Abstract
Background and Objectives:
Ischemic stroke caused by middle cerebral artery occlusion (MCAo) is the major type of stroke, but there are currently very limited options for cure. It has been shown that neural stem cells (NSCs) or neural precursor cells (NPCs) can survive and improve neurological deficits when they are engrafted in animal models of various neurological diseases. However, how the transplanted NSCs or NPCs are act in vivo in the injured or diseased brain is largely unknown. In this study, we utilized magnetic resonance imaging (MRI) techniques in order to understand the fates of human NSCs (HB1.F3) following transplantation into a rodent model of MCAo.
Methods and Results:
HB1.F3 human NSCs were pre-labeled with ferumoxides (Feridex®)-protamine sulfate complexes, which were visualized and examined by MRI up to 9 weeks after transplantation. Migration of the transplanted cells to the infarct area was further confirmed by histological methods.
Conclusions:
Based on these observations, we speculate that the transplanted NSCs have the extensive migratory ability to the injured site, which will in turn contribute to functional recovery in stroke.
Keywords: Human neural stem cells (hNSCs), Ischemic stroke, Middle cerebral artery occlusion (MCAo), Magnetic resonance imaging (MRI), Feridex, In vivo tracking
Introduction
Stroke is one of the leading causes of adult death and disability worldwide. Ischemic stroke is caused by blockade of the blood supply to part of the brain. Despite numerous research efforts, there are still currently very limited therapeutic options for the stroke-damaged patients. It has been shown that neural stem cells (NSCs) or neural precursor cells (NPCs) engrafted in animal models of various neurological diseases can survive and improve neurological deficits (1, 2). We have previously established a clonal human neural stem cell line, HB1.F3 (briefly called F3 thereafter), which was immortalized by a retroviral vector encoding v-myc oncogene (3, 4). The F3 cells showed a multipotent capacity to differentiate into neurons and glial cells (3, 4) and were able to ameliorate the neurological deficits in animal models of stroke (5-8). However, compared with these differentiation and therapeutic potentials, how the transplanted NSCs act in order to exert such effects in vivo is largely unknown. In this regard, it is worthwhile to utilize magnetic resonance imaging (MRI) approaches to non-invasively monitor cells that are labeled with MR contrast agent, which has been one of the major research directions in the past decade (9-13). In particular, the ability to image animals repeatedly using MRI can serve as useful tools to monitor the presence, migration and distribution patterns of transplanted cells in vivo. In this study, we employed a 4.7 T animal MRI to follow up the fates of F3 human NSC labeled with ferumoxides (Feridex®)-protamine sulfate complexes following transplantation into a rodent model of middle cerebral artery occlusion (MCAo) up to 9 weeks. Interestingly, we found that the majority of transplanted cells were migrated to the infarct area, showing a patho-tropism. In this study, we also described the detailed migration patterns of transplanted hNSCs.
Materials and Methods
MCAo animal models
All of the experimental animals were manipulated in accordance with the CHA University IACUC (Institutional Animal Care and Use Committee; IACUC090012). Adult male Sprague-Dawley rats (n=8) weighing 250∼300 g (Orient, Seoul, Korea) were used in this experiment. After anesthesia with 1% ketamine (30 mg/kg, i.p) and xylazine
hydrochloride (4 mg/kg, i.p), body temperature of rats were maintained at 37±1℃ by a rectal probe and heating pad. According to the method of Longa, animals were subjected to temporary middle cerebral artery occlusion (MCAo), which was induced by the retrograde insertion of a 3-0 poly-L-lysine-coated nylon suture via the external carotid artery into the internal carotid artery and MCA. Reperfusion carried out by removing the suture 60 min later (14).
Preparation of F3 hNSCs for transplantation
For cell labeling, ferumoxide and protamine sulfate were initially prepared at a concentration of 2 μg/ml of DMEM each without serum, which were subsequently mixed for 30 min at room temperature and were added with an equal volume to the culture medium containing F3 hNSCs (13). Cells were cultured with the mixture for 12∼16 hr at 37℃. At 7 days after the induction of MCAo, cells were stereotaxically transplanted into the striatum (n=4) using the following conditions: 1×105 cells in 2 μl (AP: +1.0 mm, ML: −1.5 mm, DV: −4.0 mm). As controls (n=4), a vehicle containing medium was transplanted into the same coordinates. Transplanted animals were received cyclosporine A (Sigma, 10 mg/kg, i.p) 24 h before transplantation and daily up to 9 weeks.
MRI detection of transplanted cells
To detect the Feridex®-labeled F3 hNSCs, we employed a 4.7T Bio Spec (Bruker, Germany) for animal MRI analysis using T2- and T2*-weighted imaging techniques. The MRI setting and detection methods were the same as described previously (13). To detect the transplanted stem cells, Prussian blue staining was carried out at 9 weeks following transplantation as described (13), followed by immunohistochemical staining using an antibody against human specific-nuclei (Chemicon, 1:500).
Results
Detection and migration of the Feridex®-labeled hNSCs using MRI
To investigate the migratory effects of NSC, we trans-
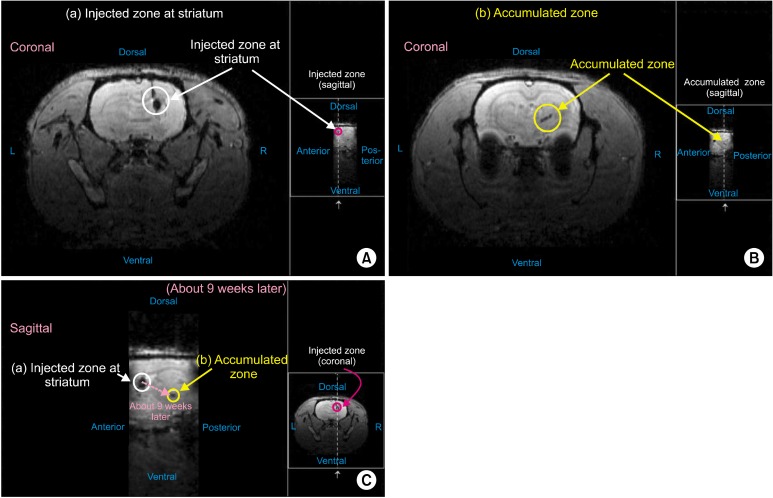
planted Feridex®-labeled hNSC into the ipsilateral side of MCAo animal models, and followed their migration patterns up to 9 weeks using MRI. Fig. 1A and 1B show the representative coronal views of T2*-weighted MR images taken from 9 weeks following transplantation. Interestingly, cells transplanted into the striatum (Fig. 1A) were also detected and accumulated in the posterior region (Fig. 1B). Fig. 1C is the sagittal view of T2*-weighted images, showing the migration and accumulation of transplanted hNSC in the posterior region. Taken together, these coronal and sagittal views of T2*-weighted MR images clearly show that the transplanted hNSCs migrated to the infarct region in anterior-posterior and dorsal-ventral directions.
Fig. 1. Detection of the Feridex®-labeled hNSCs using 4.7T animal MRI. F3 hNSCs were transplanted into the ipsilateral side of MCAo animal models, and their migration patterns were monitored up to 9 weeks using T2*-weighted MR images. (A) Coronal views taken at the injected zone in the striatum. (B) Coronal views taken at the accumulated zone. (C) Sagittal view, showing the migration and accumulation of transplanted hNSC in the posterior region.
Migration patterns of Feridex®-labeled hNSCs
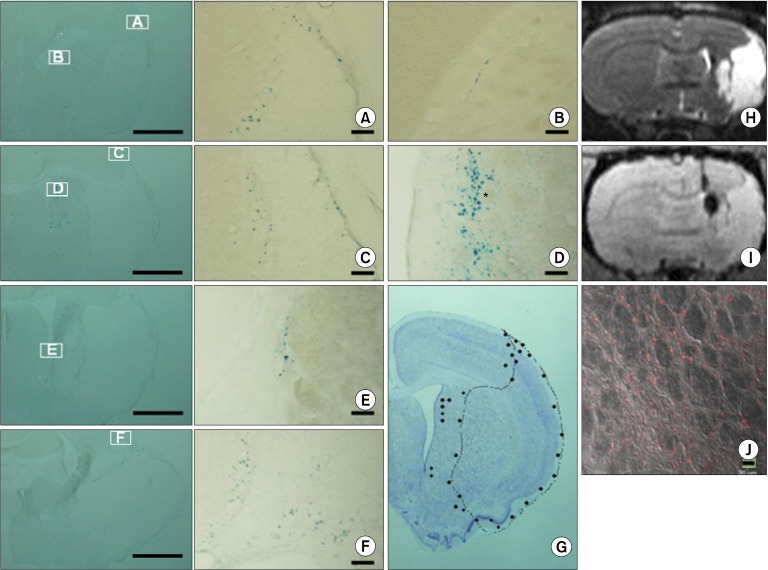
In addition to MRI analysis, we carried out histological examinations at 9 weeks in order to understand the migration patterns of transplanted hNSCs to the infarcted area. Fig. 2 shows the results of Prussian blue staining (left panel, lower magnification; Fig. 2A-F, high magnification). Fig. 2G shows the distribution patterns of Feridex®- labled hNSC, indicating the great majority of transplanted cells were localized at the periphery of infarct region. Fig. 2H and 2I show coronal view of T2 and T2*-weighted images, providing the gross morphology of infarct area and the location of transplanted cells, respectively. Fig. 2J shows that the Feridex®-containing cells (i.e., MRI-positive cells) were indeed derived from human NSCs, judged by the positive staining for an antibody against human- specific nuclei. Taken together, these results clearly indicate the transplanted hNSC migrated to the periphery of infarct region in a ventro-posterior direction.
Fig. 2. Histological analyses of Feridex®-labeled hNSCs. (A∼F) Prussian blue staining showing the presence and distribution of Feridex®- labeled hNSCs. Left panel, lower magnification; A∼F, high magnification. (G) Distribution patterns of Feridex®-labled hNSC, showing the great majority of transplanted cells were localized at the periphery of infarct region. (H, I) Coronal views of T2 and T2*-weighted images, providing the gross morphology of infarct area and the location of transplanted cells, respectively. (J) Immunohistochemical staining showing the presence of human cells. Scale bars: left panel, 1 mm; A~F, 100 μm, J, 20 μm.
Discussion
Over the past several years, there has been the great interest in the potential of stem cells for treating stroke, and studies from animal experiments and clinical trials using various cell types in recent years strongly suggest that stem cell therapy would be one of the legitimate options for the treatment of stroke (15-17). In this study, we showed that the fates of transplanted F3 hNSCs in a rodent model of ischemic stroke can be efficiently monitored by using an animal MRI. We found that the Feridex®-labeled hNSCs can survive up to 9 weeks after transplantation and were migrated to the infarct region, of which results were further confirmed by histological methods using Prussian blue staining, as well as immunohistochemical staining using a human-specific antibody. According to our observations, there is a strong tendency of transplanted hNSCs to migrate to the infarct area, showing a strong patho-tropism, which has been also observed in animal models of various other diseases, including brain tumors. Several studies have reported that neural stem cells or neural precursor cells express the chemokine receptor, CXCR4, which has the high affinity to SDF-1 (stromal cell-derived factor-1) that is strongly released in the ischemic boundary zone of the brain (18, 19).
Previous studies using F3 hNSCs indicate that they can differentiate into neurons and astrocytes and possess electrophysiological characteristics of mature neurons (20). It is also known that F3 hNSCs can secrete some neurotrophic factors (4). Therefore, it is quite likely that all of these properties will contribute to the neural protection and/or neuronal regeneration of the ischemic tissues, as well as to the amelioration of behavioral deficits in stroke animal models. Therefore, in order to develop efficient stroke cell therapy, it will be essential to determine the optimal cell types and transplantation conditions.
Taken together, the present MRI study provides useful information on the migratory routes of hNSCs following transplantation into a rodent model of ischemic stroke. Therefore, in combination with behavioral tests, in vivo tracking method using MRI will provide useful tools to closely monitor the action mode of transplanted stem cells and the extent of functional recovery following stem cell transplantation non-invasively.
Acknowledgments
This study was supported by a grant of the Korea Healthcare technology R&D project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A111016). We are grateful to the MRI facility in the Division of Magnetic Resonance, Korea Basic Science Institute, Ochang, Korea.
Potential conflict of interest
The authors have no conflicting financial interest.
References
- 1.Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441:1094–1096. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- 2.Goldman S. Stem and progenitor cell-based therapy of the human central nervous system. Nat Biotechnol. 2005;23:862–871. doi: 10.1038/nbt1119. [DOI] [PubMed] [Google Scholar]
- 3.Flax JD, Aurora S, Yang C, Simonin C, Wills AM, Billinghurst LL, Jendoubi M, Sidman RL, Wolfe JH, Kim SU, Snyder EY. Engraftable human neural stem cells respond to developmental cues, replace neurons, and express foreign genes. Nat Biotechnol. 1998;16:1033–1039. doi: 10.1038/3473. [DOI] [PubMed] [Google Scholar]
- 4.Kim SU. Human neural stem cells genetically modified for brain repair in neurological disorders. Neuropathology. 2004;24:159–171. doi: 10.1111/j.1440-1789.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- 5.Chu K, Kim M, Jeong SW, Kim SU, Yoon BW. Human neural stem cells can migrate, differentiate, and integrate after intravenous transplantation in adult rats with transient forebrain ischemia. Neurosci Lett. 2003;343:129–133. doi: 10.1016/s0304-3940(03)00174-5. [DOI] [PubMed] [Google Scholar]
- 6.Chu K, Kim M, Park KI, Jeong SW, Park HK, Jung KH, Lee ST, Kang L, Lee K, Park DK, Kim SU, Roh JK. Human neural stem cells improve sensorimotor deficits in the adult rat brain with experimental focal ischemia. Brain Res. 2004;1016:145–153. doi: 10.1016/j.brainres.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 7.Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, Masel J, Yenari MA, Weissman IL, Uchida N, Palmer T, Steinberg GK. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci USA. 2004;101:11839–11844. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee HJ, Kim KS, Kim EJ, Choi HB, Lee KH, Park IH, Ko Y, Jeong SW, Kim SU. Brain transplantation of immortalized human neural stem cells promotes functional recovery in mouse intracerebral hemorrhage stroke model. Stem Cells. 2007;25:1204–1212. doi: 10.1634/stemcells.2006-0409. [DOI] [PubMed] [Google Scholar]
- 9.Arbab AS, Bashaw LA, Miller BR, Jordan EK, Bulte JW, Frank JA. Intracytoplasmic tagging of cells with ferumoxides and transfection agent for cellular magnetic resonance imaging after cell transplantation: methods and techniques. Transplantation. 2003;76:1123–1130. doi: 10.1097/01.TP.0000089237.39220.83. [DOI] [PubMed] [Google Scholar]
- 10.Bulte JW, Douglas T, Witwer B, Zhang SC, Strable E, Lewis BK, Zywicke H, Miller B, van Gelderen P, Moskowitz BM, Duncan ID, Frank JA. Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat Biotechnol. 2001;19:1141–1147. doi: 10.1038/nbt1201-1141. [DOI] [PubMed] [Google Scholar]
- 11.Hoehn M, Küstermann E, Blunk J, Wiedermann D, Trapp T, Wecker S, Föcking M, Arnold H, Hescheler J, Fleischmann BK, Schwindt W, Bührle C. Monitoring of implanted stem cell migration in vivo: a highly resolved in vivo magnetic resonance imaging investigation of experimental stroke in rat. Proc Natl Acad Sci USA. 2002;99:16267–16272. doi: 10.1073/pnas.242435499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim D, Hong KS, Song J. The present status of cell tracking methods in animal models using magnetic resonance imaging technology. Mol Cells. 2007;23:132–137. [PubMed] [Google Scholar]
- 13.Kim D, Chun BG, Kim YK, Lee YH, Park CS, Jeon I, Cheong C, Hwang TS, Chung H, Gwag BJ, Hong KS, Song J. In vivo tracking of human mesenchymal stem cells in experimental stroke. Cell Transplant. 2008;16:1007–1012. [PubMed] [Google Scholar]
- 14.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 15.Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 16.Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8:491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindvall O, Kokaia Z. Stem cell research in stroke: how far from the clinic? Stroke. 2011;42:2369–2375. doi: 10.1161/STROKEAHA.110.599654. [DOI] [PubMed] [Google Scholar]
- 18.Hill WD, Hess DC, Martin-Studdard A, Carothers JJ, Zheng J, Hale D, Maeda M, Fagan SC, Carroll JE, Conway SJ. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J Neuropathol Exp Neurol. 2004;63:84–96. doi: 10.1093/jnen/63.1.84. [DOI] [PubMed] [Google Scholar]
- 19.Bhakta S, Hong P, Koc O. The surface adhesion molecule CXCR4 stimulates mesenchymal stem cell migration to stromal cell-derived factor-1 in vitro but does not decrease apoptosis under serum deprivation. Cardiovasc Revasc Med. 2006;7:19–24. doi: 10.1016/j.carrev.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Cho T, Bae JH, Choi HB, Kim SS, McLarnon JG, Suh-Kim H, Kim SU, Min CK. Human neural stem cells: electrophysiological properties of voltage-gated ion channels. Neuroreport. 2002;13:1447–1452. doi: 10.1097/00001756-200208070-00020. [DOI] [PubMed] [Google Scholar]