Abstract
Background and Objectives:
The transplantation of human umbilical cord blood cells (hUCBCs) has been shown to attenuate the unregulated activation of microglia in a rat model of cerebral palsy (CP). To investigate whether hUCBCs transplantation is also anti-inflammatory in humans, we performed a clinical trial in patients with CP.
Methods and Results:
Allogeneic or autologous hUCBCs and erythropoietin (EPO) were intravenously injected into human patients with CP (mean age of approximately 38 weeks), and patients were analyzed for their motor function and social behavior. Blood samples were tested for cytokine levels. The most surprising finding in the study was that the cytokine levels were dependent on the donor cell source (allogeneic or autologous). Interestingly, the allogeneic treatment group demonstrated significantly decreased levels of pro-inflammatory factors, such as IL-1α, IL-6, TNF-β, and RANTES, and showed a statistically significant improvement in motor and social behavior compared to the autologous treatment group.
Conclusions:
Given that inflammation plays a pivotal role in CP, our results suggest that allogeneic hUCBCs therapy may be an appropriate strategy for CP treatment. In addition, prior to transplantation, a detailed analysis of the amount of proinflammatory cytokines in cord blood may be needed to avoid exacerbating inflammatory responses.
Keywords: Cerebral pasly, Cord blood cell, Erythropoietin, Inflammation
Introduction
Inflammatory responses have been suggested to play a major role in central nervous system (CNS) diseases during the perinatal period and may contribute to brain impairment in conditions such as neonatal encephalopathy or cerebral palsy (CP). In particular, a majority of studies have reported that high levels of cytokines are associated with CP (1-3). Pro inflammatory cytokines have negative effects in specific brain injuries (4-6). Molecules of inflammation, such as cytokines might be major mediators in neonatal brian injury. Proinflammatory cytokines, IL-1β, TNFα, IL-6, are exacerbated in neonates with perital asphyxia, maternal infection (1, 3, 7) and in rat stroke (8). Chemokines, IP-10/CXCL10 concentration was higher in rat cerebral ischemia and human stroke (9, 10). This result indicates that these cytokines/chemokines and their receptors, have been associated in pathogenesis of cerebral palsy and stroke. Therefore, immune modulation strategies are most therapeutics for neuroprotrection in perinatal brain lesions, and also must consider continuously and accurately maintenance of this balance in their molecular environment.
Given that there seem to be an apparent difference clinically between animal model and patients with CP, we can expect the effects of hUCBCs in human that is not same as those seen in animal model. In clinical trials, hUCBCs (allogenic or autogenic) were injected intravenously into patient and blood samples were analyzed for cytokine and chemokine related to CP.
Materials and Methods
Case study of hUCBCs transplantation in seven children with CP
There were two groups in the current human case study: the autologous transplantation group and the allogeneic transplantation group. For the allogeneic hUCBCsinfused group, three enrolled children diagnosed with severe CP were randomly selected among the children enrolled in an ongoing large human trial, “Double- blind Random Control Trial to Evaluate the Efficacy and Safety of Combination Therapy With Allogeneic Umbilical Cord Blood Stem Cell and Erythropoietin” (Protocol ID in ClinicalTrials.gov is RCTUBSC). This random process was performed by a statistician in the Clinical Statistics Center at CHA University, and patient information is listed in Table 1. For the autologous hUCBC-infused group, four enrolled children diagnosed with severe CP were randomly selected among the children enrolled in a completed human trial in Bundang CHA Hospital, “Singleblind Randomized Trial to Evaluate the Efficacy and Safety of Combination Therapy With Autogenic Umbilical Cord Blood Stem Cell and Erythropoietin” (IRB # is PBC09-095). Patients were 10∼40 months with an available HLA 2 mismatched umbilical cord blood graft containing 4.25±1.29× 107 nucleated cells/kg (n=4, autolo-
Table 1.
Neonatal CP patients demographic information
| Factor | Allogeneic | Autologous | p-valuea | ||
|---|---|---|---|---|---|
|
| |||||
| N | Mean±S.E | N | Mean±S.E | ||
|
| |||||
| Gestational | 3 | 39.67±0.33 | 4 | 35.75±0.85 | 0.04b |
| age (week) | |||||
| ≥37 | 3 | 100.00% | 1 | 25.00% | 0.14c |
| <37 | 0 | 0.00% | 3 | 75.00% | |
| Birth weight (kg) | 3 | 3.50±0.35 | 4 | 2.65±0.07 | 0.11 |
| TNC (×108)d | 3 | 8.22±1.27 | 4 | 4.57±1.24 | 0.21 |
| Weight (kg) | 3 | 13.73±2.78 | 4 | 11.75±2.03 | 0.47 |
| TNC/Kg (×107)d | 3 | 6.39±1.37 | 4 | 4.25±1.29 | 0.37 |
| Age (month) | 3 | 21.64±10.74 | 4 | 33.74±6.59 | 0.37 |
aWilcoxon rank sum test; bSiginficant at the 5% level significances; cFishers' exact test; dTNC: number of nucleated cells.
gous group) or 6.39±1.37×107 nucleated cells/kg (n=3, allogeneic group) on average. Neonatal data for the children were obtained from each infant’ s hospital. Gestational age, birth weight, and sex were obtained from birth records. The erythropoietin (EPO) concentration and the IGF-1 concentration in each patient’ s plasma were analyzed before and after treatment with EPO. Two hrs before transplantation, the seven patients received recombinant EPO intravenously (250 IU/kg, Espogen prefilled inj.; LG Life Sciences). The four patients in the autogenic group received autologous hUCBCs in combination with EPO. The allogeneic group also received hUCBCs from an unrelated donor (HLA 2 mismatched) in combination with EPO. The three patients in the allogeneic group received cyclosporine (15 mg/kg) 12 hr before transplantation and once daily for 6 days post-transplantation, and thereafter received 10 mg/kg of cyclosporine for three weeks. Supportive care was standard for the recipients of autogenic or allogeneic hUCBC transplantation. The study protocol was approved by the Bundang CHA Hospital IRB, CHA University. Parents of all participants in each study provided written informed consent.
Assays for human growth factors, cytokines, and chemokines
To investigate the cytokine and chemokine profiles in the serum of CP patients, blood samples were obtained 1 hr before operation and at approximately 24 hr after transplantation. Serum was separated by centrifugation and stored in aliquots at –80℃ until analysis. Serum samples from CP patients were diluted 1:5 in 1× PBS shortly before analysis. Patient serum was analyzed using a Milliplex® assay (Human Cytokine/Chemokine Panel I kit; Millipore Corp.) using a Luminex 200 (Luminex, Texas, USA). This kit allows the simultaneous quantification of the following 42 human cytokines/chemokines: EGF, eotaxin, FGF-2, Flt-3 ligand, fractalkine/CX3CL1, granulocyte-colony stimulating factor (G-CSF), granulocyte macrophage-colony stimulating factor (GM-CSF), GROα/CXCL1, interferon (IFN) α2, interferon (IFN) γ , interleukin (IL)-1α, IL-1β, IL-1 receptor antagonist (IL-1ra), IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8/CXCL8, IL-9, IL-10, IL-12(p40), IL-12(p70), IL-1,3 IL-15, IL-17, IP-10/CXCL10, monocyte chemotactic protein (MCP)-1/ CCL2, MCP-3/CCL7, MDC/CCL22, macrophage inflammation protein (MIP)-1α/CCL3, MIP-1β /CCL5, platelet-derived growth factor (PDGF) -AA, PDGF-AB/ BB, RANTES/CCL5, sCD40L, sIL-2Rα, transforming growth factor (TGF)α, tumor necrosis factors (TNF)α, TNFβ, and vascular endothelial growth factor (VEGF).
GMFM, GMPM, MMT and social function and mobility tests
Five observational tests were performed to assess children with cerebral palsy pre- and post- transplantation.
GMFM (gross motor function measure): The aim of this test is to examine gross motor function over time. The items in the GMFM questionnaire were measured by observation and scored on a scale of 0 (does not initiate), 1 (initiates), 2 (partially completes), 3 (completes), and NT (not tested). The items were divided into 5 dimensions according to the purpose of each question: A. walking, running and jumping; B. sitting; C. crawling and kneeling; D. standing; and E. walking, running and jumping.
GMPM (gross motor performance test): This test was carried out to measure dissociated movement, coordination, alignment, and weight shift and stability, which are affected in children with CP. Based on the observations, the items in the GMPM questionnaire were scored on a scale of 1 (severely abnormal), 2 (moderately abnormal), 3 (mildly abnormal), 4 (inconsistently normal), and 5 (consistently normal).
MMT (manual muscle testing): This procedure is designed to evaluate the tone and strength of limb muscles by examining the performance of each muscle in response to gravity and manual resistance.
Social function and mobility test: For this test, parents were asked to fill in a questionnaire about the social function and mobility of their children. The items in the questionnaire were scored on a scale of 0 (the child was not able to perform the task) and 1 (the child was able to perform the task).
Statistical analysis
Statistical analyses were conducted on a CHA University mainframe computer using the Statistical Analysis System (SAS; SAS Korea Inc., Seoul, Korea), version Enterprise 4.0. For the human blood sample and demographic data, the nonparametric analysis method was used since the assumptions of normality were not met due to the small sample size. Differences in gestational age, neonatal body weight, TNC (×108), weight, and age between the allogeneic and autologous treatment groups were analyzed using the Wilcoxon rank sum test. Differences in the EPO levels and IGF values between the allogeneic and autologous groups were analyzed using the Wilcoxon rank sum test, and changes in the variables within the allogeneic and autologous groups were analyzed using the Wilcoxon sign test. Differences in the levels of EGF, eotaxin, FGF-2, Flt-3 ligand, fractalkine/CX3CL1, granulocyte-colony sti-mulating factor (G-CSF), granulocyte macrophage-colony stimulating factor (GM-CSF), GROα/CXCL1, interferon (IFN) α2, interferon (IFN) γ, interleukin (IL)-1α, IL-1 β, IL-1 receptor antagonist (IL-1ra), IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8/CXCL8, IL-9, IL-10, IL-12(p40), IL-12(p70), IL-1,3 IL-15, IL-17, IP-10/CXCL10, monocyte chemotactic protein (MCP)-1/CCL2, MCP-3/CCL7, MDC/ CCL22, macrophage inflammation protein (MIP)-1α /CCL3, MIP-1β/CCL5, platelet-derived growth factor (PDGF) -AA, PDGF-AB/BB, RANTES/CCL5, sCD40L, sIL-2Rα, transforming growth factor (TGF)α, tumor necrosis factors (TNF)α, TNFβ, and vascular endothelial growth factor (VEGF) between the allogeneic and autologous groups were analyzed using the Wilcoxon rank sum test. Significance was assigned at the p <0.05 level. Correlations between changes in interleukin levels and social function scores were analyzed using Pearson’ s correlation coefficient.
Results
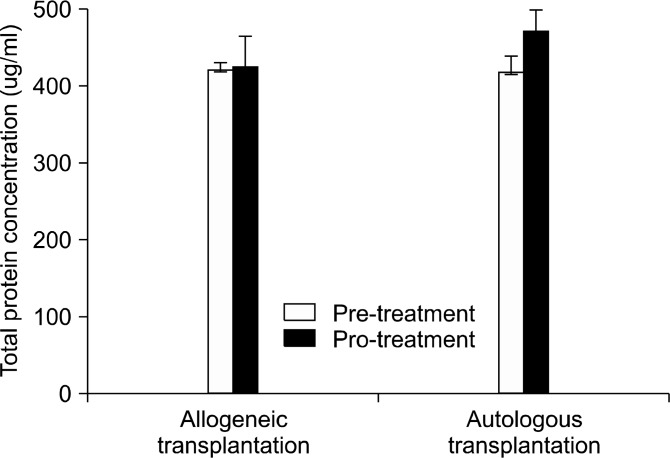
There was a significant difference in the gestational age between the two groups (p<0.04). The mean gestational age of the allogeneic group was 39 weeks (n=3), while for the autologous group it was 35 weeks (n=4). There were no significant differences in birth weight, the number of nucleated cells (TNC), or the age of the patients between the two groups (see Table 1). The EPO and IGF1 concentrations in patient plasma were analyzed before and after treatment with EPO. As shown in Table 2, there were no significant differences in the levels of EPO and IGF1 preand post-treatment between the allogeneic and autologous groups (p< 0.59 for EPO; p< 1.00 for IGF1). Blood was taken from each patient twice, the day before transplantation and the day after transplantation. Table 3 shows the difference in the levels of cytokines and chemokines in the serum of patients with CP pre- and post-allogeneic (n=3) or autogenic (n=4) transplantation. There was no difference between pre- and post- transplant con centration of total protein in the both groups (Fig. 1). However, there were significant group differences in the levels of five cytokines, IL-1α , IL-6, IL-15, RANTES, and TNF-β , among the 42 factors measured. The concentrations of the pro-inflammatory cytokines IL-1α and IL-6 were significantly decreased 24 hrs after transplantation in the allogeneic group, whereas these factors increased following treatment in the autogenic group. The differences (Post cytokine levels-pre cytokine levels) in IL-1α and IL-6 at the two time points were as follows: 1) allogeneic IL-1α, −4.75± 5.54; autogenic, 8.74± 16.02
Table 2.
EPO and IGF1 concentration in the serum of neonatal CP patients
| Variable | Allogeneic | Autologous | p-valuea | ||
|---|---|---|---|---|---|
|
| |||||
| N | Mean±S.E | N | Mean±S.E | ||
|
| |||||
| EPO | |||||
| EPO pre | 3 | 59.07±11.51 | 4 | 44.9±14.01 | |
| EPO post | 3 | 110.5±84.75 | 4 | 77.5±17.63 | |
| Difference | 3 | -51.43±78.09 | 4 | -32.6±6.95 | 0.59 |
| IGF-1 | |||||
| IGF-1 pre | 2 | 56.4±30.2 | 4 | 79.18±36.73 | |
| IGF-1 post | 3 | 49.3±24.3 | 4 | 82.63±40.06 | |
| Difference | 2 | -5.05±6.25 | 4 | -3.45±3.57 | 1 |
aWilcoxon rank sum test.
Table 3.
Concentration of cytokine and chemokine (pre-and post-) in serum from CP patients with either allogenic (n=3) or autologous (n=4)
| Allogeneic (N=3) | Autologous (N=4) | p-value | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Pre (pg/ml) | Post 24 h (pg/ml) | Difference | Pre (pg/ml) | Post 24 h (pg/ml) | Difference | ||
|
| |||||||
| IL-1aa | 29.69±33.86 | 24.94±28.38 | -4.75±5.54 | 147.15±189.88 | 155.3±204.22 | 8.14±16.02 | 0.04 |
| IL-6a | 23.81±3.94 | 8.89±10.29 | -14.92±9.07 | 59.53±77.27 | 60.13±80.01 | 0.61±4.52 | 0.04 |
| IL-15a | 10.72±2.4 | 12.73±2.29 | 2.01±0.5 | 107.42±140.18 | 104.41±139.6 | -3.01±4.02 | 0.05 |
| CCL5/RANTESa | 4,423.95±482.08 | 3,051.15±490.01 | -1,372.8±353.97 | 2,796.13±324.69 | 2,901.74±398.66 | 105.61±118.86 | 0.05 |
| TNFβa | 23.72±14.89 | 18.45±14.87 | -5.27±1.11 | 200.84±280.02 | 201.35±282.64 | 0.51±3.06 | 0.05 |
| CCL4/MIP-1β | 307.38±367.2 | 60.39±37.18 | -246.99±336.28 | 137.11±107.92 | 158.2±148.77 | 21.1±43.53 | 0.11 |
| IL-5 | 0.31±0.54 | 0.11±0.18 | -0.21±0.36 | 16.15±28.83 | 16.35±28.97 | 0.2±0.23 | 0.17 |
| CCL3/MIP-1α | 1,009.85±1,286.47 | 58.93±54.68 | -950.92±1,237.62 | 55.4±106.51 | 54.06±108.13 | -1.34±3.49 | 0.19 |
| sCD40L | 9,900.8±171.81 | 10,000±0 | -99.2±171.81 | 8,637.55±954.67 | 9,722.46±555.07 | 1,084.92±891.66 | 0.19 |
| IL-8/CXCL8 | 421.77±531.57 | 24.32±5.25 | -397.46±533.18 | 60.26±69.99 | 55.27±63.42 | -4.99±6.91 | 0.21 |
| IFNr | 31.86±36.83 | 8.91±2.88 | -22.95±34.5 | 163.65±281.49 | 169.24±305.21 | 5.59±26.15 | 0.21 |
| sIL-2Rα | 167.84±33.57 | 165.66±31.5 | -2.18±21.03 | 349.96±166.82 | 421.88±185.4 | 71.92±66.69 | 0.21 |
| Flt-3 Ligand | 58.54±34.3 | 27.95±4.75 | -30.59±32.8 | 139.97±186.59 | 143.61±185.08 | 3.64±8.73 | 0.21 |
| GM-CSF | 81.24±105.54 | 27.72±41.14 | -53.52±65.44 | 179.95±341.54 | 162.61±305.93 | -17.33±35.69 | 0.21 |
| IL-2 | 4.17±2.23 | 2.94±0.9 | -1.23±1.47 | 36.45±61.67 | 38.37±66.74 | 1.92±5.24 | 0.21 |
| IL-13 | 11.29±10.12 | 9.14±9.51 | -2.16±1.26 | 73.39±111.49 | 72.48±108.48 | -0.91±3.59 | 0.37 |
| EGF | 65.81±56.2 | 42.08±33.1 | -23.73±29.85 | 21.71±38.36 | 33.15±28.68 | 11.44±38.17 | 0.37 |
| VEGF | 218.84±175.58 | 73.41±5.29 | -145.42±172.97 | 437.95±769.7 | 412.26±673.76 | -25.69±100.2 | 0.37 |
| IL-17 | 4.37±5.61 | 0.41±0.72 | -3.96±4.92 | 84.45±158.13 | 79.84±143.8 | -4.61±15.1 | 0.52 |
| IL-1b | 8.62±14.92 | 0±0 | -8.62±14.92 | 72.58±117.46 | 73.58±119.79 | 1±2.43 | 0.55 |
| IL-9 | 0±0 | 0±0 | 0±0 | 5.48±10.96 | 4.77±9.54 | -0.71±1.42 | 0.56 |
| IFNa2 | 0±0 | 0±0 | 0±0 | 166.31±332.63 | 171.05±342.1 | 4.74±9.48 | 0.56 |
| G-CSF | 0±0 | 0±0 | 0±0 | 41.26±82.52 | 46.05±92.1 | 4.79±9.58 | 0.56 |
| PDGF-AB/BB | 10,000±0 | 10,000±0 | 0±0 | 10,000±0 | 9,187.66±1,624.68 | -812.34±1,624.68 | 0.56 |
| IL-4 | 5.57±9.01 | 2.09±3.63 | 3.48±5.39 | 88.46±151.91 | 92.01±141.74 | 3.55±19.07 | 0.58 |
| IL-10 | 34.66±4.47 | 34.07±17.48 | 0.59±14.29 | 158.22±235.78 | 157.5±237.87 | -0.72±2.18 | 0.59 |
| IL-12(p40) | 55.31±27.9 | 41.55±15.89 | 13.76±17 | 243.22±321.25 | 277.6±401.88 | 34.38±82.3 | 0.59 |
| CCL7/MCP-3 | 87.21±45.21 | 83.13±45.43 | 4.09±3.01 | 253.1±324.63 | 244.48±319.72 | -8.62±12.56 | 0.59 |
| TNFα | 22.31±19.58 | 7.79±3.11 | 14.52±16.74 | 18.72±25.2 | 17.66±26.11 | -1.06±0.94 | 0.59 |
| CCL2/MCP-1 | 468.39±75.59 | 437.75±51.21 | 30.64±126.3 | 890.61±495.35 | 819.58±523.94 | -71.03±146.34 | 0.85 |
| CCL11/Eotaxin | 78.93±26.87 | 77.01±39.15 | 1.92±12.86 | 101.47±103.04 | 103±87.51 | 1.53±23.89 | 0.85 |
| CCL22/MDC | 2,545.56±310.98 | 2,176.97±368.79 | 368.59±239.21 | 2,762.14±511.62 | 2,362.79±408.48 | -399.35±312.24 | 0.85 |
| CX3CL1 | 126.5±91.12 | 116.07±67.11 | 10.44±35.54 | 714.94±918.12 | 704.45±968.69 | -10.49±55.24 | 0.85 |
| /Fractalkine | |||||||
| TGFα | 19.97±7.22 | 18.35±4.25 | 1.62±3.55 | 72.55±114.19 | 71.61±107.82 | -0.94±8.42 | 0.85 |
| IL-1ra | 100.6±72.77 | 71.25±13.83 | 29.35±59.65 | 329.54±460.32 | 324.54±441.13 | -5±20.85 | 1 |
| IL-3 | 0±0 | 0±0 | 0±0 | 0±0 | 0±0 | 0±0 | 1 |
| IL-7 | 0±0 | 0±0 | 0±0 | 30.82±59.53 | 30.67±58.2 | 0.15±1.55 | 1 |
| IL-12(p70) | 7.14±2.13 | -5.74±1.26 | -1.4±2.65 | 80±143.97 | 74.42±132.48 | 5.58±11.53 | 1 |
| CXCL1/GROα | 792.65±333 | -814.92±302.73 | 22.27±54.61 | 896.52±358.54 | 955.57±287.98 | -59.05±114.21 | 1 |
| CXCL10/IP-10 | 237.07±27.65 | -212.79±55.96 | 24.27±74.31 | 270.85±79.38 | 230.76±73.59 | 40.09±42.78 | 1 |
| FGF-2 | 123.82±76.46 | -75.53±17.69 | 48.29±59.32 | 406.61±634.33 | 376.09±614.75 | 30.51±28.35 | 1 |
| PDGF-AA | 7,682.55±1493.7 | 8,020.52±487.87 | 337.97±1,124.51 | 6,461.26±2,424.62 | 6,645.9±2,984.82 | 184.64±1,116.36 | 1 |
Fig. 1. Total protein concentration in serum (ug/ml) : Comparison of total protein concentration in the serum of 7 neonatal CP patients. There was no significant difference between pre- and posttransplant concentration of total protein in the both groups.
(ap<0.04); and 2) allogeneic IL-6, -14.92±9.07; autogenic, 0.61±4.52 (ap<0.04). A similar pattern was also seen for RANTES and TNF-β. The difference in RANTES levels before and after transplantation were ?1,372.8±353.97 for the allogeneic group and 105.61±118.86 for the autogenic group (ap<0.05). For TNF-β, the levels were ?5.27±1.11 for the allogeneic group and 0.51±3.06 for the autogenic group (ap<0.05). In contrast to this trend, the levels of IL-15 pre- and post-transplantation changed in the other direction, such that the autogenic group showed decreased IL-15 levels 24 hrs after transplantation, whereas the allogeneic group showed increased IL-15 levels (2.01±0.5 for the allogeneic group and ?3.01±4.02 for the autogenic group, ap<0.05). In addition to the cytokines mentioned above, our data suggest that four other cytokines decreased in the allogenic group. Even though the differences were not statistically different, the levels of CCL4/MIP-1b, IL-5, CCL3/MIP-1α, and sCD40L, were lower in the serum of the allogenic group after transplantation than in that of the autologous group [CCL4/MIP-1b, ?246.99±336.28 for the allogeneic group and 21.1±43.53 for the autologous group (p<0.11); IL-5, ?0.21±0.36 for the allogeneic group and 0.2±0.23 for the autologous group (p<0.17); CCL3/MIP-1α, ?950.92±1,237.62 for the allogeneic group and ?1.34±3.49 for the autogenic group (p<0.19); and sCD40L, ?99.2±171.81 for the allogeneic group and 1084.92±891.66 for the autologous group (p<0.19).
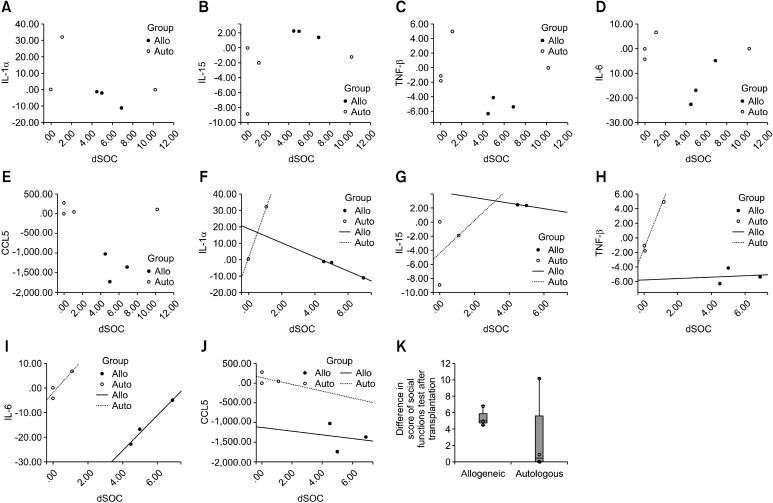
In the autologous group, the levels of MIP-1alpha and MIP-1beta tended to show an increase, whereas the allogeneic group experienced relatively decreases in the levels of these cytokines post-treatment. A similar trend was seen for IL-5. These chemokines and pro-inflammatory cytokines are upregulated under pathogenic conditions. Given that cord blood from a child with CP may have been derived from a more inflammatoryprone environment (11), it is plausible that autologous treatment augments the levels of pro-inflammatory cytokines and chemokines that are upregulated during inflammation whereas allogeneic treatment exhibited a pattern opposite to the autologous group, suggesting that allogenic treatment alleviated inflammation responses. The allogeneic (n=3) and autologous (n=4) groups were subjected to GMFM, GMPM, MMT, and social function and mobility tests to quantify changes in behavior over time. For each test, a higher score indicates that the subject showed better performance. The five observational instruments were carried out pre- and post-transplantation. For the GMFM and social function tests, there was a significant difference in test scores pre- and post-transplantation in the allogeneic group (bp<0.001 for GMFM; cp< 0.01 for social function, Table 4). However, no significant differences were observed in the autologous group, indicating that only the allogeneic transplantation group exhibited an improvement in GMFM and social interaction after transplantation. Scores in the social function test were correlated with pro-inflammatory cytokines levels. For the autologous group, three out of four patients showed a high level of pro-inflammatory cytokines and a low score in the social function test, whereas for the allogeneic group, all three patients exhibited a low level of pro-inflammatory cytokines and a high score in the social function test (Fig. 2 A∼E). After excluding data of one patient that displayed the opposite pattern to other three patients in the autologous group (Fig. 2, a strong positive correlation was found between improved scores in the social function test and changes in IL-6 and TNF-α levels
Table 4.
GMFM, GMPM, MMT and social function, mobility
| Variable | Allogeneic | Autologous |
|---|---|---|
|
| ||
| Mean±S.E | Mean±S.E | |
|
| ||
| GMFM pre | 41.67±26.3 | 27.5±16.45 |
| GMFM post | 44.67±26.3 | 29.5±16.65 |
| Difference | 3±0 | 2±0.71 |
| p-valuea | p<0.0001b | p>0.05 |
| GMPM pre | 34.33±12.81 | 29.5±14.27 |
| GMPM post | 42.6±19.79 | 36±11.52 |
| Difference | 8.27±7.19 | 6.5±3.77 |
| p-valuea | p>0.05 | p>0.05 |
| MMT pre | 103.33±36.19 | 74.5±22.29 |
| MMT post | 108.67±31.12 | 76.5±22.46 |
| Difference | 5.33±5.33 | 2±1.15 |
| p-valuea | p>0.05 | p>0.05 |
| Social fn pre | 36.73±14.43 | 36.6±5.94 |
| Social fn post | 42.2±13.99 | 39.43±7.95 |
| Difference | 5.47±0.73 | 2.83±2.47 |
| p-valuea | p<0.01c | p>0.05 |
| Mobility pre | 24.2±17.17 | 26.38±9.2 |
| Mobility post | 33.43±20.39 | 28.4±12.22 |
| Difference | 9.23±3.34 | 2.03±3.11 |
| p-valuea | p>0.05 | p>0.05 |
aWilcoxon sign test; bSignificant at the 0.01% significance level; cSignificant at the 1% significance level.
Fig. 2. Correlation between scores in the social function test and cytokine levels and distribution of differences in sores of social function test after transplantation in a box plot: Each dot indicates a patient (an open circle indicates a patient in the autologous group and a filled circle indicates a patient in the allogeneic group). The scatter plot in the upper panel shows the relationship between scores in the social function test and changes in the levels of cytokines after cell transplantation. For the autologous group, three out of four patients showed a high level of pro-inflammatory cytokines and a low score in the social function test, whereas for the allogeneic group, all three patients exhibited a low level of pro-inflammatory cytokines and a high score in the social function test (A-E). As shown in the box plot of social function scores (K), since difference in the social function score (post--pre transplantation score) of one patient in the autologous group is almost equal to 95th percentile (95th percentile=10.69, this patient score=10.2) and showed no or relatively small changes of inflammatory cytokines after transplantation (0 pg/ml for IL-1α; 0 for IL-6; -1.15 for IL-15; and 0 for TNF-β), an additional correlation analysis was performed without this one patient. After excluding data of one patient that displayed the opposite pattern to other three patients in the autologous group, a strong positive correlation was found between improved scores in the social function test and changes in IL-6 and TNF-α levels in the autologous group, according to the lines of best fit (Pearson’ s correlation coefficient=1.000 and p=0.007 between the interleukin 1α level and the score in the social function test, and Pearson’ s correlation coefficient=0.99 and p=0.05 between the TNF-α level and the score in the social function test) (F-J). It is important to note that the higher scores in the social function test were correlated closely with the lower levels of cytokines in the allogeneic groups (F-J).
in the autologous group, according to the lines of best fit (Pearson’ s correlation coefficient=1.000 and p=0.007 between the interleukin 1α level and the score in the social function test, and Pearson’ s correlation cpoefficient=0.99 and p=0.05 between the TNF α level and the score in the social function test) (Fig. 2F∼J). It is important to note that the higher scores in the social function test were correlated closely with the lower levels of cytokines in the allogeneic groups (Fig. 2F∼J).
The differences between the groups for the other tests (GMPM, MMT, and the mobility test) were not statistically significant, although the scores tended to increase
following transplantation irrespective of whether the patients belonged to the autologous or the allogeneic group.
Discussion
Our data suggest that, in CP patients, differences in the levels of inflammatory cytokines pre- and post-hUCBCs transplant depend on whether the cells are allogeneic or autologous. Allogeneic treatment significantly reduced the levels of proinflammatory cytokines, such as IL-1α, IL-6, RANTES, and TNF-β , in serum compared to the autogenic group. Consistent results were observed in the changed concentrations of CCL4/MIP-1β , IL-5, sCD40L and MIP-1 after transplantation, although the values were marginally significant (Table 3). High levels of pro-inflammatory cytokines in cord blood have been implicated in neonatal brain damage, such as that which occurs in CP or premature birth. More specifically, the levels of proinflammatory cytokines, such as IL-1β , TNF-α, and
IL-6, are higher in neonates with perinatal asphyxia or following maternal infection (1, 3, 7). It seems probable that cord blood from neonates with CP has relatively higher levels of inflammatory-related cytokines than cord blood from allogeneic sources, and therefore the levels of inflammatory factors in blood depend on the cell donor’ s health status (3, 11). Our finding that the levels of inflammatory factors in the blood of the autologous group were significantly higher than those in the allogeneic group is somewhat expected, since the autologous hUCBCs were isolated from newborn children with CP, while the allogeneic cells were from normal neonates. This result further suggests that it may be important to give serious consideration to screening hUCBCs carefully before transplantation to eliminate risk factors that would aggravate CP symptoms, such as inflammatory cytokines. Taken together, our data demonstrate that the levels of pro-inflammatory cytokines such as IL-1α, IL-6, TNF-β and RANTES were decreased more in the allogeneic treatment group than in the autologous group.
We observed a significant improvement in the allogeneic group in 2 out of 5 behavioral and social tests compared to the autologous group, suggesting that allogeneic or proinflammatory cytokine-screened cells may more efficiently treat CP. However, it could be argued that the better outcome in the allogeneic group might be due instead to the significant difference in gestational age between the two groups (39.67±0.33 vs. 35.75±0.85; allogeneic and autologous, respectively), given that the pregnancy period is an important determinant of developing CP, even though the diagnosis of the disorder requires the consideration of multiple factors (12). Injection of the immuno-suppressant, cyclosporine, may also decrease inflammatory factors in the allogeneic group. However, it is not likely that the cyclosporine is a contributing factor to produce better outcomes in the allogeneic group compared to the autologous group as cyclosporine has been known to be implicated in neurotoxicity, especially in pediatric patients (13).
These data strongly suggest that in case of infants born prematurely, the source of stem cells for therapy should be selected with extreme care. The number of neonates with CP studied in our analysis, however, was not enough (n=7) to obtain definite results, and further studies with a larger sample size are needed. There may be a potential interaction between cord blood cells and EPO such that EPO primes the damaged brain for the therapeutic effect of hUCBCs. More research is needed to elucidate a combination effect between cord blood cells and EPO in treating patients with CP effectively. Given that cord blood from a child with CP may have been derived from a more inflammatory- prone environment (11), it is plausible that autologous treatment augments the levels of pro-inflammatory cytokines and chemokines that are upregulated during inflammation whereas allogeneic treatment exhibited a pattern opposite to the autologous group, suggesting that allogeneic treatment alleviated inflammation responses. In summary, the analysis of serum from children with CP showed that the levels of pro-inflammatory cytokines were significantly higher in the allogeneic group than the autologous group after transplantation, suggesting that allogeneic hUCBCs therapy may be an appropriate strategy and should be evaluated in further human clinical trials.
Acknowledgments
This work was funded by the KOSEF (Grant 2011- 0029342 and 2011-0013280) and CHA Bio & Diostech. We are grateful to Yun-hwa Jeong and Clinical Statistics Center of CHA University for analyzing statistic.
Potential conflict of interest
The authors have no conflicting financial interest.
References
- 1.Nelson KB, Grether JK, Dambrosia JM, Walsh E, Kohler S, Satyanarayana G, Nelson PG, Dickens BF, Phillips TM. Neonatal cytokines and cerebral palsy in very preterm infants. Pediatr Res. 2003;53:600–607. doi: 10.1203/01.PDR.0000056802.22454.AB. [DOI] [PubMed] [Google Scholar]
- 2.Girard S, Kadhim H, Roy M, Lavoie K, Brochu ME, Larouche A, Sébire G. Role of perinatal inflammation in cerebral palsy. Pediatr Neurol. 2009;40:168–174. doi: 10.1016/j.pediatrneurol.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 3.Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol. 1998;44:665–675. doi: 10.1002/ana.410440413. [DOI] [PubMed] [Google Scholar]
- 4.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13:323–340. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 5.Mulcahy NJ, Ross J, Rothwell NJ, Loddick SA. Delayed administration of interleukin-1 receptor antagonist protects against transient cerebral ischaemia in the rat. Br J Pharmacol. 2003;140:471–476. doi: 10.1038/sj.bjp.0705462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Touzani O, Boutin H, Chuquet J, Rothwell N. Potential mechanisms of interleukin-1 involvement in cerebral ischaemia. J Neuroimmunol. 1999;100:203–215. doi: 10.1016/s0165-5728(99)00202-7. [DOI] [PubMed] [Google Scholar]
- 7.Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, Kim IO. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177:19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- 8.Huang J, Upadhyay UM, Tamargo RJ. Inflammation in stroke and focal cerebral ischemia. Surg Neurol. 2006;66:232–245. doi: 10.1016/j.surneu.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 9.Chaitanya GV, Schwaninger M, Alexander JS, Babu PP. Granzyme-b is involved in mediating post-ischemic neuronal death during focal cerebral ischemia in rat model. Neuroscience. 2010;165:1203–1216. doi: 10.1016/j.neuroscience.2009.10.067. [DOI] [PubMed] [Google Scholar]
- 10.Chaitanya GV, Eeka P, Munker R, Alexander JS, Babu PP. Role of cytotoxic protease granzyme-b in neuronal degeneration during human stroke. Brain Pathol. 2011;21:16–30. doi: 10.1111/j.1750-3639.2010.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matoba N, Yu Y, Mestan K, Pearson C, Ortiz K, Porta N, Thorsen P, Skogstrand K, Hougaard DM, Zuckerman B, Wang X. Differential patterns of 27 cord blood immune biomarkers across gestational age. Pediatrics. 2009;123:1320–1328. doi: 10.1542/peds.2008-1222. [DOI] [PubMed] [Google Scholar]
- 12.Reddihough DS, Collins KJ. The epidemiology and causes of cerebral palsy. Aust J Physiother. 2003;49:7–12. doi: 10.1016/s0004-9514(14)60183-5. [DOI] [PubMed] [Google Scholar]
- 13.Serkova NJ, Christians U, Benet LZ. Biochemical mechanisms of cyclosporine neurotoxicity. Mol Interv. 2004;4:97–107. doi: 10.1124/mi.4.2.7. [DOI] [PubMed] [Google Scholar]