Abstract
Background and Objectives:
Type 2 diabetes mellitus (DM) is a prevalent disorder. Diabetic keratopathy is a well-known ocular complication secondary to type 2 DM. Topical insulin application did not affect apoptosis and necrosis levels in corneal epithelium. Autologous cell transplantation is not a viable option for diabetic patients with bilateral limbal stem cell deficiency. The present study aimed at assessing the possible effect of hemopoeitic stem cell (HSC) therapy on induced diabetic keratopathy in albino rat.
Methods and Results:
Fifteen male albino rats were divided into control group of 2 rats, diabetic group of 8 rats receiving single intraperitoneal (IP) injection of 50 mg/kg streptozotocin (STZ). 3 animals were sacrificed 6 weeks following confirmation of diabetes to confirm keratopathy and 5 rats were sacrificed 4 weeks following confirmation of keratopathy. SC therapy group included 5 rats injected with HSCs 6 weeks following confirmation of diabetes and sacrificed 4 weeks following SC therapy. Cord blood collection, stem cells isolation and labeling were performed. Eye specimens were subjected to histological, histochemical, immunohistochemical, morphometric and statistical studies. In diabetic group, the central cornea showed multiple cells with vacuolated cytoplasm and dark nuclei, focal epithelial discontinuity, reduced corneal thickness and less number of layers of corneal and conjunctival epithelia. In stem cell therapy group, few cells with vacuolated cytoplasm and dark nuclei were found in the corneal and conjunctival epithelia with more number of epithelial layers.
Conclusions:
A definite ameliorating effect of HSC therapy was detected on diabetic keratopathy. The therapeutic cells were effective in limiting corneal epithelial changes.
Keywords: Hemopoeitic stem cells, Diabetes, Cord blood, Keratopathy
Introduction
Type 2 diabetes mellitus (DM) is a prevalent disorder that affects children, adolescents, and adults worldwide (1). Diabetic keratopathy is a well-known ocular complication secondary to type 2 diabetes mellitus (2). Diabetes adversely affects corneal sensory nerves and consequently impairs their function, with vision loss being the inevitable consequence of severe corneal neurotrophic ulceration. However, current standard treatment regimens are often ineffective (3).
Following topical insulin application diabetic rats had corneal sensitivity values comparable to the healthy group. However, apoptosis and necrosis levels were similar (4). Naltrexone topically treated diabetic rabbits had residual corneal defects that were 23% smaller than diabetic rabbits (5).
Umbilical cord blood transplantation (CBT) has been widely used as an alternative source of hematopoietic cell support for SC transplant patients. CBT offers several advantages over traditional stem cell sources, such as immediate availability and lower risk of acute graft-versus-host disease. Recent studies suggest that CBT is a safe and effective strategy for adult patients lacking a suitable related or unrelated donor (6). Autologous cell transplantation is not a viable option for diabetic patients with bilateral limbal SC deficiency (7).
The present work aimed at assessing the possible effect of CB HSC therapy on induced diabetic keratopathy in albino rat.
Materials and Methods
Fifteen male albino rats weighing 150∼200 g were used and divided into 3 groups placed in separate cages in the Animal House of Kasr El Aini. The rats were treated in accordance with guidelines approved by the Animal Use Committee of Cairo University. Group 1 (control group): 2 control rats, 1 for each experimental group. Each animal received single intraperitoneal (IP) injection of citrate buffer.
Group 2 (diabetic group): Eight rats, each received single IP injection of 50 mg/kg (8) streptozotocin (STZ) (Sigma Chemical Corporation., St. Louis) dissolved in citrate buffer. Diabetes was confirmed 3 days later by blood glucose level greater than 250 mg/dl. Blood samples were obtained from the right eye and analysis was performed in Biochemistry Department, Faculty of Medicine, Cairo University. Early diabetic eye changes develop 6 weeks following confirmation of diabetes (8). Three animals were sacrificed 6 weeks following confirmation of diabetes to check for keratopathy and 5 animals were sacrificed 4 weeks following confirmation of keratopathy.
Group 3 (stem cell therapy group): 5 rats were subjected to induction of diabetes in the same way as in the second group. In addition, they were injected with 0.5 ml of isolated and labeled SCs suspended in phosphate buffer saline (PBS) in the tail vein (9) following confirmation of diabetic keratopathy. SCs were isolated from cord blood (10).
Cord blood collection was performed at Gynaecology Department, Faculty of Medicine, Cairo University. Stem cell isolation and labeling were performed at Clinical Pathology Department, Elmonira Hospital, Cairo University.
The animals were sacrificed 4 weeks following stem cell therapy (11).
Cord blood collection (12)
The storage and transport temperature was 15∼22℃ , transport time was 8∼24 hours, sample volume was 65∼ 250 ml, and no sample had signs of coagulation or hemolysis.
Mononuclear cell fraction isolation (12)
The mononuclear cell fraction (MNCF) was isolated by carefully loading 30 ml of whole blood onto 10 ml of Ficoll density media (Healthcare Bio-Sciences) in 50 ml polypropylene tubes. Centrifuge for 30 minutes at room temperature at 450×g and the interphase collected after aspirating and discarding the supernatant. The interphase was washed with 20 ml PBS and centrifuged at 150×g for 5 minutes at room temperature. The supernatant was aspirated and the cells were washed with PBS a second time. The cells were re-suspended in the isolation media to prevent adherence of monocytic cells. The isolation media was low-glucose DMEM (Dulbecco's modified Eagles medium) (Cambrex Bio Science), penicillin (100 IU/ml) (Invitrogen), streptomycin (0.1 mg/ml) (Invitrogen), and ultraglutamine (2 mM) (Cambrex Bio-Science). Incubation was at 38.5℃ in humidified atmosphere containing 5% CO2.
Labeling (13)
Hemopoeitic stem cells were labeled by incubation with ferumoxides injectable solution (25 μgFe/ml, Feridex, Berlex Laboratories) in culture medium for 24 hours with 375 nanogram/ml poly L lysine added 1 hour before cell incubation. Labeling was histologically assessed using Prussian blue. Feridex labeled HSCs were washed in PBS, trypsinized, washed and resuspended in 0.01 Mol/L PBS at concentration of 1×1,000,000 cells/ml.
Cell viability analysis
Cell viability was done using trypan blue dye exclusion test. This method is based on the principle that viable cells do not take up certain dyes, whereas dead cells do.
Flow cytometry (14)
Flow cytometric analyses were performed on a Fluorescence Activated Cell Sorter (FACS) flow cytometer (Coulter Epics Elite, Miami, FL, USA). HMSC were trypsinized and washed twice with PBS. A total number of 1×105 HSC were used for each run. To evaluate the HSC marker profile, cells were incubated in 100 μl of PBS with 3 μl of CD34-FITC for 20 min at room temperature. Antibody concentration was 0.1 mg ml-1. Cells were washed twice with PBS and finally diluted in 200 μl of PBS. The expression of surface marker was assessed by the mean fluorescence. CD44 (mesenchymal stem cell marker), CD133 (early hematopoietic & endothelial progenitor stem cell marker) and CD45 (panleucocytic marker) were also used. The percentage of cells positive for CD34 was determined by subtracting the percentage of cells stained non-specifically with isotype control antibodies.
Histological study
The animals were sacrificed using lethal dose of ether. Left eye specimens were removed and fixed in 10% formol saline for 24 hours. Paraffin blocks were prepared and 5μm thick sections were subjected to hematoxylin and eosin (H&E) stain (15).
Histochemical study
Eye sections were stained with Prussian blue stain (16) for demonstration of iron oxide labeled therapeutic stem cells.
Immunohistochemical study (17) and (18)
CD34 immunostaining is the marker for HSCs. Mouse Monoclonal Antibody (Lab Vision Corporation, CA 94539, USA, catalogue number MS-363-R7). It was supplied as (7.0 ml) of antibody (200 ug/ml) prediluted in 0.05 mol/L Tris-HCl, pH 7.6 containing stabilizing protein and 0.015 mol/L sodium azide. It was stored at 2∼8℃. No special pretreatment is required for immuno-histochemical staining of formalin-fixed tissues. 2 drops or 100 μl for each section, incubation with the primary antibody took place in humidity chamber for 60 minutes at room temperature. Tonsil used as positive control specimens. Cellular localization is the cytoplasm. On the other hand, one of the eye sections was used as a negative control by passing the step of applying the primary antibody.
Morpthometric study
Using Leica Qwin 500 LTD image analysis, assessment of the thickness of corneal epithelium, the area of dark nuclei and the area of vacuolated cytoplasm detected in
the corneal epithelial cells was performed in H&E stained sections using interactive measurements menu. The measurments were done in 10 high power fields (HPF) in control and experimental groups.
Statistical analysis
Quantitative data were summarized as means and standard deviations and compared using one-way analysis- of-variance (ANOVA). P-values <0.05 were considered statistically significant. Calculations were made on SPSS software (19).
Results
Haematoxylin and eosin (H&E) stained sections
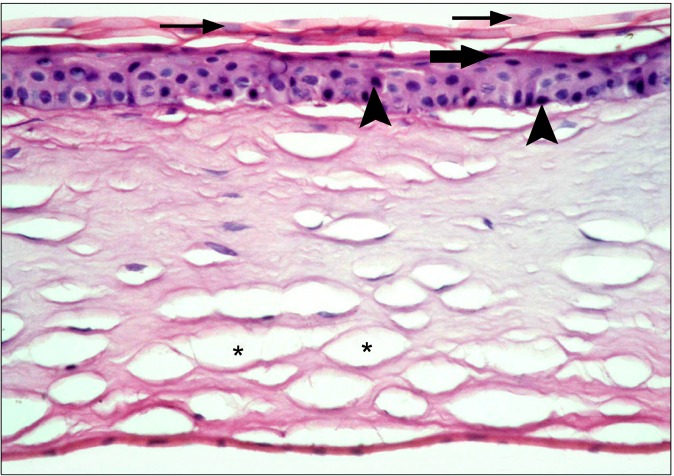
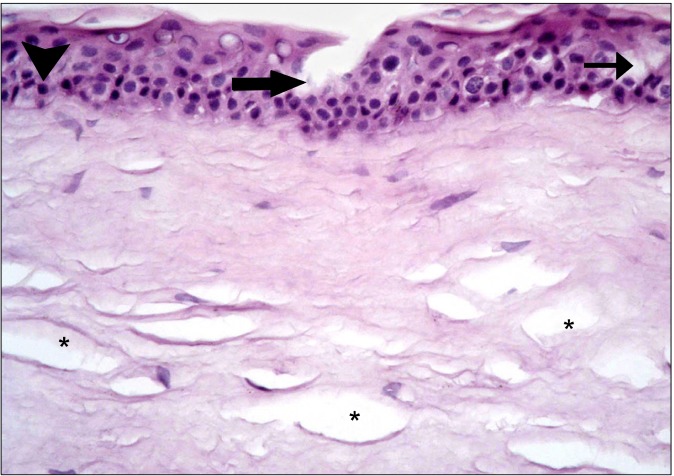
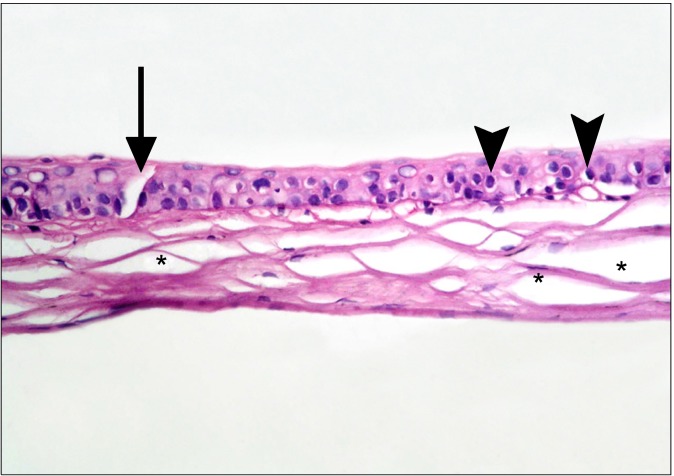
In the diabetic group, the central cornea of rats showed separation of the surface epithelial layers that appeared more acidophilic. Some of the surface nuclei appeared dark, while others appeared as ghost nuclei. Dark nuclei were seen in multiple basal and intermediate cells, in addition separation of the substantia fibers was noticed (Fig. 1). Other fields revealed focal epithelial discontinuity. Dark nuclei and vacuolated cytoplasm of some epithelial cells were evident, in addition to separation of substantia fibers (Fig. 2). Some other fields demonstrated reduced corneal thickness. Focal discontinuity of the epithelial cells, multiple cells showing dark nuclei and vacuolated cytoplasm were seen. Obvious separation of the substantia fibers was found (Fig. 3). The limbus recruited corneal and conjunctival epithelia formed of less number of layers in comparison to control group. Some epithelial cells contained
Fig. 1. The surface epithelial layers at the central cornea appearing separated and more acidophilic. Some of the surface nuclei appear dark (thick arrows) and others appear as ghost nuclei (thin arrows). Multiple dark nuclei (arrowheads) are seen in the basal and intermediate layers. Note separation of substantia fibers (*) (H&E, ×400).
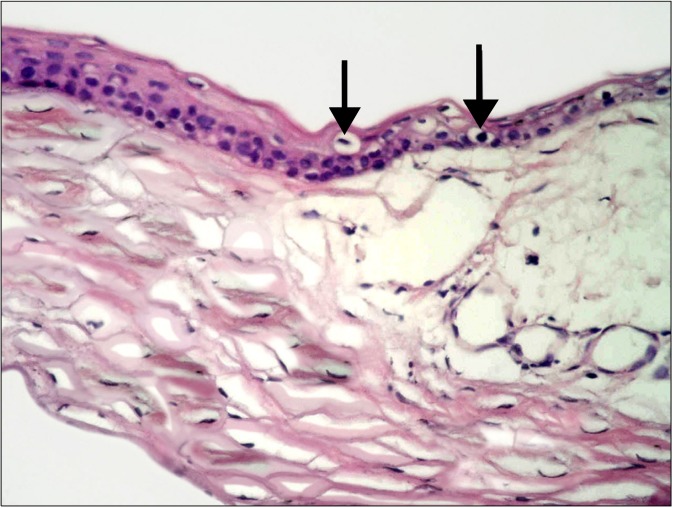
Fig. 2. Focal epithelial discontinuity (thick arrow) at the central cornea. Dark nuclei (arrowhead) and vacuolated cytoplasm (thin arrow) of some epithelial cells are obvious. Note separation of substantia fibers (*) (H&E, ×400).
Fig. 3. educed corneal thickness is seen in comparison to Fig. 1. Focal epithelial discontinuity (arrow) is noted. Dark nuclei and vacuolated cytoplasm of multiple epithelial cells are evident (arrowheads). Note obvious separation of substantia fibers (*) (H&E, ×400).
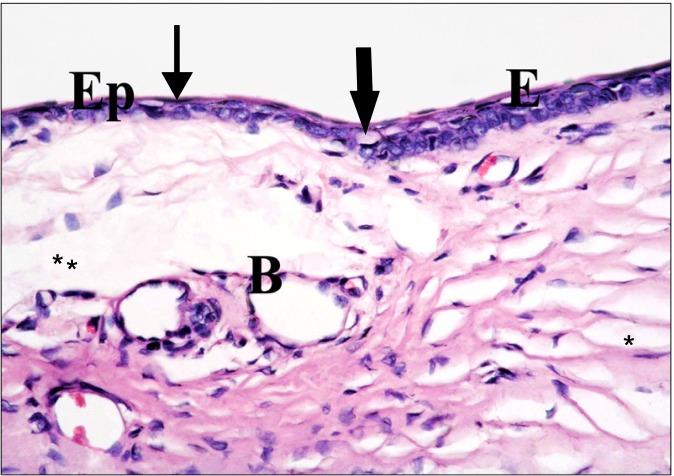
dark nuclei and vacuolated cytoplasm. Obvious separation of substantia fibers was seen. The connective tissue of the sclera showed multiple dilated blood vessels, in addition to separation of substantia and scleral fibers (Fig. 4).
Fig. 4. The corneal (E) and the conjunctival (Ep) epithelia at the limbus are formed of less number of layers in comparison to Fig. 2. Some cells exhibit dark nuclei and vacuolated cytoplasm (thick arrows), others have dark nuclei (thin arrow). Note multiple blood vessels (B), separation of substantia (*) and scleral (**) fibers (H&E, ×400).
In SC therapy group, few cells with vacuolated cytoplasm and dark nuclei were found in the corneal and conjunctival epithelia at the limbus. In addition, more number of epithelial layers was evident in comparison to the diabetic group (Fig. 5). Sections demonstrating the central cornea showed vacuolation of the cytoplasm and dark nuclei of few epithelial cells. No surface keratinization, no focal discontinuity of the epithelium and no reduction of
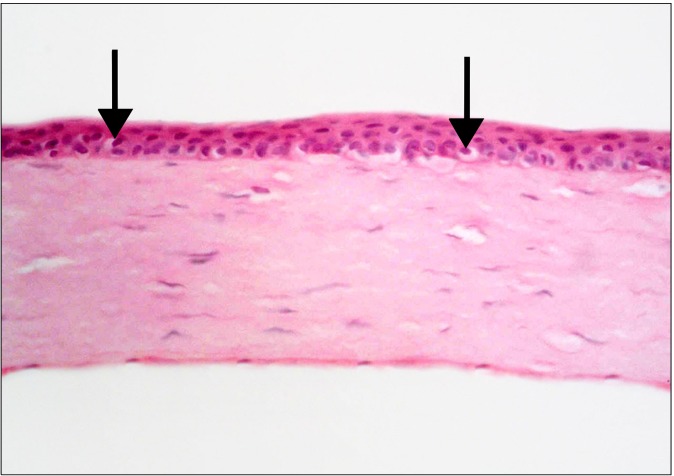
Fig. 5. At the limbus, more number of epithelial layers compared to Fig. 6 is noticed. Few epithelial cells with vacuolated cytoplasm and dark nuclei (arrows) are seen (H&E, ×400).
the corneal thickness were noticed in comparison to the diabetic group (Fig. 6).
Fig. 6. At the central part of the cornea, vacuolated cytoplasm and dark nuclei are seen in few cells (arrows) compared to Fig. 3, 4 and 5 (H&E, ×400).
Prussian blue stained sections
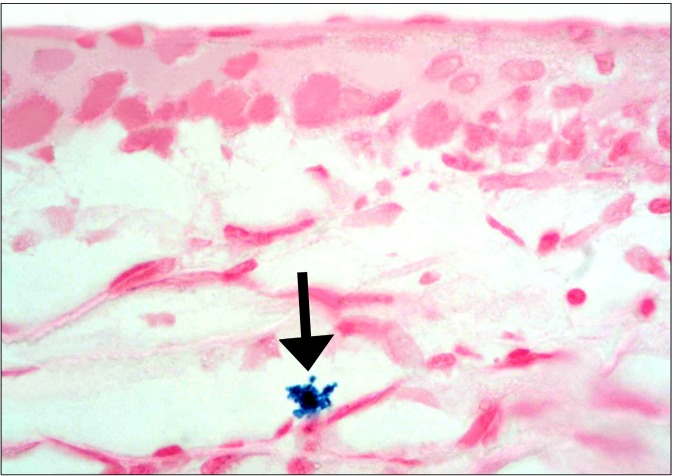
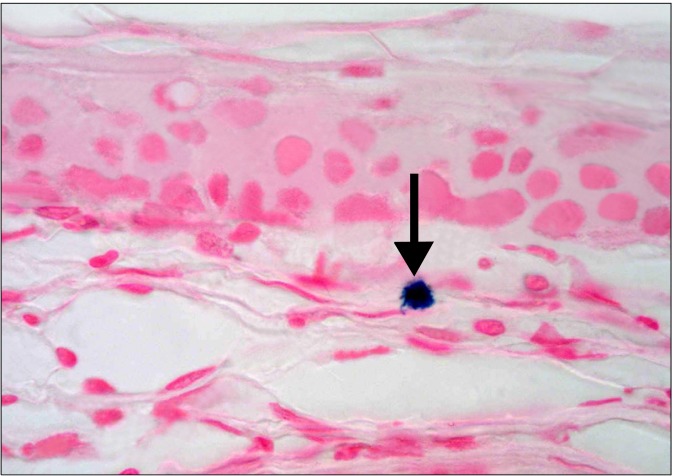
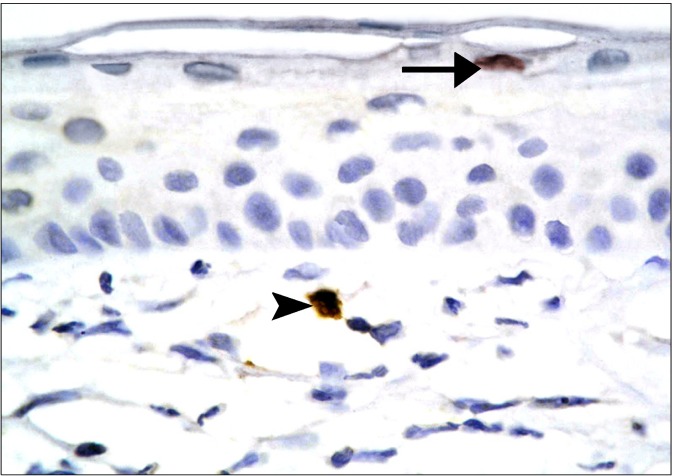
The SC group revealed occasional Prussian blue positive (+ve) branched cells between the connective tissue fibers at the limbus (Fig. 7). Other fields in the limbus recruited occasional branched +ve cells close to the conjunctival epithelium and other +ve cells of variable shapes between the connective tissue fibers of the sclera (Fig. 8). Some fields in the central part of the cornea revealed occasional +ve branched cells with multiple processes deep in the substantia propria (Fig. 9). Other fields showed occasional +ve cuboidal cell in the substantia propria near the
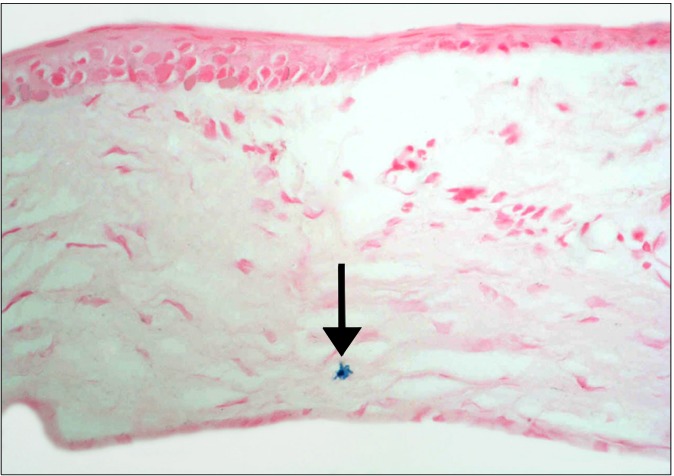
Fig. 7. A diabetic rat in SC therapy group showing a Prussian blue +ve cell (arrow) between the connective tissue fibers of the sclera (Prussian blue, ×400).
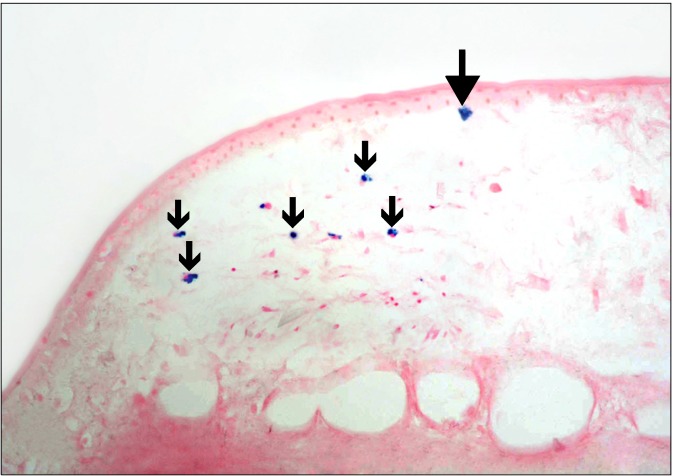
Fig. 8. A diabetic rat in SC therapy group showing a Prussian blue +ve cell close to the conjunctival epithelium and some +ve cells between the connective tissue fibers of the sclera (arrows) (Prussian blue, ×400).
Fig. 9. A diabetic rat in SC therapy group showing at the central part of the cornea a Prussian blue +ve branched cell with multiple processes (arrow) deep in the substantia propria (Prussian blue, ×1,000).
epithelium (Fig. 10).
Fig. 10. A diabetic rat in SC therapy group showing at the central part of the cornea a Prussian blue +ve cuboidal cell (arrow) in the substantia propria near the epithelium (Prussian blue, ×1,000).
CD34 immunostained sections
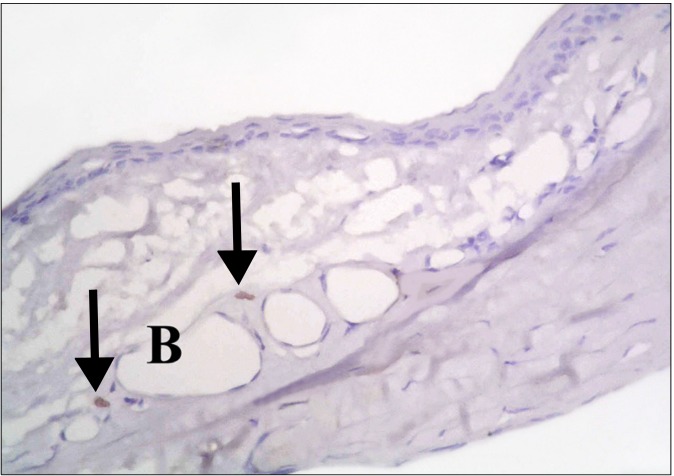
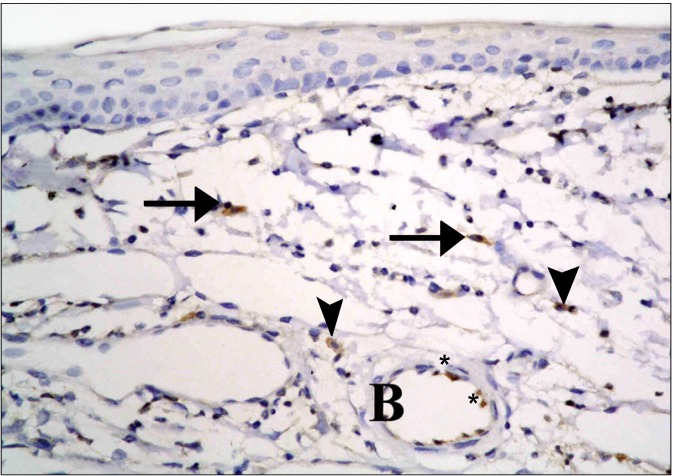
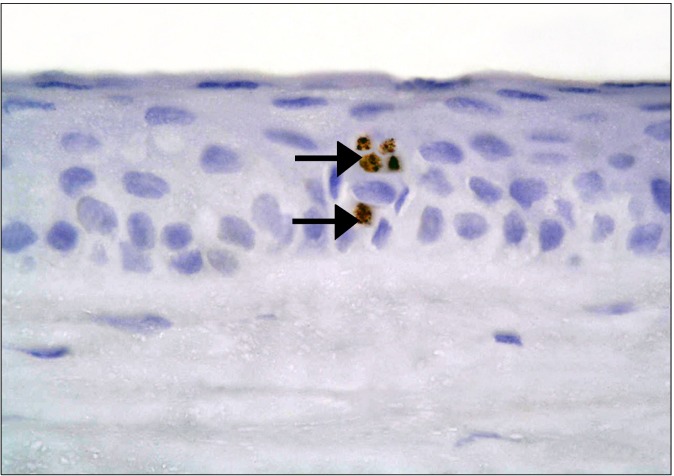
In the diabetic group, few CD34 +ve spindle cells were found at the limbus (Fig. 11). The stem cell therapy group showed multiple +ve cells of variable shapes between the connective tissue fibers, near blood vessels and inside a blood vessel (Fig. 12). The central part of the cornea, showed some cuboidal +ve cells in the epithelium (Fig. 13), occasional spindle +ve cells at the surface of the epithelium and occasional cuboidal +ve cells in the substantia propria (Fig. 14).
Fig. 11. A diabetic rat showing two CD34 +ve spindle cells (arrows) at the limbus (CD34, ×400).
Fig. 12. A diabetic rat in SC therapy group showing multiple +ve cells of variable shapes at the limbus. They appear between stromal fibers (arrows), others (arrowheads) near blood vessels (B) and some others (*) inside a blood vessel (B) (CD34, ×400).
Fig. 13. A diabetic rat in SC therapy group showing some +ve cuboidal cells (arrows) in the epithelium of central cornea (CD34, ×1,000).
Fig. 14. A diabetic rat in SC therapy group showing two CD34 positive cells at the central cornea. A spindle one at the surface of the epithelium (arrow) and a cuboidal one in the substantia propria (arrowhead) (CD34, ×1,000).
Morphometric results
The mean thickness of the corneal epithelium denoted nonsignificant difference between the different groups. Statistical analysis by ANOVA test indicated a significant increase (P<0.05) in the mean area of dark nuclei and vacuolated cytoplasm of corneal epithelial cells compared to stem cell therapy group.
Discussion
The current study demonstrated modulating effect of cord blood stem cell therapy on induced diabetic keratopathy in albino rat. This was evidenced by histological, histochemical, immunohistochemical and morphometric studies.
In the diabetic group, multiple epithelial cells recruited dark nuclei, denoting pyknosis. Pyknosis was described as irreversible condensation of chromatin in the nucleus of a cell undergoing necrosis (20). On the other hand, pyknosis was related to apoptosis (21). Progressive nuclear changes in the form of ghost nuclei were detected and referred to as karyolysis. The dissolution of the chromatin matter of a dying cell, known as karyolysis develops in necrosis due to the activity of DNase (22).
Cytoplasmic changes found in diabetic rats, included the appearance of some surface keratocytes with pale cytoplasm. Woolf (23) explained this by reduced activity of cellular adenosine triphosphatase resulting in failure of sodium pump mechanism that is responsible for water and electrolytes control. So, cells accumulate water and cel-
lular degeneration develops.
Epithelial cells revealed vacuolated cytoplasm, indicative of necrosis. In accordance, it was deduced that some of the major morphological changes that occur with necrosis include cell swelling with formation of cytoplasmic vacuoles (22).
Separation of the surface epithelial cells that appeared more acidophilic was found indicating keratinization. This separation was explained by decreased epithelial adherence in diabetic cornea (24). It was stated that surface keratinization represents a pathologic process whereby the non-keratinized stratified ocular epithelium is replaced by keratinized. This process is known as squamous metaplasia, which is a serious and challenging clinical problem that can cause severe discomfort and vision loss (25).
In the present study, focal epithelial discontinuity was recorded diabetic group. This was in agreement with Messmer and his colleagues (26) who deduced that advanced diabetic keratopathy includes impaired corneal sensation and reduced tear secretion. Conjunctival squamous metaplasia, and goblet cell loss, as well as susceptibility to corneal erosions and ulcerations can develop.
In the present study, dilatation of the limbal blood vessels was detected in diabetic group. It was documented that nitric oxide is an important molecule that involves many vascular functions. Diabetes induced inactivation of nitric oxide led to diabetic vascular complications (27).
Separation of the fibers of substantia propria and scleral connective tissue was recorded in diabetic rats. This can be attributed to limbal blood vessels dilatation and compromised endothelial integrity that led to edema.
In the diabetic group, reduced corneal thickness was reported. It was commented that oxidative stress has been described in human and animal diabetic corneas (28).
In the present study, few CD34 +ve cells were noticed in the diabetic group. In accordance it was documented that diabetic humans and animals exhibit fewer numbers of circulating progenitor cells (PCs) at rest and in response to ischemia. This can be refered to reduced ability of CD34 +ve endothelial PCs to proliferate, differentiate and integrate into the vessel (29).
There is now a large belief that not only do limbal stem cells (LSCs) undergo continual self-renewal to maintain the corneal epithelium. They are also responsible for epithelial tissue replacement, repair and regeneration in an adult cornea (30). This explained the increased need to in vitro stem cell therapy in diabetic patients.
In the present study, hemopoeitic stem cell therapy resulted in obvious decrease in the vacuolation of corneal epithelial cells and the appearance of dark nuclei. In addition, no epithelial erosions, keratinization, reduced corneal thickness or separation of substantia fibers were found. The regression of the area of vacuolated cytoplasm and that of dark nuclei were confirmed morphometrically, in comparison to diabetic group. Stem cell therapy group revealed Prussian blue +ve cells between the connective tissue fibers of the sclera, close to the conjunctival epithelium, in the substantia propria deep and near the epithelium of the central cornea.
It was recorded that hematopoietic stem cells have been attracting ever increasing attention for their potential use in regenerative medicine and tissue engineering. They can be harvested from healthy donors by bone marrow aspiration, by peripheral stem cell mobilization or from umbilical cord blood (UCB) (31).
In the recent years, UCB is also considered an accessible and less immunogenic source for stem cells with multipotent properties. The latter is capable of differentiating into a wide variety of cell types (32).
In the stem cell therapy group, CD34 HSC +ve cells were observed between the connective tissue fibers of the sclera and close to the conjunctival epithelium at the limbus. Other positive cells were detected in the substantia propria of the central part of the cornea, deep and near the epithelium. In addition, CD34 positive cells were seen near and inside the blood vessels. The existence near blood vessels correlates with the route of administration which is intravenous.
A definite ameliorating effect of hemopoeitic stem cell therapy was detected on diabetic keratopathy. This was evidenced by limiting nuclear and cytoplasmic changes of epithelial cells confirmed by morphometric results. In addition, stem cell therapy abolished epithelial discontinuity and reduction of corneal thickness.
Potential conflict of interest
The authors have no conflicting financial interest.
References
- 1.Davidson JA. Incorporating incretin-based therapies into clinical practice: differences between glucagon-like Peptide 1 receptor agonists and dipeptidyl peptidase 4 inhibitors. Mayo Clin Proc. 2010;85:S27–37. doi: 10.4065/mcp.2010.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagai N, Murao T, Okamoto N, Ito Y. Kinetic analysis of the rate of corneal wound healing in Otsuka long-evans Tokushima Fatty rats, a model of type 2 diabetes mellitus. J Oleo Sci. 2010;59:441–449. doi: 10.5650/jos.59.441. [DOI] [PubMed] [Google Scholar]
- 3.Abdelkader H, Patel DV, McGhee CNj, Alany RG. New therapeutic approaches in the treatment of diabetic keratopathy: a review. Clin Experiment Ophthalmol. 2011;39:259–270. doi: 10.1111/j.1442-9071.2010.02435.x. [DOI] [PubMed] [Google Scholar]
- 4.Zagon IS, Klocek MS, Sassani JW, McLaughlin PJ. Use of topical insulin to normalize corneal epithelial healing in diabetes mellitus. Arch Ophthalmol. 2007;125:1082–1088. doi: 10.1001/archopht.125.8.1082. [DOI] [PubMed] [Google Scholar]
- 5.Zagon IS, Sassani JW, Carroll MA, McLaughlin PJ. Topical application of naltrexone facilitates reepithelialization of the cornea in diabetic rabbits. Brain Res Bull. 2010;81:248–255. doi: 10.1016/j.brainresbull.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bashir Q, Robinson SN, de Lima MJ, Parmar S, Shpall E. Umbilical cord blood transplantation. Clin Adv Hematol Oncol. 2010;8:786–801. [PubMed] [Google Scholar]
- 7.Vasania VS, Prasad P, Gill RK, Mehta A, Viswanathan C, Sarang S, Majumdar AS. Molecular and cellular characterization of expanded and cryopreserved human limbal epithelial stem cells reveal unique immunological properties. Exp Eye Res. 2011;92:47–56. doi: 10.1016/j.exer.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Obrosova IG, Drel VR, Kumagai AK, Szábo C, Pacher P, Stevens MJ. Early diabetes-induced biochemical changes in the retina: comparison of rat and mouse models. Diabetologia. 2006;49:2525–2533. doi: 10.1007/s00125-006-0356-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blau HM. A twist of fate. Nature. 2002;419:437. doi: 10.1038/419437a. [DOI] [PubMed] [Google Scholar]
- 10.Stocum DL, Zupanc GK. Stretching the limits: stem cells in regeneration science. Dev Dyn. 2008;237:3648–3671. doi: 10.1002/dvdy.21774. [DOI] [PubMed] [Google Scholar]
- 11.Dua HS, Joseph A, Shanmuganathan VA, Jones RE. Stem cell differentiation and the effects of deficiency. Eye (Lond) 2003;17:877–885. doi: 10.1038/sj.eye.6700573. [DOI] [PubMed] [Google Scholar]
- 12.Koch TG, Heerkens T, Thomsen PD, Betts DH. Isolation of mesenchymal stem cells from equine umbilical cord blood. BMC Biotechnol. 2007;7:26. doi: 10.1186/1472-6750-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kraitchman DL, Heldman AW, Atalar E, Amado LC, Martin BJ, Pittenger MF, Hare JM, Bulte JW. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 2003;107:2290–2293. doi: 10.1161/01.CIR.0000070931.62772.4E. [DOI] [PubMed] [Google Scholar]
- 14.Haasters F, Prall WC, Anz D, Bourquin C, Pautke C, Endres S, Mutschler W, Docheva D, Schieker M. Morphological and immunocytochemical characteristics indicate the yield of early progenitors and represent a quality control for human mesenchymal stem cell culturing. J Anat. 2009;214:759–767. doi: 10.1111/j.1469-7580.2009.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiernan JA. Histological and histochemical methods: theory and practice. Arnold Publisher; London, New York & New Delhi: 2001. pp. 111–162. [Google Scholar]
- 16.Ellis R. Perls prussian blue stain protocol. Queen Elizabeth Hospital; South Australia: 2007. [Google Scholar]
- 17.Bancroft JD, Cook HC. Immunocytochemistry. In: Manual of histological techniques and their diagnostic applications. Churchill Livingstone; Edinburgh: 1994. [Google Scholar]
- 18.Filobbos SA, Salama NM, Abo Elnour RK. Evaluation of two medium depth peels: glycolic acid 70% versus trichloroacetic acid 35%, a histological and immunohistochemichal study on rat skin. Master Thesis in Histology. 2010 [Google Scholar]
- 19.Emsley R, Dunn G, White IR. Mediation and moderation of treatment effects in randomised controlled trials of complex interventions. Stat Methods Med Res. 2010;19:237–270. doi: 10.1177/0962280209105014. [DOI] [PubMed] [Google Scholar]
- 20.Kumar V, Abbas AK, Fausto N, Mitchell R. Robbins basic pathology. Saunders/Elsevier; philadelphia, PA: 2007. pp. 9–10. [Google Scholar]
- 21.Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nuñez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G. Nomenclature Committee on Cell Death 2009. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woolf N. Cell and tissue death. In: Cell, tissue and diseases, the basis of pathology. Saunders Ltd; London: 2000. pp. 37–43. [Google Scholar]
- 24.McDermott AM, Xiao TL, Kern TS, Murphy CJ. Non-enzymatic glycation in corneas from normal and diabetic donors and its effects on epithelial cell attachment in vitro. Optometry. 2003;74:443–452. [PubMed] [Google Scholar]
- 25.Li S, Gallup M, Chen YT, McNamara NA. Molecular mechanism of proinflammatory cytokine-mediated squamous metaplasia in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2010;51:2466–2475. doi: 10.1167/iovs.09-4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Messmer EM, Schmid-Tannwald C, Zapp D, Kampik A. In vivo confocal microscopy of corneal small fiber damage in diabetes mellitus. Graefes Arch Clin Exp Ophthalmol. 2010;248:1307–1312. doi: 10.1007/s00417-010-1396-8. [DOI] [PubMed] [Google Scholar]
- 27.Rungseesantivanon S, Thenchaisri N, Ruangvejvorachai P, Patumraj S. Curcumin supplementation could improve diabetes- induced endothelial dysfunction associated with decreased vascular superoxide production and PKC inhibition. BMC Complement Altern Med. 2010;10:57. doi: 10.1186/1472-6882-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLaughlin PJ, Sassani JW, Klocek MS, Zagon IS. Diabetic keratopathy and treatment by modulation of the opioid growth factor (OGF)-OGF receptor (OGFr) axis with naltrexone: a review. Brain Res Bull. 2010;81:236–247. doi: 10.1016/j.brainresbull.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tepper OM, Carr J, Allen RJ Jr, Chang CC, Lin CD, Tanaka R, Gupta SM, Levine JP, Saadeh PB, Warren SM. Decreased circulating progenitor cell number and failed mechanisms of stromal cell-derived factor-1alpha mediated bone marrow mobilization impair diabetic tissue repair. Diabetes. 2010;59:1974–1983. doi: 10.2337/db09-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trounson A, Thakar RG, Lomax G, Gibbons D. Clinical trials for stem cell therapies. BMC Med. 2011;9:52. doi: 10.1186/1741-7015-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jing D, Fonseca AV, Alakel N, Fierro FA, Muller K, Bornhauser M, Ehninger G, Corbeil D, Ordemann R. Hematopoietic stem cells in co-culture with mesenchymal stromal cells--modeling the niche compartments in vitro. Haematologica. 2010;95:542–550. doi: 10.3324/haematol.2009.010736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arien-Zakay H, Lazarovici P, Nagler A. Tissue regeneration potential in human umbilical cord blood. Best Pract Res Clin Haematol. 2010;23:291–303. doi: 10.1016/j.beha.2010.04.001. [DOI] [PubMed] [Google Scholar]