa Cruz (Santa Cruz Biotechnology Inc., Santa Cruz CA, USA). PKD1 rabbit polyclonal (H-260) antibody was provided by Santa Cruz (Santa Cruz Biotechnology Inc., Santa Cruz CA, USA). Goat anti mouse IgG2b-Alexa 568 conjugate, goat anti mouse IgG2b-Alexa 633 conjugate, goat anti mouse IgG1-Alexa 568 conjugate, goat anti rabbit IgG-Rhod conjugate, goat anti mouse IgG-HRP conjugate were used as secondary antibodies.
Tissues
Normal kidney fragments was obtained from a cadaveric kidney donor. The kidney was not suitable for transplantation due to the incidental discovery of a neoplastic nodule of 5 cm at the lower pole upon macroscopic examination. The pathologic examination diagnosed a clear cell carcinoma (grade 2 according to Fuhrman classification; T1bN0M0 according to the International Union Against Cancer classification); the lower pole was excised and the remaining parenchyma was used in the following procedures. The remaining parenchyma did not reveal any evidence of clear cell carcinoma.
Polycystic kidney tissue was obtained from 2 ADPKD patients underwent to nephrectomy in preparation for receiving renal transplantation. All procedures were preceded by the approval of our Local Ethical Committee on human experimentation and informed consent was obtained from the patients.
Colture
Human Kidney 2 cells (HK-2) were maintained in DMEM F-12 (Invitrogen/GIBCO, Cralsbad, California) supplementated with 10% FBS (PAA laboratories GmbH, Pasching, Austria), 2,000 U/ml penicillin (PAA laboratories GmbH, Pasching, Austria), 2,000 ug/ml streptomycin (PAA laboratories GmbH, Pasching, Austria), and 20 mM L-glutamine (Invitrogen/GIBCO, Cralsbad, California).
Human Embryonic Kidney 293 cells (HEK-293) were manteined in DMEM (Invitrogen/GIBCO, Cralsbad, California) supplementated with 5% FBS (PAA laboratories GmbH, Pasching, Austria), 2,000 U/ml penicillin (PAA laboratories GmbH, Pasching, Austria), 2,000 ug/ml streptomycin (PAA laboratories GmbH, Pasching, Austria), and 20 mM L-glutamine (Invitrogen/GIBCO, Cralsbad, California).
HK-2 and HEK-293 were grown two weeks and medium was changed every two days. After every week, cells were washed with PBS (PAA laboratories GmbH, Pasching, Austria) and were trypsinizated (Invitrogen/GIBCO, Cralsbad, California). Cells harvested were, in part, seeded on chambers slides and the others were used to extract proteins.
Cystic cells were obtained by fragments of renal cysts deprived of external membrane. Each cysts was picked up, was divided in some little fragments and were digested by collagenase (Invitrogen/GIBCO, Cralsbad, California) for one hours. Every pieces of a single cysts were seeded on a plate, previously treated by collagene type I (Invitrogen/ GIBCO, Cralsbad, California), the same medium used for HK-2.
Immunofluorescence
Three-micrometer frozen kidney samples mounted on Super Frost Plus slides (Menzel-Gläser, Thermo Scientific, Waltham, MA) were cut by a cryostat (Leica 1720, Leica Mycrosystems, Heerbrog, Germany). The sections, or cellular spots, were fixed with PFA for 10 min, washed with PBS. Slides were coated (3% Bovine Serum Albumin in PBS buffer) for 1 h at 20℃. Slides were incubated by primary antibody for 1 h at 37℃, washed with PBS and successively incubated by secondary antibody for 1 h at 20℃. Primary and secondary antibodies were diluted in coating solution. Slides were closed with Vectashield® Mounting Medium with DAPI (Vector Laboratories, Inc., Burlingame, CA, USA). Tissue samples, or cells, were examined by immunofluorescence microscopy (Olympus BX41 Microscopy). Confocal images were obtained with Leica TCS SP2 (Leica Microsystems, Heerbrog, Germany).
Image analysis
Images of immunofluorescence-marked kidney were sampled by an acquisition system composed by Olympus BX41 microscope, Olympus XC30 and CELL B 3.0 analysis image processing (Olympus Europa GmbH, Hamburg Germany). Images were saved in tagged image file format (.tiff), a file type better handled by graphic software. The files were analyzed with Inspector Matrox (Matrox Electronic System Ltd., Quebec Canada), a professional graphic software. ROI defines a portion of image that contains areas to analyze. ROIs of each renal component (tubules, glomeruli and cysts) were sampled. The ROIs were converted in a list of single grey tone amount, from white (highest value) to black (zero). Background fluorescence was obtained sampling spot of negative structure, such mesangium or negative tubules, in every image.
Statistical analysis
Total brightness/positive area ratio was calculated for each ROI. The following analysis of expression were performed: CD24+ in tubules of normal tissue, CD24+ in glomeruli of normal tissue, CD24+ in tubules of ADPKD samples, CD24+ in glomeruli of ADPKD samples, CD24+ in cysts of ADPKD samples, CD133+ in tubules of normal samples, CD133+ in glomeruli of normal tissue, CD133+ in tubules of ADPKD samples, CD133+ in glomeruli of ADPKD samples, CD133+ in cysts of ADPKD samples. Normal and ADPKD tissue components were compare by Mann-Whitney test. Results were considered statistically significant for p<0.01.
Western blot analysis
Total proteins were extracted from cellular pellets with Triton X-100 based lysis buffer. Proteins were loaded on SDS-PAGE (3% stacking, 7,5% resolving), previously denatured with 4X Reducing Loading Buffer (200 mM TrisHCl, 20% β-Mercapto Ethanol, 8% SDS, 0.4% Bromofenol Blue, 40% glycerol). After SDS-PAGE run and transblot, nitrocellulose membranes were previously incubated by antibodies anti-CD133 and anti-CD24 and subsequently by horse radish peroxidise conjugated secondary antibodies. The detection was obtained by exposition of radiological plates to peroxidise substrate activated by the enzyme.
Results
Expression of CD133 and CD24 in normal kidney
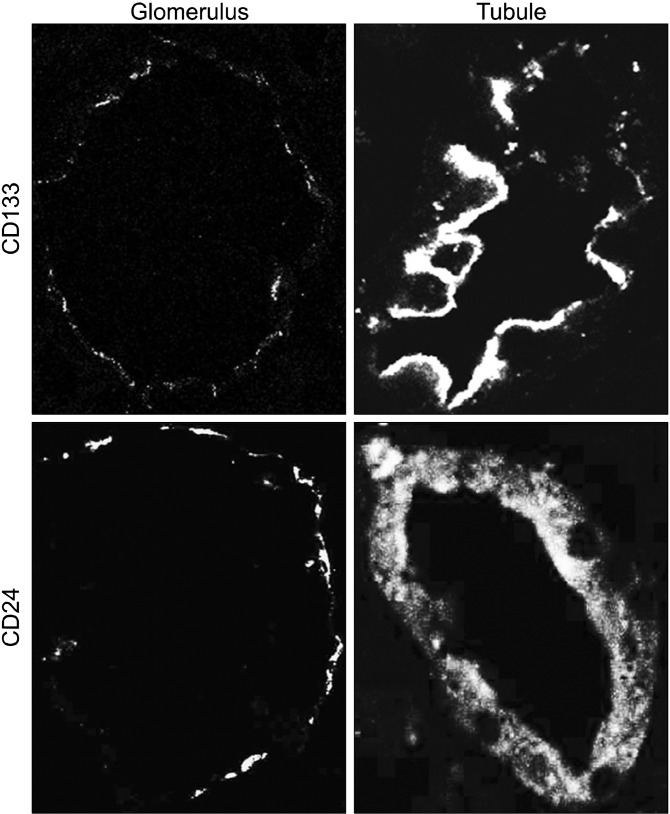
Confocal microscopy analysis shows that CD133 and CD24 are expressed by a subset of PECs of Bowman’s capsule in normal kidney glomeruli (Fig. 1: on line publication). Moreover, tubular distribution of CD133 and CD24 is focal and segmental. Interestingly, CD133 is polarized to the luminary side of the tubular cells and positive PECs, whereas CD24 is not clearly polarized showing a diffuse distribution (membrane and cytoplasm) (Fig. 1: on line publication).
Fig. 1. (On line publication). CD133 and CD24 expression in normal kidney. The first lane shows tissue expression of CD133 in Bowman's capsules and tubules. CD133 is expressed in a subset of PEC in the Bowman's capsule and in the luminal side of positive tubule cells. The second lane shows tissue expression of CD24 in Bowman's capsules and tubules. CD24 is expressed in a subset of PEC in the Bowman's capsule and in the cytoplasm of tubule cells.
Expression of CD133+ and CD24+ in ADPKD kidney
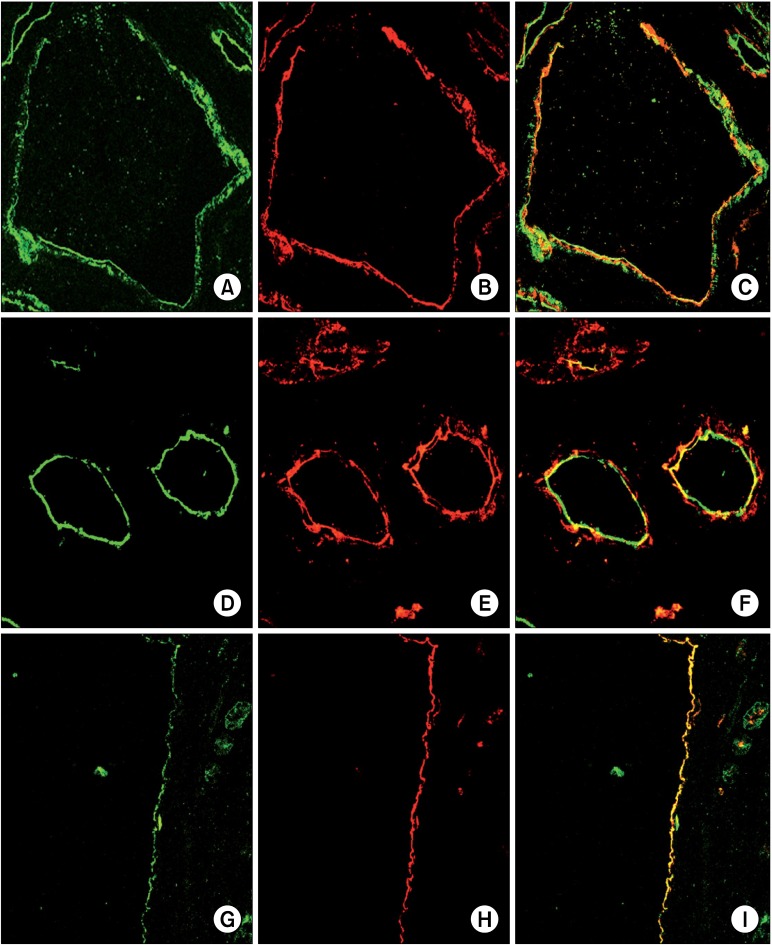
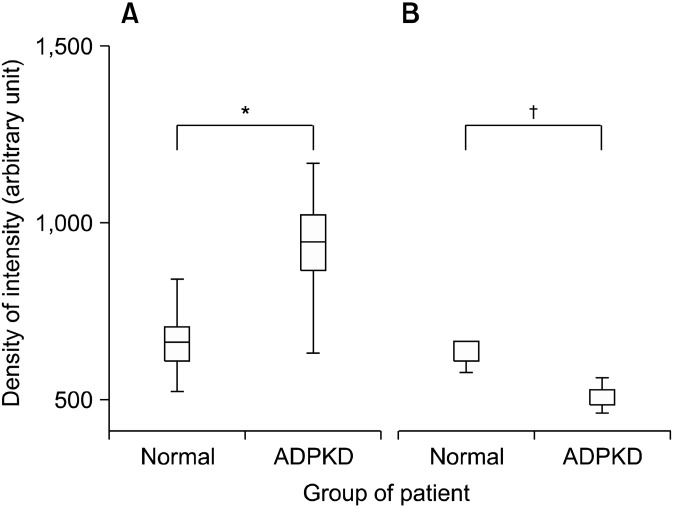
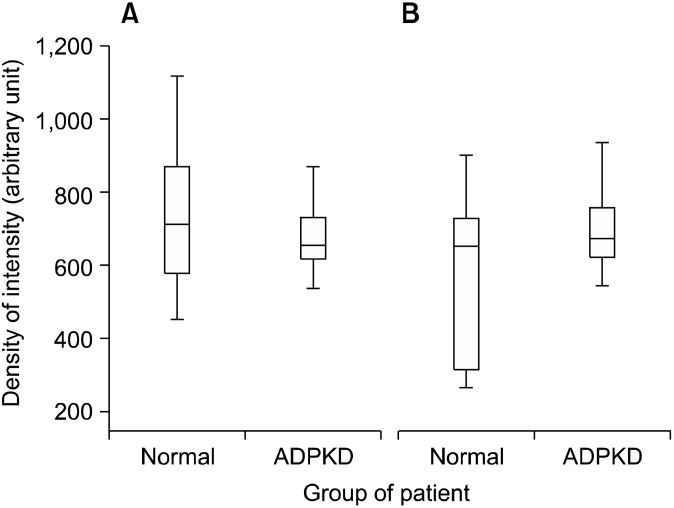
Confocal microscopy analysis of ADPKD kidneys revealed that CD133 and CD24 were expressed by the same subset of glomerular (Fig. 2A∼C) and tubular (Fig. 2D∼ F) cells and they showed the same tissue and cellular localization (Fig. 3: on line publication) of normal kidney: PECs in Bowman’s capsule and focal and segmental expression in tubules. Nevertheless, the quantitative comparison between normal kidney and ADPKD kidney revealed an increased CD133 expression in tubules and a reduced CD133 expression in glomeruli of ADPKD samples (Fig. 4). After image analysis Mann-Whitney test resulted statistically significant for these comparisons (ADPKD tubules vs Normal tubules: z=−7.681 p<0.001; ADPKD Glomerula vs Normal Glomerula: z= 3.162 p=0.0016). CD 24 expression in glomeruli and tubules does not show quantitative difference between normal and ADPKD samples (Fig. 5: on line publication).
Fig. 2. CD133 and CD24 expression in ADPKD samples. The first lane shows glomerular expression of CD133 (A) and CD24 (B). CD133 and CD24 are co-localized and co-expressed by a subset of PEC in the Bowman's capsule (C). The second lane shows tubular expression of CD133 (D) and CD24 (E). CD133 and CD24 are co-localized and co-expressed by the luminal side of tubules (F). The third lane shows cystic expression of CD133 (G) and CD24 (H). CD133 and CD24 are co-localized and co-expressed by the cystic cells (I).
Fig. 3. (On line publication). CD133 and CD24 expression in cystic cells. CD133 is closely expressed by the cellular membrane (A). CD24 is expressed in the cytoplasm (B). CD133 and CD24 are co-expressed by the cystic cells (C).
Fig. 4. Morphometric analysis of CD133 expression in tubules and glomerula of normal kidney and ADPKD kidney. CD133 expression is higher in ADPKD tubules than in normal tubules (*p <0,001) (A). CD133 expression is higher in normal glomerula than ADPKD glomerula (†p=0,0016) (B).
Fig. 5. (On line publication). Morphometric analysis of CD24 expression in tubules and glomerula of normal kidney and ADPKD kidney. CD24 expression is not statistically different in both normal tubules vs ADPKD tubules (A) and normal glomerula vs ADPKD glomerula (B) comparison.
The cysts of ADPKD samples were diffusely positive for CD133 and CD24. The cells of large cysts show a flatted morphology with co-localization of CD133 and CD24 (Fig. 2G∼I).
Expression of CD133 and CD24 in cultured cells
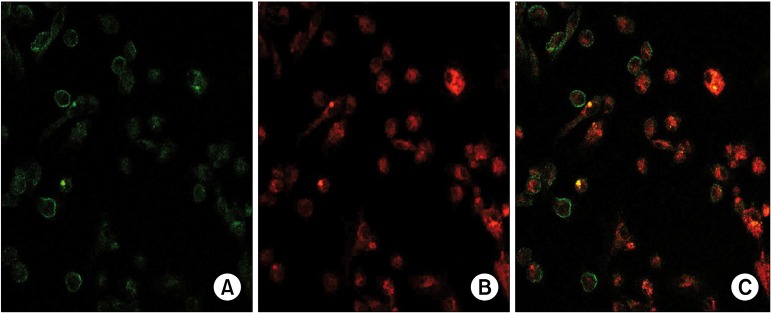
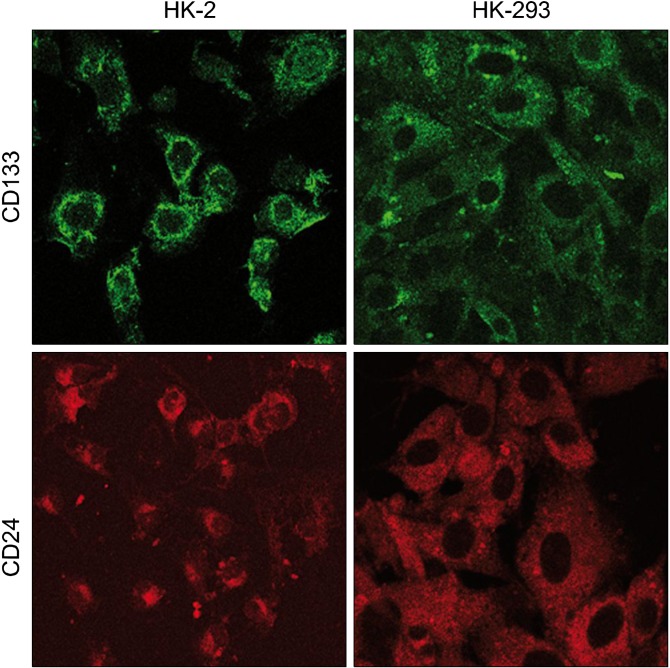
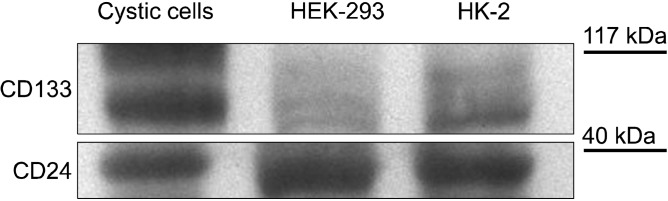
Cystic cultured cells showed a characteristic expression pattern for CD133 and CD24 (Fig. 3C: on line publication): CD133 localized on cellular membrane (Fig. 3A: on line publication) whereas CD24 was globally diffused into cytoplasm (Fig. 3B: on line publication). The same pattern was appreciable in HK-2 cells; on the other hand, both antigens presented a cytoplasmic distribution in HEK-293 (Fig. 6: on line publication). Western blot analysis revealed that CD133 is highly expressed in cystic cells, while HK-2 and HEK-293 showed a reduced level of the protein (Fig. 7, higher bands). Analogous amounts of CD24 were detected in the three cellular populations (Fig. 7, lower bands).
Fig. 6. (On line publication). CD133 and CD24 expression in cellular lines. HK-2 show a similar antigen-expression patternto cystic cells: CD133 is localized on the cellular membrane and CD24 is localized in the cytoplasm. HEK-293 have a diffused and low cellular expression of CD133 and CD24.
Fig. 7. Western blot analysis of CD133 and CD24 expression in untreated HEK-293, HK-2 and cystic cells. Cystic cells express the highest amount of CD133 whereas HEK-293 the lowest. CD24 amount is similar in the three populations.
Discussion
The presence of PECs in ADPKD kidney suggests that the pathologic tissue tries to repair damages with cellular hyperproliferation. This is a common reaction of many tissue to the injuries. The results show unchanged high-amount of CD24 in ADPKD tubules and ADPKD glomeruli compared by the healthy component, but increased amounts of CD133 in ADPKD tubules and decreased amount of CD133 in ADPKD glomeruli. These evidences suggest a gradual depletion of CD133+ CD24+ progenitors from glomeruli and their accumulation in tubules, the tissue component which is directly involved in ADPKD. It also interesting to note the double positivity to CD133 and CD24 of the cysts. We think that the cysts derived from damaged ADPKD tubules wherein chemoactracted-CD133+CD24+ progenitors try to repair the tissue with no success, but on the contrary triggering the uncontrolled proliferation charactering cyst development.
Obviously many aspects contribute to ADPKD development. Hyperplasia and cysts growth depend also on the lack of inhibition of STAT 6 and mTOR by mutated PC1 (4, 19, 20). The inability of polycystin complex to recover calcium ions from lumen increased the osmolarity of the fluid which flows in the tubules, causing attraction of water and the increase of volume.
In B lymphocytes CD24 is an important co-stimulus of cell activation and is involved in Ca++ uptake (21). CD133 localization on cellular membrane suggests an implication in cell signaling, in particular the ability to link specific ligand or act like sensor for alteration of liquid contained in fold. CD133 is mutated in several pathology charac-
terized by retinal degeneration, such as retinitis pigmentosa and Stargardt disease (22, 23). The most common forms of Stargardt disease are associated with mutation of gene coding ATP-binding cassette transporters. It is possible that CD133 could be involved in cellular molecule exchanges. In a cellular subset where polycystin are not able to maintain Ca++ gradient and other systems of Ca++ flow control are physiologically switch off, PECs are potentially and paradoxically dangerous for kidney.
ADPKD pathogenesis is an unsolved paradigm. It is not
clear if ADPKD late onset is due to haploinsufficiency or loss of heterozygosity (LOH). Theory of haploinsufficiency is supported by the progressive worsening of ADPKD phenotype, e.g., major frequency of cerebral aneurism, associated with less-conservative mutation of pkd1 gene. Theory of LOH is supported by the evidence that not all tubules become cysts. We think that cellular hyperproliferation is the base of cyst formation. Tubular damage is the event which trigs cyst development. Without injuries, ADPKD tubules have a normal behavior. However, during renal filtration tubules could be damaged and this event draws PECs and activates cell proliferation. Probably, more truncated proteins compromise membrane stability, make cells more sensitive to injuries and cyst formation becomes easier. From this point of view PECs assume a crucial role in ADPKD pathogenesis.
Nevertheless, it is also likely that a local block of cell proliferation risks to worsen kidney health because of damages persistence.
We conclude that PECs could have a complex role in ADPKD pathogenesis and it is important to control their hyperproliferation. In order to deepen this issue, we are trying to evaluate several antiproliferative drugs used in nephrologic practice with the aim to control cell proliferation and stabilize ADPKD.
Abbreviations
Autosomal Dominant Polycystic Kidney Disease (ADPKD)
Parietal Epithelial Cell (PEC)
Delayed graft function (DGF)
Human Kidney 2 cells (HK-2)
Human Embryonic Kidney 293 cells (HEK-293)
Loss Of Heterozygosity (LOH)
Disclosures
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Acknowledgments
This study was supported by the Research Program “Regione-Università 2007-2009 - Innovative Research” granted by the Regional Sanitary Service of Emilia Romagna, Italy. Thanks to nephrology department of Modena Polyclinic, in particular to Prof. Gianni Cappelli and Riccardo Magistroni.
Potential conflict of interest
The authors have no conflicting financial interest.
References
- 1.Chang MY, Ong AC. Autosomal dominant polycystic kidney disease: recent advances in pathogenesis and treatment. Nephron Physiol. 2008;108:1–7. doi: 10.1159/000112495. [DOI] [PubMed] [Google Scholar]
- 2.Wei W, Hackmann K, Xu H, Germino G, Qian F. Characterization of cis-autoproteolysis of polycystin-1, the product of human polycystic kidney disease 1 gene. J Biol Chem. 2007;282:21729–21737. doi: 10.1074/jbc.M703218200. [DOI] [PubMed] [Google Scholar]
- 3.Delmas P, Padilla F, Osorio N, Coste B, Raoux M, Crest M. Polycystins, calcium signaling, and human diseases. Biochem Biophys Res Commun. 2004;322:1374–1383. doi: 10.1016/j.bbrc.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 4.Weimbs T. Polycystic kidney disease and renal injury repair: common pathways, fluid flow, and the function of polycystin- 1. Am J Physiol Renal Physiol. 2007;293:F1423–1432. doi: 10.1152/ajprenal.00275.2007. [DOI] [PubMed] [Google Scholar]
- 5.Tirino V, Desiderio V, Paino F, De Rosa A, Papaccio F, Fazioli F, Pirozzi G, Papaccio G. Human primary bone sarcomas contain CD133+ cancer stem cells displaying high tumorigenicity in vivo. FASEB J. 2011;25:2022–2030. doi: 10.1096/fj.10-179036. [DOI] [PubMed] [Google Scholar]
- 6.Lorico A, Rappa G. Phenotypic heterogeneity of breast cancer stem cells. J Oncol. 2011;2011:135039. doi: 10.1155/2011/135039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janikova M, Skarda J, Dziechciarkova M, Radova L, Chmelova J, Krejci V, Sedlakova E, Zapletalova J, Langova K, Klein J, Grygarkova I, Kolek V. Identification of CD133+/nestin+ putative cancer stem cells in non-small cell lung cancer. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2010;154:321–326. doi: 10.5507/bp.2010.048. [DOI] [PubMed] [Google Scholar]
- 8.Miraglia S, Godfrey W, Yin AH, Atkin K, Warnke R, Holden JT, Bray RA, Waller EK, Buck DW. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997;90:5013–5021. [PubMed] [Google Scholar]
- 9.Kobayashi SD, Voyich JM, Whitney AR, DeLeo FR. Spontaneous neutrophil apoptosis and regulation of cell survival by granulocyte macrophage-colony stimulating factor. J Leukoc Biol. 2005;78:1408–1418. doi: 10.1189/jlb.0605289. [DOI] [PubMed] [Google Scholar]
- 10.Vlková M, Fronková E, Kanderová V, Janda A, Ruzicková S, Litzman J, Sedivá A, Kalina T. Characterization of lymphocyte subsets in patients with common variable immunodeficiency reveals subsets of naive human B cells marked by CD24 expression. J Immunol. 2010;185:6431–6438. doi: 10.4049/jimmunol.0903876. [DOI] [PubMed] [Google Scholar]
- 11.Berga-Bolaños R, Drews-Elger K, Aramburu J, López-Rodríguez C. NFAT5 regulates T lymphocyte homeostasis and CD24-dependent T cell expansion under pathologic hypernatremia. J Immunol. 2010;185:6624–6635. doi: 10.4049/jimmunol.1001232. [DOI] [PubMed] [Google Scholar]
- 12.Jiang W, Sui X, Zhang D, Liu M, Ding M, Shi Y, Deng H. CD24: a novel surface marker for PDX1-positive pancreatic progenitors derived from human embryonic stem cells. Stem Cells. 2011;29:609–617. doi: 10.1002/stem.608. [DOI] [PubMed] [Google Scholar]
- 13.Myung JH, Gajjar KA, Pearson RM, Launiere CA, Eddington DT, Hong S. Direct measurements on CD24- mediated rolling of human breast cancer MCF-7 cells on E-selectin. Anal Chem. 2011;83:1078–1083. doi: 10.1021/ac102901e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, Romagnani P. Isolation and characterization of multipotent progenitor cells from the Bowman's capsule of adult human kidneys. J Am Soc Nephrol. 2006;17:2443–2456. doi: 10.1681/ASN.2006010089. [DOI] [PubMed] [Google Scholar]
- 15.Romagnani P. Toward the identification of a “renopoietic system”? Stem Cells. 2009;27:2247–2253. doi: 10.1002/stem.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazzeri E, Crescioli C, Ronconi E, Mazzinghi B, Sagrinati C, Netti GS, Angelotti ML, Parente E, Ballerini L, Cosmi L, Maggi L, Gesualdo L, Rotondi M, Annunziato F, Maggi E, Lasagni L, Serio M, Romagnani S, Vannelli GB, Romagnani P. Regenerative potential of embryonic renal multipotent progenitors in acute renal failure. J Am Soc Nephrol. 2007;18:3128–3138. doi: 10.1681/ASN.2007020210. [DOI] [PubMed] [Google Scholar]
- 17.Romagnani P, Kalluri R. Possible mechanisms of kidney repair. Fibrogenesis Tissue Repair. 2009;2:3. doi: 10.1186/1755-1536-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loverre A, Capobianco C, Ditonno P, Battaglia M, Grandaliano G, Schena FP. Increase of proliferating renal progenitor cells in acute tubular necrosis underlying delayed graft function. Transplantation. 2008;85:1112–1119. doi: 10.1097/TP.0b013e31816a8891. [DOI] [PubMed] [Google Scholar]
- 19.Low SH, Vasanth S, Larson CH, Mukherjee S, Sharma N, Kinter MT, Kane ME, Obara T, Weimbs T. Polycystin-1, STAT6, and P100 function in a pathway that transduces ciliary mechanosensation and is activated in polycystic kidney disease. Dev Cell. 2006;10:57–69. doi: 10.1016/j.devcel.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Weimbs T. Regulation of mTOR by polycystin-1: is polycystic kidney disease a case of futile repair? Cell Cycle. 2006;5:2425–2429. doi: 10.4161/cc.5.21.3408. [DOI] [PubMed] [Google Scholar]
- 21.Fischer GF, Majdic O, Gadd S, Knapp W. Signal transduction in lymphocytic and myeloid cells via CD24, a new member of phosphoinositol-anchored membrane molecules. J Immunol. 1990;144:638–641. [PubMed] [Google Scholar]
- 22.Maw MA, Corbeil D, Koch J, Hellwig A, Wilson-Wheeler JC, Bridges RJ, Kumaramanickavel G, John S, Nancarrow D, Röper K, Weigmann A, Huttner WB, Denton MJ. A frameshift mutation in prominin (mouse)-like 1 causes human retinal degeneration. Hum Mol Genet. 2000;9:27–34. doi: 10.1093/hmg/9.1.27. [DOI] [PubMed] [Google Scholar]
- 23.Shmelkov SV, St Clair R, Lyden D, Rafii S. AC133/CD133/ Prominin-1. Int J Biochem Cell Biol. 2005;37:715–719. doi: 10.1016/j.biocel.2004.08.010. [DOI] [PubMed] [Google Scholar]