Abstract
Background and Objectives:
Heparin-conjugated fibrin (HCF) is suitable for the release and localization of bFGF. We analyzed the effects of a bFGF delivery system using HCF with human bone marrow-derived mesenchymal stem cells (HBM- MSCs) in a dog ischemic limb model.
Methods and Results:
Animals were divided into HBM-MSCs, HBM-MSCs+HCF, bFGF-HCF, and HBM-MSCs+ bFGF-HCF groups. A total of 1×107 HBM-MSCs were injected per animal, and the amount of bFGF was 1 mg per dog. Ischemic muscles were harvested at eight weeks and six months after injection of cells. The HBM-MSCs+ bFGF-HCF group exhibited decreased proportions of capillaries and arterioles six months after transplantation. However, there were more cells positive for the angiogenic factors, VEGF and PDGF, in the eight-week specimens compared with those harvested six months after transplantation.
Conclusions:
Our results demonstrated that a single injection of HBM-MSCs did not have significant long-term angiogenic effects; however, a bFGF delivery system using HCF exerted prolonged angiogenic effects when combined with HBM-MSCs.
Keywords: Angiogenesis, Ischemia, Heparin-conjugated fibrin, HBM-MSCs, Vascular disease
Introduction
Angiogenesis in ischemic limbs or in cases of myocardial ischemia can been induced by injection of numerous angiogenic growth factors such as vascular endothelial growth factor (VEGF) (1, 2), basic fibroblast growth factor (bFGF) (3, 4), and hepatocyte growth factor (HGF) (5, 6). In addition, therapeutic angiogenesis can be achieved by injection of stem cells such as endothelial progenitor cells (7), bone marrow cells (BMCs) (8, 9), and adipose-derived stromal cells (ADSCs) (10). However, therapies consisting of single injections of angiogenic growth factors or stem cells have a limited ability to promote angiogenesis due to poor survival rates of injected stem cells and limited duration of the biological activity of injected growth factors (11-14). In response to this lack of efficacy of single agents, many studies have utilized combined injections of growth factors and stem cells; however, in such cases, the duration of the biological activity of growth factors in vivo is extremely short (10, 15, 16). Thus, additional methods to improve the biological activity of growth factors in vivo have been studied (17-19).
To further improve upon these studies, we selected bFGF as a model growth factor. Specifically, bFGF is a primary promoter of cell proliferation and has applications in the treatment of wounds and angiogenesis. Heparin binds bFGF to form a stable complex that maintains bFGF biological activity (20) and can inhibit its release (21). However, current controlled release systems that incorporate heparin are associated with unfavorable side effects due to hemorrhage, resulting from in vivo degradation of heparin-containing materials (18). In a previous study, heparin was conjugated to fibrinogen to release heparin-binding growth factors (22); however, such fibrin gels do not elicit sustained release of the protein (23). We previously demonstrated that heparin binds fibrinogen directly via covalent bonding between the carboxyl group of heparin and the amine group of fibrinogen (24). In the present study, we evaluated the effects of a heparin- conjugated fibrin (HCF) gel as a bFGF delivery vehicle in stem cell therapy in improving the biological activity of growth factors. Specifically, we determined whether the HCF delivery system could enhance the angiogenic efficacy of bFGF2 and HBM-MSCs compared with vehicle or single-agent treatment groups in a dog limb ischemia model.
Materials and Methods
Ethics and approval
Animal studies were approved by the Institutional Animal Care and Use Committee of Samsung Biomedical Research Institute (SBRI). SBRI is a member of the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC) International Accredited Facilities and abides by the Institute of Laboratory Animal Resource’ s guidelines.
Preparation of the bFGF delivery system
HCF was prepared as previously described (24). Briefly, heparin (molecular weight=4,000∼6,000; Sigma, St. Louis, MO, USA) was covalently bound to plasminogen-free fibrinogen (Sigma) using a procedure employing standard carbodiimide chemistry. Carboxyl groups in hep arin were activated using N-hydroxysuccinimide (NHS, 0.04 mM; Sigma) and 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC, 0.08 mM; Sigma) in a buffer solution (100 ml, pH 6) of 0.05 M 2-morpholinoethanesulfonic acid (MES; Sigma). Initially, fibrinogen (100 mg) was dissolved in phosphate buffered saline (PBS, 20 ml, pH 7.4) and reacted with the activated carboxyl acid groups of heparin (60 mg). The product was then precipitated with a large excess of acetone and lyophilized. The resulting white powder was completely dissolved in PBS and dialyzed through a porous membrane bag (12,000∼14,000 Da molecular weight cutoff; Spectrum Labs Inc., Rancho Dominguez, CA, USA) at 4℃ for 24 hours to remove the residual heparin. HCF was formed by mixing heparin-conjugated fibrinogen (40 mg/ml), normal fibrinogen (60 mg/ml) with factor XIII, aprotinin (100 KIU/ml), calcium chloride (6 mg/ml), and thrombin (500 IU/mg).
HBM-MSC preparation
HBM-MSCs were obtained from the Adult Stem Cell Research Center of the College of Veterinary Medicine at Seoul National University. HBM samples were obtained from Berger’ s disease patients at the Samsung Medical Center, the isolation protocol for which was approved by the Boramae Hospital Institutional Review Broad (IRB). HBM-MSCs were obtained as described previously with slight modification (25). Briefly, HBM-MSCs were isolated from mixed HBMs with HetaSepTM solution (Stem Cell Technologies, Vancouver, Canada) by Ficoll density- gradient centrifugation. Cells were then washed with 1×PBS and seeded in D-media (formula no. 78-5470EF; Gibco) containing 10% FBS (Gibco). The media was subsequently changed three times a week. Adherent cells formed colonies approximately 10∼14 days after seeding and then grew rapidly thereafter with a spindle-shaped morphology (26).
Dog ischemic model and experimental groups
Dog ischemic models were generated as follows. Under general anesthesia, the femoral arteries in both lower limbs were dissected and encircled by an ameroid constrictor (RISW, CA, USA) with an inner diameter of 1.5 mm. Cefazolin (20 mg/kg, Chong Kun Dong, Korea) was subsequently injected intra-muscularly (I.M.) twice a day for three days. After four weeks, we identified complete occlusion of the femoral artery by Doppler examination. Stem cells and growth factors were injected intramuscularly in the right limbs of the dogs, while an equal volume of normal saline was injected into the same muscle location in the control (left) ischemic limb. A total of 1×107 HBM-MSCs were injected and a total of 1 mg of bFGF was injected.
Angiography and muscle harvest
Dogs were re-anesthetized eight weeks after cell transplantation. After exposure of the abdominal aorta, a contrast dye (Ultravist 370, Schering, Korea) was injected into the abdominal aorta and angiograms of the limbs were obtained. After angiography, ischemic muscles that underwent cell-injection were harvested for histologic analysis.
Immunohistochemical staining for detection of angiogenic factors
Blocked sections were incubated with primary antibodies (anti-human vWF, anti-human SMA, anti-human VEGF, anti-human PCNA, and anti-human PDGF) for 1 hour at RT (Dako, Glostrup, Denmark). Sections were then incubated with EnVision+ Systems polymer-conjugated secondary antibody (Dako) and were finally incubated with diaminobenzidine (DAB) for visualization.
Western blotting for detection of angiogenic factors
Proteins separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were blotted on membranes and incubated with specific antibodies overnight at 4℃ Next, blotted proteins were reacted with HRP goat anti-mouse Ig (BD, Franklin Lakes, NJ, USA), polyclonal goat anti-rabbit antibody (Dako), or polyclonal rabbit anti-goat antibody (Dako). The reaction was developed using an Enhanced Chemiluminescence Detection System (ECL, Amersham, Piscataway, NJ, USA) and detected on X-ray film.
Ratio of angiogenesis- and cytokine-positive cell counts
Ten fields of each section of sampled muscle were randomly selected, and the number of capillaries, arterioles, and cytokine-positive cells (VEGF, PDGF and PCNA) per field were counted and measured using the Image-pro plus 6.2 program (Media Cybrernetics). The angiogenesis ratio of the treated limbs was determined for comparison with control limbs.
Statistical analysis
Results are expressed as means±SDs. Comparisons of results between the different groups were performed using unpaired Student’ s t-tests. All calculations were performed with the computer program, Sigmaplot. p-values less than 0.05 were considered statistically significant.
Results
Dog ischemic limb model subjects were grouped as follows: HBM-MSCs injection group, HBM-MSCs+FM injection group, bFGF-HCF injection group, and HBMMSCs+ bFGF-HCF injection group. For each group, specimens were harvested at eight weeks and six months after transplantation. The control limb for each animal was the contralateral limb.
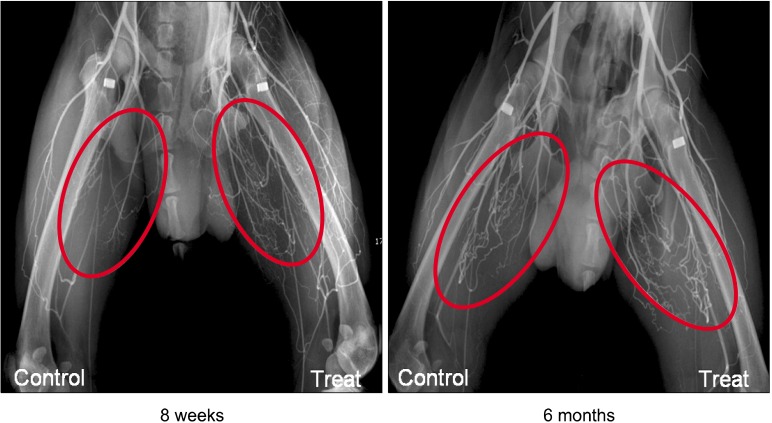
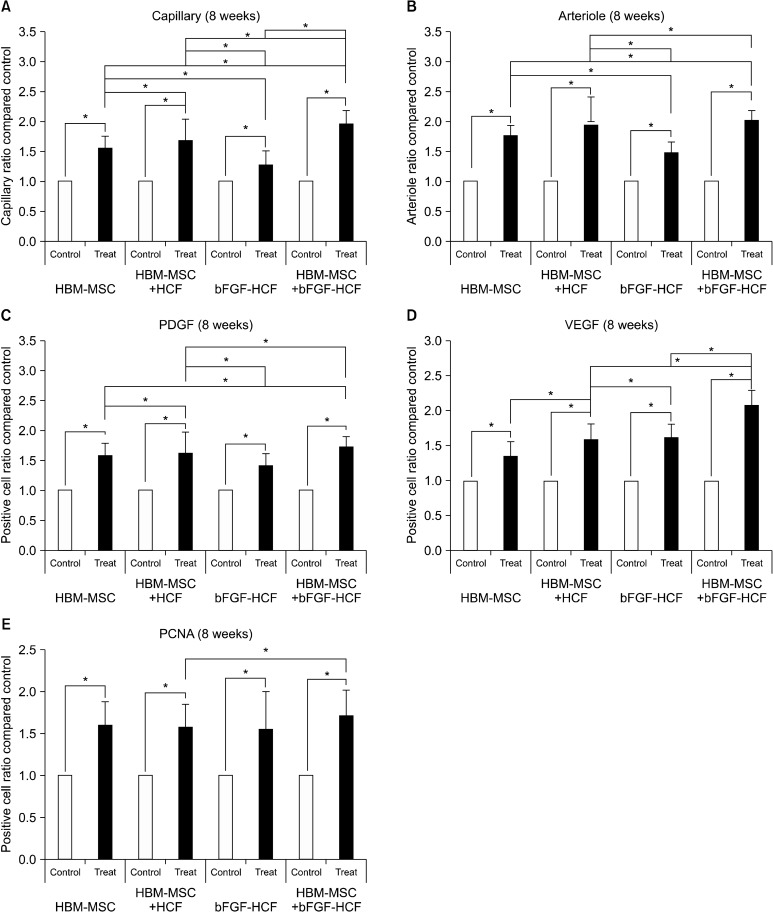
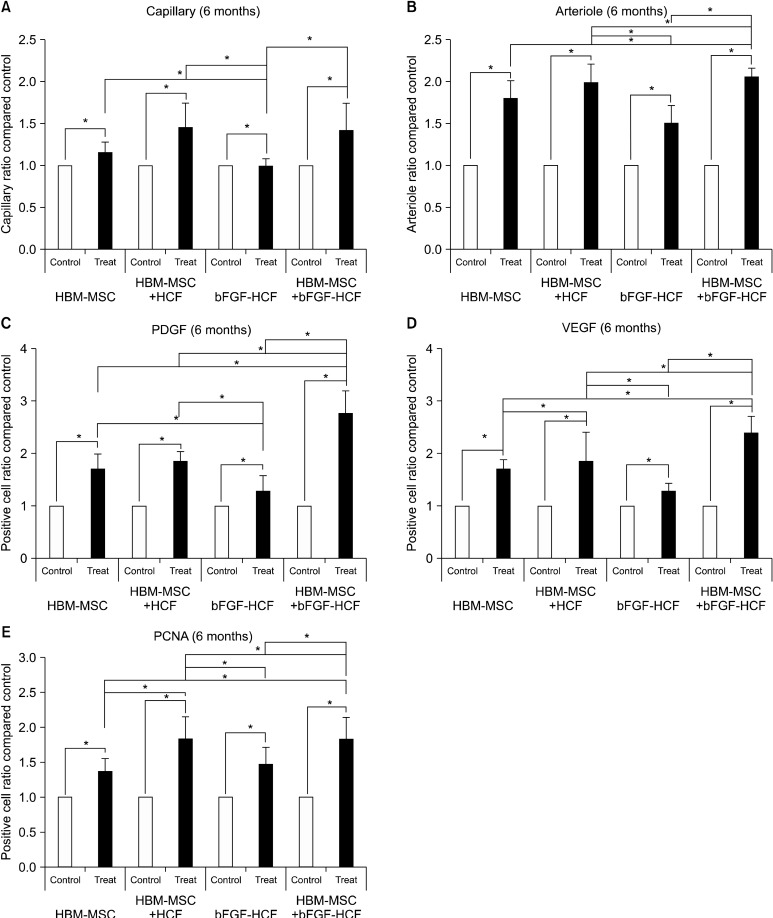
Angiography of treated limbs revealed an increased presence of capillaries as compared to control limbs for all groups at both eight weeks and six months post-treatment (Fig. 1). Specifically, at eight weeks post-transplantation, the capillary and arteriole ratios were significantly increased in treated limbs compared to the control limbs (Fig. 2A, B). Likewise, the counts of cells positive for angiogenic factors (VEGF and PDGF) and of proliferating cells (PCNA) were significantly higher in all treated limbs compared with control limbs (Fig. 2C-E). In addition, at six months, the capillary and arteriole ratios as well as the absolute number of angiogenic factors and proliferating cells were significantly increased in treated limbs compared with control limbs (Fig. 3). The HBM-MSC+bFGF-HCF group exhibited the highest levels of capillaries, arterioles, and angiogenic factors compared to the other groups (HBM-MSC, HBM-MSC+HCF and bFGF-HCF) at both eight weeks
Fig. 1. Angiography for confirmation of angiogenesis effects. All of the treated limbs exhibited improved vascularity compared to the control limbs.
Fig. 2. Short-term (eight weeks) effect of the bFGF delivery system using HCF with HBM-MSCs. (A) Capillary ratio, (B) arteriole ratio, (C) PDGF-positive cell count, (D) VEGF-positive cell count, and (E) PCNA-positive cell count. Capillaries, arterioles, angiogenic factors (PDGF, VEGF), and proliferating cell (PCNA) ratios in the treated limbs were higher than those in the control limbs in all groups. bFGF-HCPNs promoted angiogenesis, but the effect was limited. The effect of HBM-MSCs+bFGF-HCPN treatment maximized pro-angiogenic effects. Error bars: standard deviation; (*p<0.05).
Fig. 3. Long-term (six months) effect of the bFGF delivery system using HCF with HBM-MSCs. (A) Capillary ratio, (B) arteriole ratio, (C) PDGF-positive cell count, (D) VEGF-positive cell count, and (E) PCNA-positive cell count. Treated limbs had higher levels than control limbs in all groups. HBM-MSCs+ bFGF-HCPNs had the highest level of angiogenic effectiveness. Error bars: standard deviation; (*P<0.05).
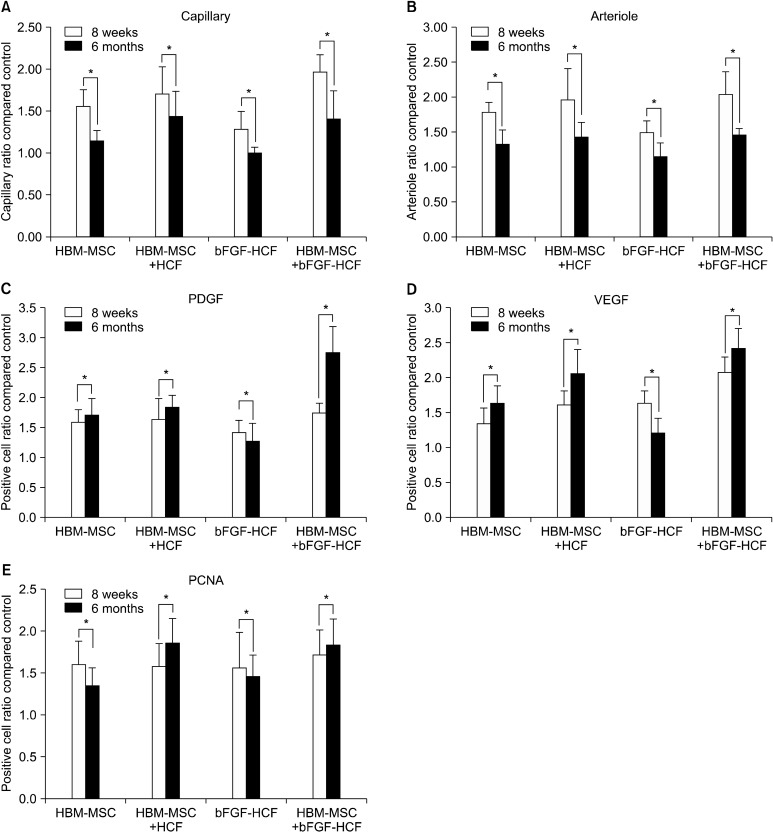
Fig. 4 shows the difference between results at eight weeks and six months after the procedures. The capillary and arteriole ratios were significantly decreased six months after transplantation compared to eight weeks after transplantation; however, angiogenic factors were significantly increased at six months compared to eight weeks, except for the bFGF-HCF group. In addition,
Fig. 4. Comparison between short- and long-term results. (A) Capillary ratio, (B) arteriole ratio, (C) PDGF-positive cell count, (D) VEGF-positive cell count, and (E) PCNA-positive cell count. The capillary and arteriole ratios were decreased at six months compared to eight weeks in all groups. However, angiogenic factors were significantly increased at six months compared to eight weeks. Error bars: standard deviation; (*P<0.05).
PDGF levels at six months were the highest in the HBM-MSC+bFGF-HCF group. Likewise, the number of PCNA-positive cells was significantly increased in the HBM-MSCs+HCF and HBM-MSCs+bFGF-HCF injection groups (Fig. 4E).
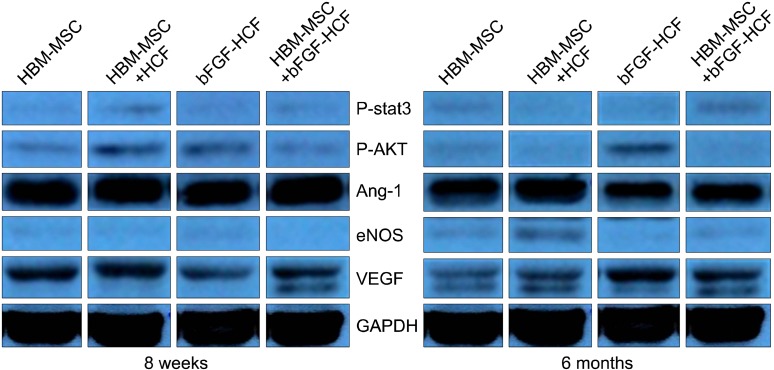
Lastly, Western blot analysis revealed that the HBM-
MSCs+bFGF-HCF group had elevated VEGF and p-STAT3 protein expression at both eight weeks and six months compared to the other groups (Fig. 5). In addition, VEGF protein levels were higher at six months than at eight weeks in the HBM-MSCs+bFGF-HCF group.
Fig. 5. Detection of angiogenic factors by Western blotting. Expression of the angiogenic factors, p-stat3, p-AKT, and Ang-1 was decreased at six months compared to that at eight weeks. However, eNOS and VEGF levels were increased at six months compared to those at eight weeks.
Discussion
Induction of angiogenesis via injection of various angiogenic growth factors (4, 5) and stem cells (9, 10) has been shown to improve ischemic conditions. However, growth factors are generally not sufficiently stable to exert significant biological activity due to a short half-life, whereas stem cells exhibit poor cell survival after transplantation
(11, 14). Thus, a single treatment with either stem cells or growth factors is not recommended.
In order to prolong the biological activity of growth factors, heparin-conjugated fibrin (HCF) was developed to elicit sustained release of heparin-binding growth factors (22, 24). A previous study showed that local delivery of bFGF to ischemic tissue to which ADSCs had been previously transplanted improves the survival of transplanted ADSCs for a short duration (10), suggesting that bFGF binding of HCF may increase its stability and prolong its half-life. Further, HCF delivery systems enhance the angiogenic efficacy of bFGF compared with normal fibrin in a mouse limb ischemia model (24, 27).
In the present study, a HCF gel was used as a local bFGF delivery vehicle. Specifically, we determined whether dual implantation of a bFGF local delivery system consisting of HCF and HBM-MSCs into ischemic hind limbs of dogs could improve the angiogenic therapeutic efficacy of transplanted HBM-MSCs as compared to an injection of cells alone. The bFGF delivery system employing HCF enhanced angiogenic efficacy compared to control limbs in an ischemia dog model (Fig. 2, 3). At six months post-transplantation, the capillary to artery ratio was lower than that at eight weeks in all treatment groups; however, VEGF and PDGF at six months exhibited high positive cell counts compared with eight weeks in all treatment groups. Specifically, PDGF levels at six months were the highest in the HBM-MSC+bFGF-HCF group, indicating the efficacy of the combined HBM-MSCs and bFGF delivery system using HCF. This was likely due to the maintenance of bFGF bioactivity and long-term release of bFGF in the bFGF-HCF delivery system, which resulted in the long-term release of VEGF and PDGF (Fig. 3C, D) that may have stimulated angiogenesis for a longer period of time, thereby yielding more extensive angiogenesis than short-term release. The long-term delivery of bFGF using HCF stimulated angiogenesis to a significantly greater extent than bFGF-HCF and HBM-MSCs+HCF treatments. PCNA-positive cell counts were not different between eight weeks and six months post-transplantation. Together, these results demonstrated that HBM-MSCs+ bFGF-HCF maintained long-term angiogenesis effects.
Our results suggest that HBM-MSC transplantation had high angiogenic efficacy, but did not have long term effects. However, HBM-MSCs combined with a bFGF local delivery system using HCF transplantation sustained long-term angiogenic factor release for six months in a dog model. Thus, the complex of HBM-MSCs and bFGF-HCF should be considered a useful source for angiogenesis enhancement by promoting long-term release of angiogenic proteins.
Acknowledgments
This study was supported by a grant of the Korea Health-Care Technology R&D Project, Ministry of Health, Welfare and Family Affairs (A110552).
Potential conflicts of interest
The authors have no conflicting financial interests.
References
- 1.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow- derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takeshita S, Zheng LP, Brogi E, Kearney M, Pu LQ, Bunting S, Ferrara N, Symes JF, Isner JM. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Invest. 1994;93:662–670. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baffour R, Berman J, Garb JL, Rhee SW, Kaufman J, Friedmann P. Enhanced angiogenesis and growth of collaterals by in vivo administration of recombinant basic fibroblast growth factor in a rabbit model of acute lower limb ischemia: dose-response effect of basic fibroblast growth factor. J Vasc Surg. 1992;16:181–191. [PubMed] [Google Scholar]
- 4.Yanagisawa-Miwa A, Uchida Y, Nakamura F, Tomaru T, Kido H, Kamijo T, Sugimoto T, Kaji K, Utsuyama M, Kurashima C. Salvage of infarcted myocardium by angiogenic action of basic fibroblast growth factor. Science. 1992;257:1401–1403. doi: 10.1126/science.1382313. [DOI] [PubMed] [Google Scholar]
- 5.Aoki M, Morishita R, Taniyama Y, Kida I, Moriguchi A, Matsumoto K, Nakamura T, Kaneda Y, Higaki J, Ogihara T. Angiogenesis induced by hepatocyte growth factor in non-infarcted myocardium and infarcted myocardium: up-regulation of essential transcription factor for angiogenesis, ets. Gene Ther. 2000;7:417–427. doi: 10.1038/sj.gt.3301104. [DOI] [PubMed] [Google Scholar]
- 6.Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A, Comoglio PM. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992;119:629–641. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dzau VJ, Gnecchi M, Pachori AS, Morello F, Melo LG. Therapeutic potential of endothelial progenitor cells in cardiovascular diseases. Hypertension. 2005;46:7–18. doi: 10.1161/01.HYP.0000168923.92885.f7. [DOI] [PubMed] [Google Scholar]
- 8.Comerota AJ, Link A, Douville J, Burchardt ER. Upper extremity ischemia treated with tissue repair cells from adult bone marrow. J Vasc Surg. 2010;52:723–729. doi: 10.1016/j.jvs.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Kim DI, Kim MJ, Joh JH, Shin SW, Do YS, Moon JY, Kim NR, Lim JE, Kim AK, Eo HS, Kim BS, Cho SW, Yang SH, Park CJ, Shim JS. Angiogenesis facilitated by autologous whole bone marrow stem cell transplantation for Buerger's disease. Stem Cells. 2006;24:1194–1200. doi: 10.1634/stemcells.2005-0349. [DOI] [PubMed] [Google Scholar]
- 10.Bhang SH, Cho SW, Lim JM, Kang JM, Lee TJ, Yang HS, Song YS, Park MH, Kim HS, Yoo KJ, Jang Y, Langer R, Anderson DG, Kim BS. Locally delivered growth factor enhances the angiogenic efficacy of adipose-derived stromal cells transplanted to ischemic limbs. Stem Cells. 2009;27:1976–1986. doi: 10.1002/stem.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46:1339–1350. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 12.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 13.Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907–921. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 14.Zhu W, Chen J, Cong X, Hu S, Chen X. Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Stem Cells. 2006;24:416–425. doi: 10.1634/stemcells.2005-0121. [DOI] [PubMed] [Google Scholar]
- 15.van Weel V, van Tongeren RB, van Hinsbergh VW, van Bockel JH, Quax PH. Vascular growth in ischemic limbs: a review of mechanisms and possible therapeutic stimulation. Ann Vasc Surg. 2008;22:582–597. doi: 10.1016/j.avsg.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 16.Maurer MH, Thomas C, Bürgers HF, Kuschinsky W. Transplantation of adult neural progenitor cells transfected with vascular endothelial growth factor rescues grafted cells in the rat brain. Int J Biol Sci. 2007;4:1–7. doi: 10.7150/ijbs.4.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bulpitt P, Aeschlimann D. New strategy for chemical modification of hyaluronic acid: preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. J Biomed Mater Res. 1999;47:152–169. doi: 10.1002/(sici)1097-4636(199911)47:2<152::aid-jbm5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 18.Cai S, Liu Y, Zheng Shu X, Prestwich GD. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials. 2005;26:6054–6067. doi: 10.1016/j.biomaterials.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Tabata Y, Ikada Y. Vascularization effect of basic fibroblast growth factor released from gelatin hydrogels with different biodegradabilities. Biomaterials. 1999;20:2169–2175. doi: 10.1016/s0142-9612(99)00121-0. [DOI] [PubMed] [Google Scholar]
- 20.Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991;64:841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- 21.Liu LS, Ng CK, Thompson AY, Poser JW, Spiro RC. Hyaluronate-heparin conjugate gels for the delivery of basic fibroblast growth factor (FGF-2). J Biomed Mater Res. 2002;62:128–135. doi: 10.1002/jbm.10238. [DOI] [PubMed] [Google Scholar]
- 22.Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J Control Release. 2000;65:389–402. doi: 10.1016/s0168-3659(99)00221-7. [DOI] [PubMed] [Google Scholar]
- 23.Jeon O, Ryu SH, Chung JH, Kim BS. Control of basic fibroblast growth factor release from fibrin gel with heparin and concentrations of fibrinogen and thrombin. J Control Release. 2005;105:249–259. doi: 10.1016/j.jconrel.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 24.Yang HS, Bhang SH, Hwang JW, Kim DI, Kim BS. Delivery of basic fibroblast growth factor using heparin-conjugated fibrin for therapeutic angiogenesis. Tissue Eng Part A. 2010;16:2113–2119. doi: 10.1089/ten.TEA.2009.0673. [DOI] [PubMed] [Google Scholar]
- 25.Cooper K, Sen Majumdar A, Viswanathan C. Derivation, expansion and characterization of clinical grade mesenchymal stem cells from umbilical cord matrix using cord blood serum. Int J Stem Cells. 2010;3:119–128. doi: 10.15283/ijsc.2010.3.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun B, Roh KH, Park JR, Lee SR, Park SB, Jung JW, Kang SK, Lee YS, Kang KS. Therapeutic potential of mesenchymal stromal cells in a mouse breast cancer metastasis model. Cytotherapy. 2009;11:289–298. doi: 10.1080/14653240902807026. [DOI] [PubMed] [Google Scholar]
- 27.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]