Abstract
Background and Objectives
The rapidly increasing number of diabetic patients across the world drew the attention to develop more effective therapeutic approaches. Recent investigations on newly differentiated insulin producing cells (IPCs) revealed that they could be derived from embryonic, adult mesenchymal and hematopoietic stem cells. This work was planned to evaluate the role of StemEnhance (Aphanizomenon flos-aquae [AFA] plant extract) in mobilizing naturally occurring bone marrow stem cells as well as in improving streptozotocin-induced diabetic rats.
Methods and Results
Twenty adult male albino rats were divided into four groups namely the control, the diabetic, the positive control-StemEnhance and the diabetic-StemEnhance groups. After diabetes induction by streptozotocin (STZ), rats received StemEnhance for four weeks. The mean number of blood CD34 immunopositive cells was measured by flowcytometry and random blood sugar was measured weekly. The pancreas was removed from the sacrificed rats and processed for staining with H&E and immunohistochemical staining for CD34+ve and insulin +ve cells. CD34+ve cells increased in the blood after introduction of StemEnhance. CD34+ve cells were observed in the pancreas and the insulin producing cells in the islets of Langerhans were increased from the second to the fourth week of treatment. Blood glucose level improved but it was still higher than the control level after four weeks of StemEnhance treatment.
Conclusions
This work points to the significant role of StemEnhance in stem cell mobilization and the improvement of diabetes mellitus.
Keywords: Diabetes, Hematopoietic stem cells, CD34, Aphanizomenon flos aquae
Introduction
Diabetes mellitus is a major health problem affecting more than 200 million of adult populations worldwide and it is expected to affect at least 5% of the global population by the year 2025. The association of diabetes with micro and macrovascular complications as well as cardiomyopathy makes it a major cause of morbidity and mortality in the world (1, 2).
Treatments of diabetes by different regimens of insulin injections failed to offer complete cure and could not prevent secondary complications associated with diabetes (3, 4).
Early treatment of patients by restoration of their β-cell function through pancreas or islet transplantation can relieve the patients from their dependency on insulin, achieve lifelong normoglycemia and prevent related complications (5). However, transplantation requires major surgery with general anaesthesia, prolonged hospital stays, immunosuppressive therapy and considerable financial costs (6). One of the new promising islet sources is the replacement by stem cells which could be done using embryonic and adult stem cell types, but this requires a combination of advanced clinical skills and highly equipped laboratory support (6).
Scientists have been working on the potential use of mobilized in situ adult stem cells (ASCs) as a noninvasive form of stem cell treatment. Many researches have led to the idea that endogenous or in situ ASCs can be mobilized from their niches (inhabitant areas) in the body. The result is migration of ASCs to various organs where they share in tissue repair or regeneration. This noninvasive method has an obvious advantage over the processes of harvesting and reintroduction of ASCs. It also represents a new horizon in stem cell treatments that could make stem cell therapies more portable and cost limited (7, 8).
Several pharmaceuticals and natural substances can mobilize ASCs from human bone-marrow deposits. Mobilized ASCs can home into damaged tissue and produce the desired tissue repair or regeneration. However, diseased or damaged tissues provide complex signals which attract migrating regenerative stem cells (8).
One of the natural products that can mobilize ASCs from human bone marrow deposits is StemEnhance (STEM Tech HealthSciences San Clemente, CA, USA). It is extracted from Aphanizomenon flos aquae (AFA) plant. StemEnhance capsules contain a blend of the cytoplasmic and cell wall fractions of AFA plant which is enriched by L-selectin ligand (LSL). L-selectin ligand supports the release of stem cells (CD34+ cells) from the bone marrow. Its effect was detected on B.M stem cell mobilization (9).
This work was planned to evaluate the role of Stem- Enhance in mobilizing naturally occurring bone marrow stem cells in addition to the improvement of streptozotocin- induced diabetic rats. Histological and immunohistochemical techniques were applied in this study.
Materials and Methods
Drugs
Streptozotocin (STZ): an antibiotic was purchased from Sigma Company (St. Louis, MO, USA) in the form of powder. StemEnhance: capsules were purchased from STEM Tech HealthSciences (San Clemente, CA, USA) in the form of a bottle containing 50 capsules each containing 500 mg of L-selectin ligand enriched fractions of Aphanizomenon flos-aquae (AFA).
Administration of drugs
STZ 60 mg/kg was solubilized in sodium citrate buffer in Biochemistry Department, Faculty of Medicine, Cairo University. The solution was prepared at pH 4.5, and injected i.p. within 15 min. of its preparation (10). The aim of this procedure is to induce type I DM (11). StemEnhance was solubilized in distilled water and was given using gastric gavage at a dose of 270 mg/kg. StemEnhance was given daily after the blood glucose test confirmed that the animals became diabetic (nearly on the third day) and continued till the day of sacrificing.
Experimental design
Twenty adult male albino rats (160∼200 g) were housed in Kasr ElAiny Animal House, Faculty of Medicine, Cairo University according to the guide for the care and use of laboratory animals. They were divided into four groups; Group I: (control group) 4 rats received no treatment. Group II: (diabetic group) 4 rats. Diabetes was induced by single intraperitoneal (i.p.) injection of streptozotocin (STZ) 60 mg/kg; this group was left untreated. The animals were considered diabetic when blood glucose level was higher than 200 mg/dl (11). Group III: (positive control- StemEnhance group) 4 rats treated daily orally with StemEnhance 270 mg/kg body weight dissolved in distilled water by gastric gavage till the day of sacrificing. Group IV: (diabetic-StemEnhance group) 8 rats. Diabetes was induced as in GII then rats received (on the 3rd day) daily StemEnhance as in GIII till the day of sacrificing. This dose was equivalent to the human dose of 6 capsules 3,000 mg/day as recommended by STEM Tech Health- Sciences (San Clemente, CA, USA). The dose was calculated as follows: human equivalent dose (mg/kg)=animal dose (mg/kg) multiplied by (animal km divided by human km). Km factor is the representative surface area (m2) to body weight (kg) ratio. km of rat=5.9 while km of adult human=37 (12, 13). The animals of group IV were subdivided into 2 subgroups; each subgroup included 4 rats; Subgroup IVa=GIVa: sacrificed after 2 weeks, subgroup IVb=GIVb: sacrificed after 4 weeks. Ten rats were sacrificed after 2 and 4 weeks, four rats from the diabetic- StemEnhance group (group IV) in addition to two rats from each of the groups I, II and III.
Laboratory investigation
Random blood sugar was measured weekly for all groups in the Clinical Pathology Department (Kasr ElAiny Hospital). The blood samples were obtained from the retro- orbital vein. CD34 was measured in the blood by flowcytometry (Coulter Epics xl-BECKMAN coulter) using FITC-Conjugated CD34 Class II, Clone QBEnd 10 (Dako- Cytomation, Denmark, catalogue number F 7166) in Kasr ElAiny Hospital, Flowcytometry Unit. CD34 was measured once per animal one hour after StemEnhance administration to check for the mobilization of stem cells from the bone marrow to the peripheral blood.
Specimens taken and sectioning
The animals were sacrificed by using chloroform inhalation and the pancreas was immediately dissected out, fixed in 10% formol saline for 24 hours at room temperature, dehydrated in ascending grades of alcohol (70%, 95%, 100%), cleared in of xylene then embedded into paraffin wax (Department of Histology, Faculty of Medicine, Cairo University). Sections of 5 μm thickness were stained with hematoxylin and eosin (14). Immunohistochemical staining (15) was done using anti CD34 antibodies to detect stem cells and anti insulin antibodies to detect pancreatic β cells.
Immunohistochemistry
Anti CD34 immunohistochemical staining was done according to Bancroft and Cook (15). Paraffin sections were deparaffinized in xylene for 1∼2 minutes and then rehydrated in descending grades of ethanol then brought to distilled water for 5 minutes. Sections were incubated in hydrogen peroxide for 30 minutes then rinsed in PBS (3 times, 2 minutes each). Each section was incubated for 60 minutes with 2 drops (=100 μl) of the primary antibody CD34 Ab-1, Clone QBEnd/10 (Lab Vision Corporation Laboratories, CA 94539, USA, catalogue number MS-363- R7). Slides were rinsed well in PBS (3 times, 2 min. each), incubated for 20 minutes with 2 drops of biotinylated secondary antibody for each section then rinsed well with PBS. Each section was incubated with 2 drops (100 μl) enzyme conjugate "streptavidin-horseradish peroxidase" for 10 minutes at room temperature then washed in PBS. Substrate-chromogen (DAB) mixture 2 drops was applied to each section and incubated at room temperature for 5∼ 10 min. then rinsed well with distilled water. Slides were counterstained with hematoxylin, dehydrated and mounted. CD34+ve cells showed brown deposits. Insulin immunohistochemical staining was done according to Bancroft and Cook (15) using Insulin Ab-6 (INS04+INS05) (Lab Vision Corporation Laboratories, CA 94539, USA, catalogue number MS-1379-P) in the same way as CD34 immunohistochemical staining except for using AEC chromogen and slides were mounted with aqueous mounting media (glycerine), 2 drops to each slide and covered with a coverslip. Insulin+ve cells showed reddish brown deposits. All steps were performed in a humidity chamber to prevent drying of the tissues. Non-specific background elimination step was omitted.
Morphometric study
Data were obtained using "Leica Qwin 500 C" Image Analyzer Computer System Ltd. (Cambridge, England). Mean number of pancreatic CD34 immunopositive cells and mean area percent of insulin immunopositive cells were measured in all immunostained pancreas sections. From each section 10 non overlapping fields were examined using an objective lens ×10 (=total magnification ×100) and the mean value for each slide was obtained.
Statistical methods
Data were tabulated and statistically analyzed to evaluate the difference between the groups under study as regards the various parameters. The mean, standard deviation and analysis of variance (ANOVA) were calculated using EXCEL and SPSS 9.0 Software. Results were considered statistically significant when p<0.05 (16).
Results
General observation
No deaths or abnormal behavior were observed in all rats. The biochemical and histological results of control (GI) and positive control-StemEnhance (GIII) were similar (except for an increase in blood CD34+ve cells in GIII). The biochemical and histological results of all diabetic rats in GII (untreated, sacrificed after 2 and 4 weeks) showed the same findings.
Blood glucose level (Table 1)
Table 1.
The mean±SD values of blood glucose level of control and experimental groups
| Group | Values of blood glucose level (mean±SD) | |||
|---|---|---|---|---|
| 1st week | 2nd week | 3rd week | 4th week | |
| Control (GI) | 96.8±8.1 | 97.8±6.6 | 100.3±8.3 | 98.5±9.1 |
| Diabetic (GII) | 464.5±45.9a | 645.8±21.4a | 650±34.3a | 648±41.9a |
| Positive control-StemEnhance (GIII) | 98.3±10.9 | 95.8±16.1 | 96.3±12.3 | 107.8±8.5 |
| Diabetic-StemEnhance (GIV) | 446.1±36.8a | 632.4±34.7a | 532.4±35.4a,b | 354.6±41.4a,b |
ap<0.05 as compared to GI, bp<0.05 as compared to GII.
The mean values of blood glucose level of diabetic rats were significantly increased when compared to control rats. The mean values of blood glucose level of diabetic- StemEnhance rats revealed no significant change after two weeks with significant decrease after four weeks when compared with diabetic rats. However, the blood glucose level after StemEnhance administration for four weeks had not returned to the normal level and it showed a significant increase when compared with the control group.
Flowcytometry of CD34 cells in the blood (Table 2)
The percent of circulating CD34+ve cells showed significant increase after one hour of oral intake of StemEnhance in positive control-StemEnhance and diabetic-Stem- Enhance groups when compared to control and diabetic groups.
Histological results
Hematoxylin and Eosin stained pancreatic sections: Control & positive control-StemEnhance groups revealed normal structure of the pancreas (Figs. 1, 2). The diabetic group revealed swelling, vacuolation and cytoplasmic degranulation of some islet cells, while others showed dark acidophilic cytoplasm. Nuclei were small darkly stained with condensed, fragmented or dissoluted chromatin (Fig. 3). Sections of GIVa showed distorted shape of the islets and congested capillaries in some locations of the exam ined slides. Whereas GIVb showed a structure nearly similar to that of the control group (Fig. 4).
Fig. 1. A photomicrograph of a section in the pancreas of an albino rat (GI) showing two pale stained islets rich in capillaries (c) surrounded by many deeply stained pancreatic acini (a). The islet contains large acidophilic cells (blue arrow) and small basophilic cells (green arrow). The acini have basal nuclei surrounded by basophilic cytoplasm with apical acidophilic granules (H&E, ×400).
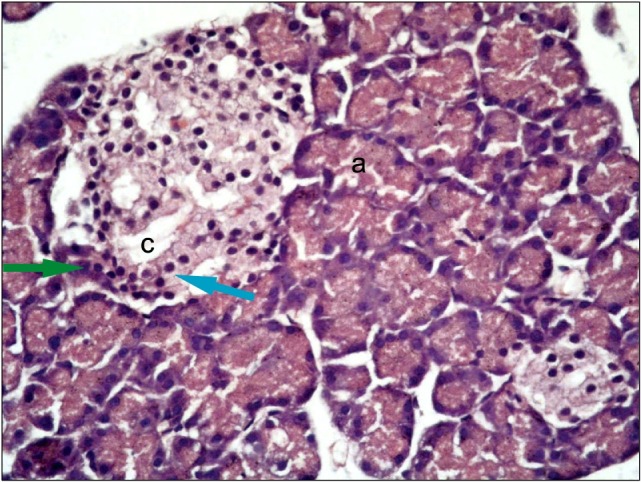
Fig. 2. A photomicrograph of a section in the pancreas of an albino rat (GIII); showing one pale stained islet rich in capillaries, which is surrounded by many deeply stained pancreatic acini (H&E, ×400).
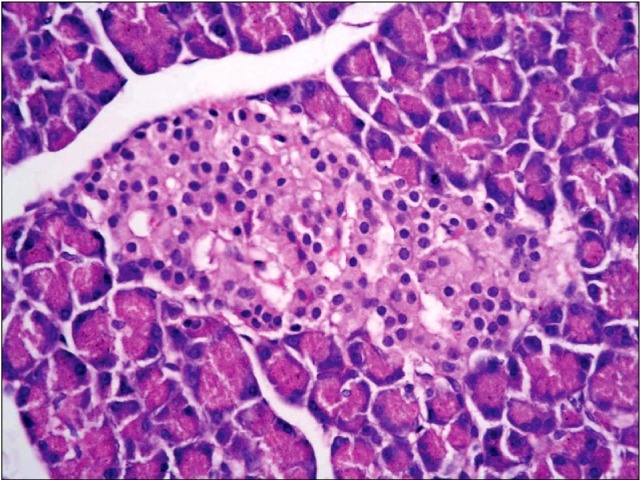
Fig. 3. A photomicrograph of a section in the pancreas of an albino rat (GII) showing swelling, vacuolation and degranulation in the cytoplasm of islet cells (v). Other cells show small darkly stained nuclei with condensed chromatin and acidophilic cytoplasm (black arrows). Some nuclei show fragmented chromatin and dissolution (red arrow) (H&E, ×1,000).
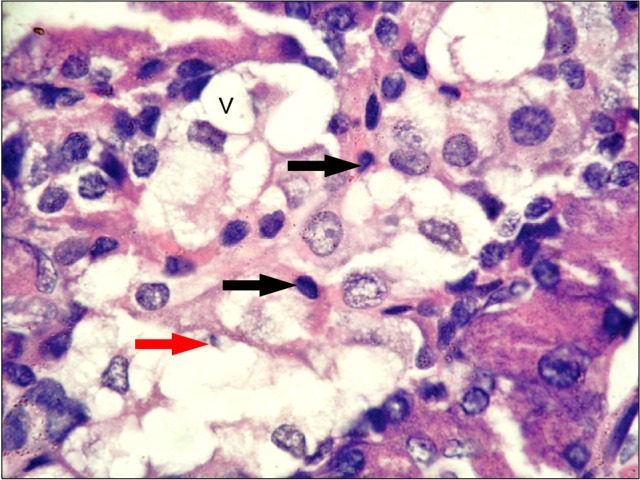
Fig. 4. A photomicrograph of a section in the pancreas of an albino rat (GIVb) showing one pale stained islet rich in capillaries (c), some islet cells are large (blue arrow) while others are small (green arrow) (H&E, ×400).
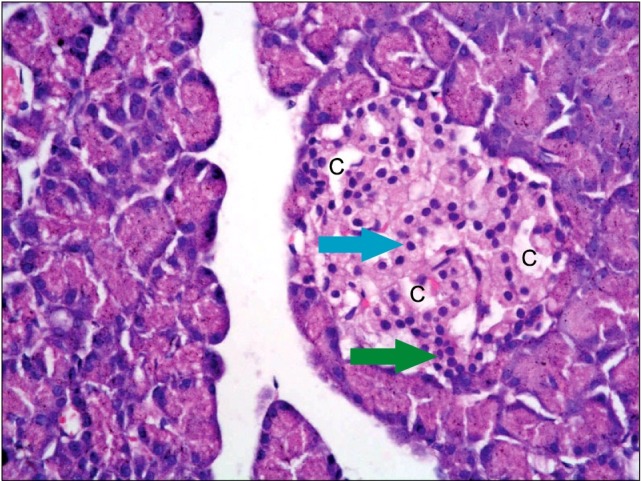
Table 2.
The mean±SD values of blood CD34 immunopositive cells percentage by flowcytometry in the control and experimental groups
| Group | Mean±SD |
|---|---|
| Control | 0.35%±0.00013 |
| Diabetic | 0.31%±0.00008 |
| Positive control-StemEnhance | 0.50%±0.00018a,b |
| Diabetic-StemEnhance (2 weeks) | 0.46%±0.00058a,b |
| Diabetic-StemEnhance (4 weeks) | 0.45%±0.00046a,b |
ap<0.05 as compared to GI, bp<0.05 as compared to GII.
Anti-CD34 stained pancreatic sections: Sections of control group & positive control-StemEnhance group revealed no immunoreactivity (Figs. 5, 6) while sections of diabetic group revealed localized mild immunoreactivity in the form of brown cytoplasmic granules that were detected in cells interspersed between the pancreatic acini and closely related to blood vessels (Fig. 7).
Fig. 5. A photomicrograph of a section in the pancreas of an albino rat (GI) showing no immunoreactivity (anti-CD34 immunostaining ×400).
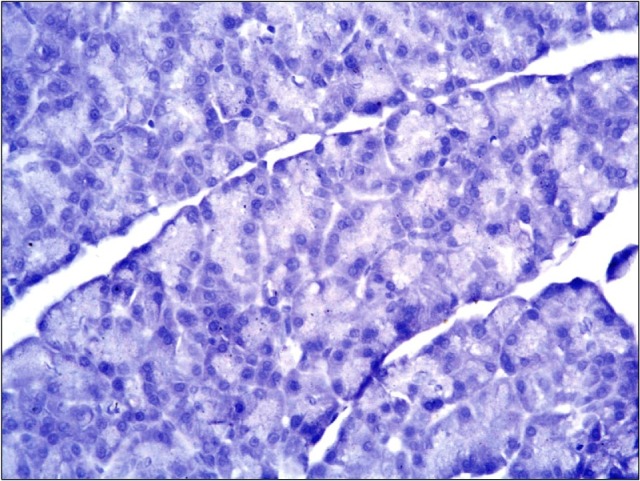
Fig. 6. A photomicrograph of a section in the pancreas of an albino rat (group III) showing no immunoreactivity (anti-CD34 immunostaining ×400).
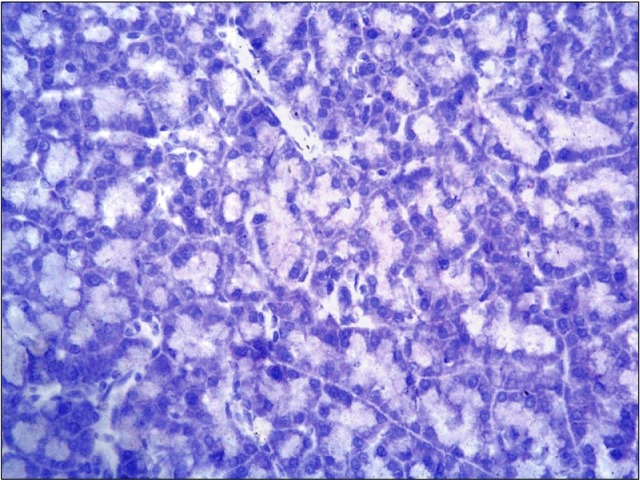
Fig. 7. A photomicrograph of a section in the pancreas of an albino rat (GII) showing brown cytoplasmic granules in two cells (arrows) adjacent to a blood vessel (b). One immunoreactive cell (arrowhead) is seen inside the blood vessel (b) (Anti-CD 34 immunostaining ×1,000).
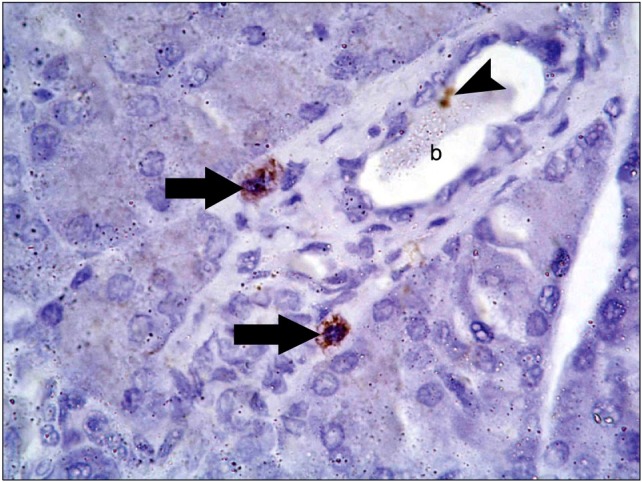
Sections of diabetic-StemEnhance group after 2 weeks revealed widespread moderate to dense cytoplasmic immunoreactivity that was detected in cells present in pancreatic connective tissue septa closely related to blood vessels as well as between the pancreatic acini. The immunopositive cells had different shapes; oval, triangular and irregular (Fig. 8). Moderate immunoreactive endothelial cells were seen lining a capillary (Fig. 8). Few cells in sections from GIVb revealed moderate to dense immunoreactivity between pancreatic acini and closely related to islets of Langerhans (Fig. 9).
Fig. 8. A photomicrograph of a section in the pancreas of an albino rat (GIVa) showing moderate to dense widespread immunoreactive cells in connective tissue adjacent to capillaries (c). Moderate immunoreactive endothelial cells are seen lining a capillary (black arrow). Immunoreactive cells are triangular (red arrow), oval (blue arrow) and flat (green arrow) (anti-CD34 immunostaining ×400).
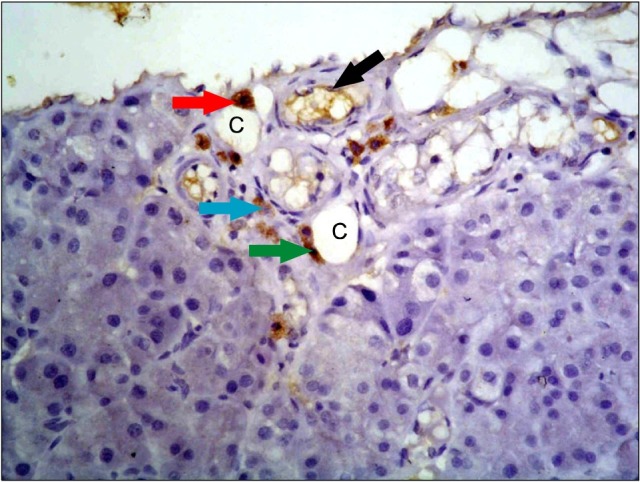
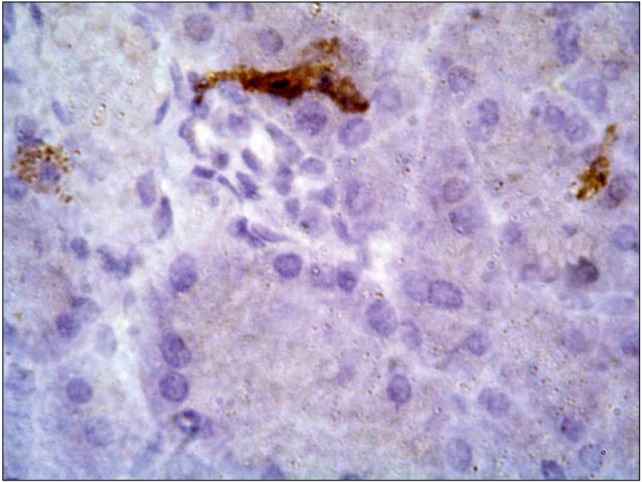
Fig. 9. A photomicrograph of a section in the pancreas of an albino rat (GIVb) showing moderate to dense granular immunoreactive cells between pancreatic acini and closely related to an islet of Langerhans (anti-CD34 immunostaining ×1,000).
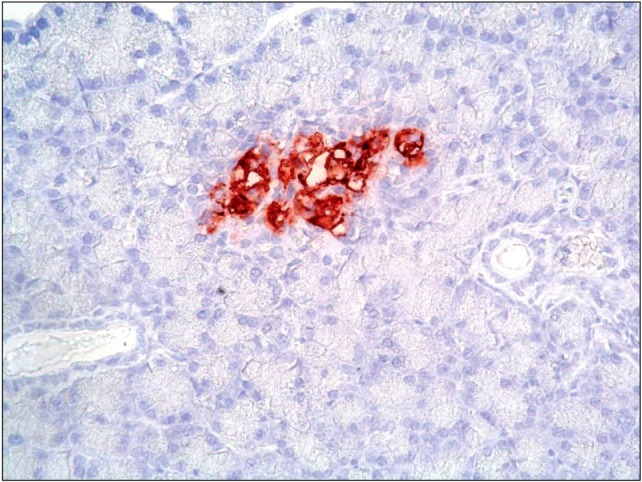
Anti-insulin stained pancreatic sections: Sections of control & Positive control-StemEnhance group revealed dense reddish brown cytoplasmic immunoreactivity that was detected in the cells of the pancreatic islets of Langerhans. The immunoreactivity was observed in most of the islet cells (Figs. 10,11).
Fig. 10. A photomicrograph of a section in the pancreas of an albino rat (GI) showing dense reddish brown cytoplasmic immunoreactivity in most of islet cells (anti-insulin immunostaining ×400).
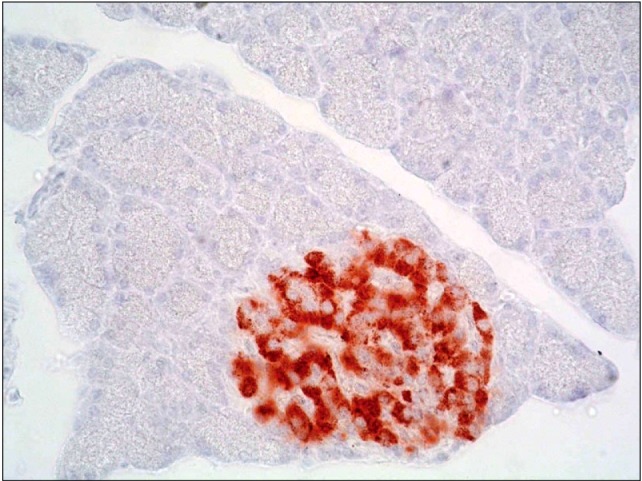
Fig. 11. A photomicrograph of a section in the pancreas of an albino rat (GIII) showing dense cytoplasmic immunoreactivity in an islet of Langerhans, the immunoreactivity occupies most of islet cells (immunohistochemical stain for insulin ×400).
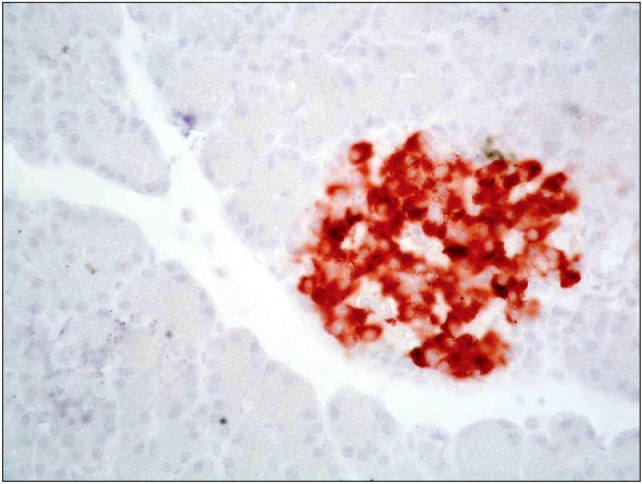
Sections of diabetic group revealed markedly decreased immunoreactivity that was detected mainly in peripheral part of pancreatic islets of Langerhans (Fig. 12). Sections of diabetic-StemEnhance group after 2 weeks revealed slightly increased immunoreactivity that was detected in the peripheral part of the islet of Langerhans (Fig. 13). Sections of diabetic-StemEnhance group after 4 weeks revealed marked increased immunoreactivity that was detected in the central part of the islet of Langerhans (Fig. 14).
Fig. 12. A photomicrograph of a section in the pancreas of an albino rat (GII). It shows markedly decreased immunoreactivity in the peripheral part of an islet of Langerhans (circle). One mildly stained immunoreactive cell is seen between acini (arrow) (anti-insulin immunostaining ×400).
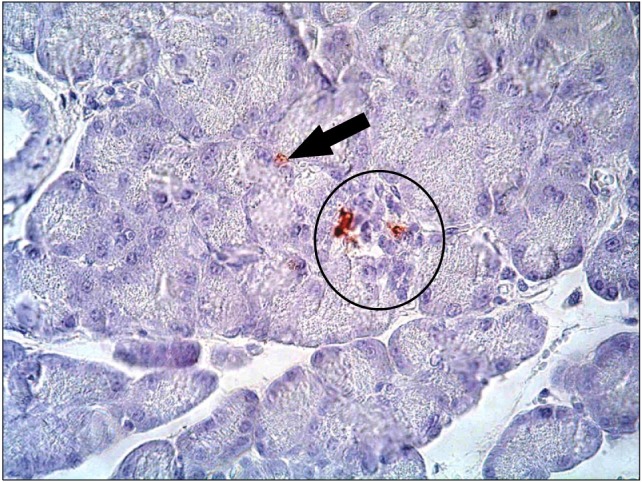
Fig. 13. A photomicrograph of a section in the pancreas of an albino rat (GIVa) showing slightly moderate to dense immunoreactivity which is detected in the peripheral part of an islet of Langerhans (anti-insulin immunostaining ×400).
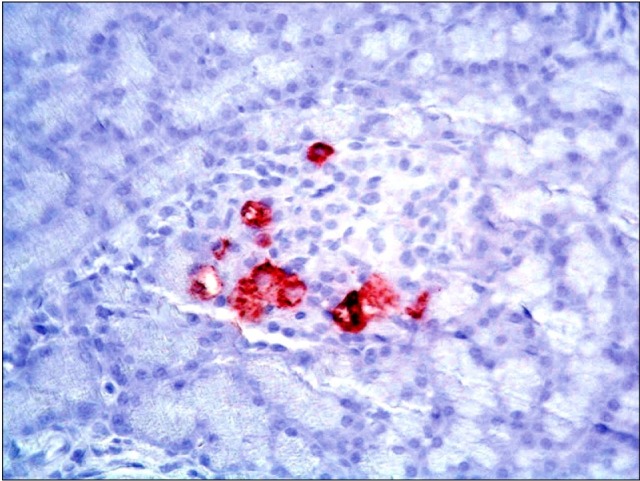
Fig. 14. A photomicrograph of a section in the pancreas of an albino rat (GIVb) showing dense immunoreactivity detected in the central zone of an islet of Langerhans (anti-insulin immunostaining ×400).
Morphometric results
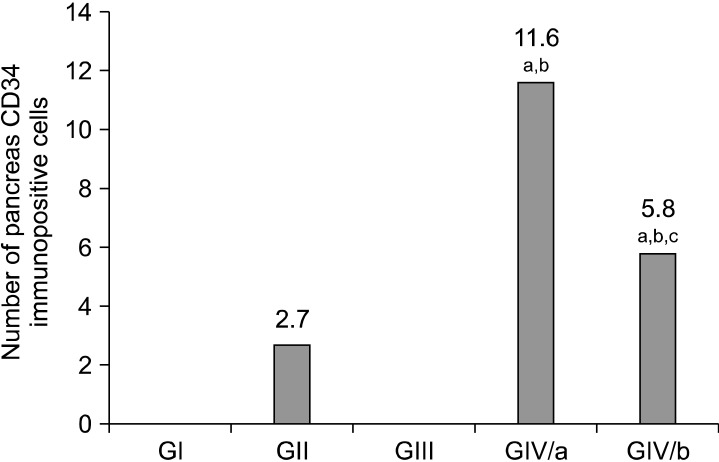
I. Mean number of CD34 immunopositive cells in pancreas (Fig. 15): CD34+ve cells were not detected in control or positive control-StemEnhance pancreas, whereas few CD34+ve cells were detected in the diabetic pancreas (GII). The number of CD34+ve cells increased significantly when StemEnhance was given to diabetic rats in the first two weeks (GIVa). This increase in number of CD34+ve cells was not maintained; it declined in the fourth week but remained significantly higher than the control values.
Fig. 15. Histogram comparing the mean values of pancreatic CD34 immunopositive cell’s number in the control and experimental groups. ap<0.05 as compared to GI. bp<0.05 as compared to GII. cp<0.05 compared to GIVa.
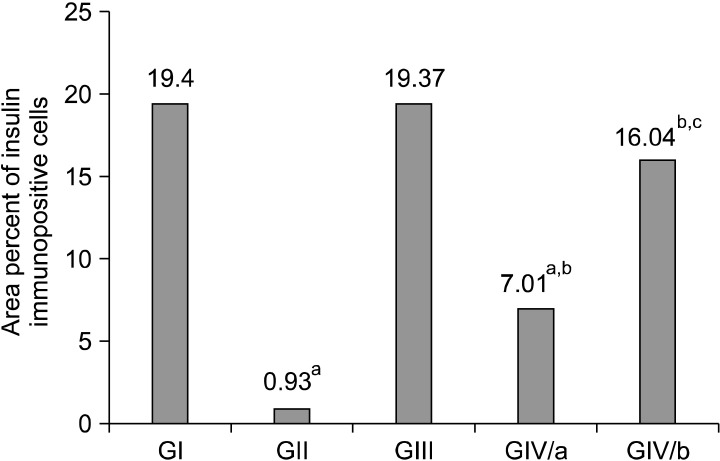
II. Mean area percent of insulin immunopositive cells (Fig. 16): The present study demonstrated that the area percent of insulin+ve cells significantly decreased in the diabetic pancreas. Insulin+ve cells progressively increased after StemEnhance administration to diabetic rats as demonstrated by significant progressive increase in the area percent of insulin+ve cells after two and four weeks.
Fig. 16. Histogram comparing the mean values of area percent of insulin immunopositive cells in the control and experimental groups. ap<0.05 as compared to GI. bp<0.05 as compared to GII. cp<0.05 compared to subGIVa.
Discussion
In this study, an attempt was done to investigate the changes which occurred in the pancreas of streptozotocin- induced diabetic rats after administration of bone marrow stem cell mobilizer (StemEnhance).
Diabetic rat pancreatic sections revealed swelling, vacuolation and degranulation in the cytoplasm of some islet cells denoting necrotic changes. Other cells exhibited small darkly stained nuclei with condensed chromatin together with darkly acidophilic cytoplasm probably denoting apoptosis. Moreover, some nuclei showed fragmented chromatin and dissolution. These findings were in accordance with Akbarzadeh et al. (17) who reported that STZ injection caused β cell damage in rat pancreas.
CD34 antibody was used to immunostain hematopoietic stem cells. The expression of the cell surface sialomucin CD34 has for a long time, been considered the basis for the selection of HSCs (18).
Mobilization of stem cells was detected by estimating the percent of circulating CD34+ve cells using flowcytometry. The present work demonstrated that oral intake of StemEnhance in positive control-StemEnhance rats and diabetic-StemEnhance rats produced significant increase in circulating CD34+ve cells percentage after one hour as compared to the percentage of circulating CD34+ve cells in control rats.
The previous findings were in accordance with Jensen et al. (9) who performed a study on 12 patients and revealed that the consumption of one gram of StemEnhance led to a significant increase in the circulating CD34+ve cells percentage after one hour. They also proved that mobilization of BM CD34+ve cells was related to L-selectin ligand contained in StemEnhance which caused down regulation of CXCR4 chemokine receptor. This interrupted the binding of SDF-1 with CXCR4 chemokine receptor leading to mobilization of CD34+ve stem cells.
Stimulation of L-selectin leads to the externalization of pre-formed CXCR4 chemokine receptors. Binding of SDF-1 to CXCR4 leads to the externalization of adhesion molecules that anchor the stem cell in the bone marrow. Therefore, any compound that interferes with CXCR4 or SDF-1 has the potential of acting as a stem cell mobilizer (9, 19).
The increase in the number of circulating CD34+ cells peaked within 1 hour after the consumption of StemEnhance. This was in contrast with the response time seen with granulocyte colony stimulating factor (G-CSF) which peaked after a few days of injection (20). More comparable to StemEnhance was the response to AMD3100 that peaked around 6 hours after injection (21). However, the magnitude of the CD34 cells mobilization obtained with StemEnhance (18∼25%) was much smaller than that reported with G-CSF and AMD3100 which was about 20 to 200-fold (20).
An extreme increase in the number of circulating stem cells might not be required to achieve health benefits. Moreover, Werner et al. (22) correlated the levels of circulating stem cells with the risk of cardiovascular incidents in 519 patients with coronary artery disease. They concluded that the level of circulating CD34+ progenitor cells predicted the occurrence of cardiovascular events and death from cardiovascular causes.
Furthermore, Cottler-Fox et al. (23) emphasized that the previously mentioned compounds could only be used for short periods of time due to severe side effects. In our study, no deaths or abnormal behavior were observed in all rats treated with StemEnhance. This was in accordance with Drapeau et al. (13) who reported neither death nor toxicity and animal growth was normal while receiving 300 mg/kg of StemEnhance (SE), which is about 10 times the dose given to humans.
Our observations were also consistent with Schaeffer et al. (24) who reported that consumption of 16,666 mg/kg of AFA (equivalent to >3,000 mg/kg SE) did not show any toxicity in mice.
Pancreatic sections of diabetic-StemEnhance rats after four weeks revealed nearly similar structure to the control. The area of the islets of Langerhans decreased in both the diabetic and the diabetic-StemEnhance groups due to decreased β cell mass mostly secondary to necrosis and apoptosis which occurred with sustained hyperglycemia. This was supported by Donath and Halban (25). Another explanation was the development of fibrosis secondary to hyperglycemia since the latter was known to stimulate the secretion of fibronectin, collagen I and III by endothelial cells and/or vascular smooth muscle cells (26).
In the present study the migration of bone marrow stem cells to the diabetic pancreas after StemEnhance administration was demonstrated by immunohistochemical staining of CD34 cells using antibodies against CD34 antigen. A striking difference in the number of CD34+ve cells was observed between treated & control pancreas after two & four weeks.
CD34+ve cells were detected mainly in the pancreatic CT septa near the blood vessels as well as in between the acini, whereas few cells were found in the islets. No CD34+ve cells were detected in control pancreas whereas the number of CD34+ve cells increased when StemEnhance was given for two weeks. This increase in the number of CD34+ve cells was not maintained, but it declined, in the fourth week of treatment but still remaining higher than the control values. In our opinion the decline in the number of stem cells observed in the fourth week may be related to their differentiation to islet β cells.
Few CD34+ve cells were also detected in the diabetic pancreas without intake of StemEnhance. This may be due to attraction of stem cells by the diabetic pancreas. This finding was explained by Körbling et al. (27) who mentioned that diseased or degenerating cells produce a variety of chemical messengers or have alterations of cell surface receptors that may attract reparative ASCs.
Sordi et al. (28) provided evidence that BM- MSCs were attracted by pancreatic islets in vitro and in vivo, and confirmed that CXCL12 (SDF-1α) and its ligand CXCR4 played an important role in the homing process. The MSCs were found mainly inside and around islets.
In the present work detection of differentiated islet β cells was achieved by using anti insulin antibody to immunostain islet β cells. Insulin+ve cells were present mainly in the islet of Langerhans, while few cells were observed between the acini. This was in accordance to Stacey and Mills (29) who stated that there were a small number of individual endocrine cells scattered among the acini.
The present study demonstrated that the area percent of insulin+ve cells in the pancreas dropped in the diabetic pancreas after two weeks but had not shown any difference after four weeks. A striking difference in the area percent of insulin+ve cells was observed between treated and diabetic pancreas where Insulin+ve cells progressively increased after StemEnhance administration for two and four weeks.
The increase in the area percent of insulin+ve cells was accompanied by a decline in pancreatic CD34+ve cells during the fourth week of treatment. This might denote the differentiation of BM-derived HSCs (CD34+ve cells) to insulin+ve cells. However, the results of the present study do not exclude the possible role of resident pancreatic stem cells. Ianus et al. (30) reported that BM-GFP (green fluorescent protein)-labelled murine cells, transplanted into lethally irradiated mice differentiated to β cells evidenced by their ability to express insulin, GLUT2 and transcription factors typically found in β cells. They also could not exclude the role of resident pancreatic stem cells.
Butler et al. (31) concluded that hematopoietic stem cells derived from adult donors differentiated minimally to pancreatic β cells in nondiabetic adult humans. However, they did not exclude the possibility that hematopoietic stem cells could differentiate to pancreatic β cells in individuals with type 1 diabetes.
Although the area percent of insulin+ve cells progressively increased from the second to fourth week, it did not reach the control level. This explained the presence of a high blood glucose level after four weeks of treatment with StemEnhance.
Concerning the blood glucose level, the mean values of blood glucose level of diabetic-StemEnhance rats were significantly increased in comparison to the control rats. However, the mean values of blood glucose level revealed no significant change after one and two weeks of StemEnhance intake with highly significant decrease after three and four weeks when compared with diabetic group. This decrease might be related to secretion of insulin by the newly differentiated β cells from mobilized HSCs. However, the blood glucose level after StemEnhance administration for four weeks did not return to the normal level and showed a significant increase when compared with the control group. This could be supported by the smaller area percent of insulin+ve cells which was observed by the fourth week of StemEnhance administration compared to the control.
Our results were consistent with Hess et al. (32) who reported a decrease in the blood glucose level and enhanced islet regeneration in STZ-induced diabetic mice after transplantation of green fluorescent protein (GFP)- labelled c-kit+ BM murine cells.
Lee et al. (33) performed repeated transplantation of STZ-induced diabetic mice with human MSCs via intracardiac infusion. An increased production of endogenous β cells and higher levels of mouse circulating insulin were obtained, with improvement of the hyperglycemia.
Urbán et al. (34) reported that administration of BMCs with MSCs normalized the blood glucose level and serum insulin levels in STZ-induced diabetic mice. This allowed regeneration of recipient-derived pancreatic insulin-secreting cells. They also stated that neither BMC nor MSC transplantation was effective alone and successful treatment of diabetic animals was not due to the reconstitution of the damaged islet cells from the transplant. They suggested that two aspects of this successful treatment regimen operate in parallel and synergistically in their model. First, BMCs and MSCs induce the regeneration of recipient- derived pancreatic insulin-secreting cells. Second, MSCs inhibit T cell mediated immune responses against the newly formed beta-cells.
Voltarelli et al. (35) induced insulin independence in patients with early onset DM type I after high dose of immunosuppression which was followed by autologous hematopoietic stem cell transplantation. The insulin independent state was achieved during a mean of 30 months of follow up after transplantation and was maintained for a mean of 31 months in 12 patients whereas 8 patients relapsed and resumed insulin use. Moreover, complications related to immunosuppression chemotherapy included infections and autoimmune diseases. They suggested that improvement of DM was related to immune tolerance and immunomodulatory effect of HSCs and not related to their differentiation to β cells.
Replacement by stem cells which could be done using embryonic and adult stem cell types is a promising source for improvement of DM but this requires a combination of advanced clinical skills and highly equipped laboratory support (6).
Autologous human MSCs need few weeks for generation from patients (36). Malignant transformations were observed with MSCs following long-term in vitro culture for 4∼5 months (37). In mice, MSCs enhanced the growth of cancers (38), and therefore there is a risk that administration of autologous human MSCs to a patient might enhance the growth of an unsuspected tumor. Also, there is a risk of pulmonary emboli if the cells were allowed to aggregate in suspension before i.v. infusion (33). In addition, culture of human MSCs is usually performed in media containing fetal calf serum (FCS) where the cells can internalize (39) and can produce immune reactions with repeated administrations of the cells (40). The above mentioned data concerning the risk of administration of Autologous human MSCs support our trend in using StemEnhance as a simple procedure for the replacement of injured β cells in diabetic animals.
In conclusion, StemEnhance successfully mobilized BM stem cells which migrated to diabetic pancreas with subsequent increase in pancreatic islet β cells area.
Potential conflict of interest
The authors have no conflicting financial interest.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Joshua IG, Zhang Q, Falcone JC, Bratcher AP, Rodriguez WE, Tyagi SC. Mechanisms of endothelial dysfunction with development of type 1 diabetes mellitus: role of insulin and C-peptide. J Cell Biochem. 2005;96:1149–1156. doi: 10.1002/jcb.20620. [DOI] [PubMed] [Google Scholar]
- 3.Vija L, Farge D, Gautier JF, Vexiau P, Dumitrache C, Bourgarit A, Verrecchia F, Larghero J. Mesenchymal stem cells: Stem cell therapy perspectives for type 1 diabetes. Diabetes Metab. 2009;35:85–93. doi: 10.1016/j.diabet.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Mandrup-Poulsen T. Diabetes. BMJ. 1998;316:1221–1225. doi: 10.1136/bmj.316.7139.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Diabetes Association. Robertson P, Davis C, Larsen J, Stratta R, Sutherland DE. Pancreas transplantation in type 1 diabetes. Diabetes Care. 2004;27(Suppl 1):S105. doi: 10.2337/diacare.27.2007.s105. [DOI] [PubMed] [Google Scholar]
- 6.Roche E, Enseñat-Waser R, Reig JA, Jones J, León-Quinto T, Soria B. Therapeutic potential of stem cells in diabetes. Handb Exp Pharmacol. 2006;174:147–167. [PubMed] [Google Scholar]
- 7.Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, Henegariu O, Krause DS. Liver from bone marrow in humans. Hepatology. 2000;32:11–16. doi: 10.1053/jhep.2000.9124. [DOI] [PubMed] [Google Scholar]
- 8.Jensen GS, Drapeau C. The use of in situ bone marrow stem cells for the treatment of various degenerative diseases. Med Hypotheses. 2002;59:422–428. doi: 10.1016/s0306-9877(02)00147-0. [DOI] [PubMed] [Google Scholar]
- 9.Jensen GS, Hart AN, Zaske LA, Drapeau C, Gupta N, Schaeffer DJ, Cruickshank JA. Mobilization of human CD34+ CD133+ and CD34+ CD133(-) stem cells in vivo by consumption of an extract from Aphanizomenon flosaquae-- related to modulation of CXCR4 expression by an L-selectin ligand? Cardiovasc Revasc Med. 2007;8:189–202. doi: 10.1016/j.carrev.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Dong QY, Chen L, Gao GQ, Wang L, Song J, Chen B, Xu YX, Sun L. Allogeneic diabetic mesenchymal stem cells transplantation in streptozotocin-induced diabetic rat. Clin Invest Med. 2008;31:E328–E337. doi: 10.25011/cim.v31i6.4918. [DOI] [PubMed] [Google Scholar]
- 11.Cesaretti ML, Ginoza M, Ribeiro AB, Kohlmann O Jr. Systemic hemodynamic and left ventricular function of diabetic- induced hypertensive rats. Arq Bras Endocrinol Metabol. 2010;54:842–851. doi: 10.1590/s0004-27302010000900011. [DOI] [PubMed] [Google Scholar]
- 12.Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep. 1966;50:219–244. [PubMed] [Google Scholar]
- 13.Drapeau C, Antarr D, Ma H, Yang Z, Tang L, Hoffman RM, Schaeffer DJ. Mobilization of bone marrow stem cells with StemEnhance improves muscle regeneration in cardiotoxin- induced muscle injury. Cell Cycle. 2010;9:1819–1823. doi: 10.4161/cc.9.9.11540. [DOI] [PubMed] [Google Scholar]
- 14.Kiernan JA. Histological and histochemical methods: theory and practice. 3rd ed. Arnold Publisher; London, New York & New Delhi: 2001. pp. 111–162. [Google Scholar]
- 15.Bancroft J, Cook HC. Immunocytochemistry. In: Manual of histological techniques and their diagnostic applications. 2nd ed. Churchill Livingstone; Edinburgh, London, Madrid, Melbourne, New York and Tokyo: 1994. pp. 263–325. [Google Scholar]
- 16.Emsley R, Dunn G, White IR. Mediation and moderation of treatment effects in randomised controlled trials of complex interventions. Stat Methods Med Res. 2010;19:237–270. doi: 10.1177/0962280209105014. [DOI] [PubMed] [Google Scholar]
- 17.Akbarzadeh A, Norouzian D, Mehrabi MR, Jamshidi SH, Farhangi A, Verdi AA, Mofidian SM, Rad BL. Induction of diabetes by Streptozotocin in rats. J Clin Biochem. 2007;22:60–64. doi: 10.1007/BF02913315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alison MR, Brittan M, Lovell MJ, Wright NA. Markers of adult tissue-based stem cells. Handb Exp Pharmacol. 2006;174:185–227. [PubMed] [Google Scholar]
- 19.Pusic I, DiPersio JF. Update on clinical experience with AMD3100, an SDF-1/CXCL12-CXCR4 inhibitor, in mobilization of hematopoietic stem and progenitor cells. Curr Opin Hematol. 2010;17:319–326. doi: 10.1097/MOH.0b013e328338b7d5. [DOI] [PubMed] [Google Scholar]
- 20.Bodine DM, Seidel NE, Orlic D. Bone marrow collected 14 days after in vivo administration of granulocyte colonystimulating factor and stem cell factor to mice has 10-fold more repopulating ability than untreated bone marrow. Blood. 1996;88:89–97. [PubMed] [Google Scholar]
- 21.Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, Liles WC, Li X, Graham-Evans B, Campbell TB, Calandra G, Bridger G, Dale DC, Srour EF. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Böhm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 23.Cottler-Fox MH, Lapidot T, Petit I, Kollet O, DiPersio JF, Link D, Devine S. Stem cell mobilization. Hematology Am Soc Hematol Educ Program. 2003:419–437. doi: 10.1182/asheducation-2003.1.419. [DOI] [PubMed] [Google Scholar]
- 24.Schaeffer DJ, Malpas PB, Barton LL. Risk assessment of microcystin in dietary Aphanizomenon flos-aquae. Ecotoxicol Environ Saf. 1999;44:73–80. doi: 10.1006/eesa.1999.1816. [DOI] [PubMed] [Google Scholar]
- 25.Donath MY, Halban PA. Decreased beta-cell mass in diabetes: significance, mechanisms and therapeutic implications. Diabetologia. 2004;47:581–589. doi: 10.1007/s00125-004-1336-4. [DOI] [PubMed] [Google Scholar]
- 26.Homo-Delarche F, Calderari S, Irminger JC, Gangnerau MN, Coulaud J, Rickenbach K, Dolz M, Halban P, Portha B, Serradas P. Islet inflammation and fibrosis in a spontaneous model of type 2 diabetes, the GK rat. Diabetes. 2006;55:1625–1633. doi: 10.2337/db05-1526. [DOI] [PubMed] [Google Scholar]
- 27.Körbling M, Katz RL, Khanna A, Ruifrok AC, Rondon G, Albitar M, Champlin RE, Estrov Z. Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N Engl J Med. 2002;346:738–746. doi: 10.1056/NEJMoa3461002. [DOI] [PubMed] [Google Scholar]
- 28.Sordi V, Malosio ML, Marchesi F, Mercalli A, Melzi R, Giordano T, Belmonte N, Ferrari G, Leone BE, Bertuzzi F, Zerbini G, Allavena P, Bonifacio E, Piemonti L. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005;106:419–427. doi: 10.1182/blood-2004-09-3507. [DOI] [PubMed] [Google Scholar]
- 29.Mills SE. Pancreas. In: Histology for pathologists. 3rd ed. Lippincott, Williams and Wilkins; Philadelphia: 2007. pp. 724–760. [Google Scholar]
- 30.Ianus A, Holz GG, Theise ND, Hussain MA. In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Invest. 2003;111:843–850. doi: 10.1172/JCI16502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butler AE, Huang A, Rao PN, Bhushan A, Hogan WJ, Rizza RA, Butler PC. Hematopoietic stem cells derived from adult donors are not a source of pancreatic beta-cells in adult nondiabetic humans. Diabetes. 2007;56:1810–1816. doi: 10.2337/db06-1385. [DOI] [PubMed] [Google Scholar]
- 32.Hess D, Li L, Martin M, Sakano S, Hill D, Strutt B, Thyssen S, Gray DA, Bha M. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat Biotechnol. 2003;21:763–770. doi: 10.1038/nbt841. [DOI] [PubMed] [Google Scholar]
- 33.Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, Prockop DJ. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A. 2006;103:17438–17443. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urbán VS, Kiss J, Kovács J, Gócza E, Vas V, Monostori E, Uher F. Mesenchymal stem cells cooperate with bone marrow cells in therapy of diabetes. Stem Cells. 2008;26:244–253. doi: 10.1634/stemcells.2007-0267. [DOI] [PubMed] [Google Scholar]
- 35.Voltarelli JC, Couri CE, Stracieri AB, Oliveira MC, Moraes DA, Pieroni F, Coutinho M, Malmegrim KC, Foss-Freitas MC, Simões BP, Foss MC, Squiers E, Burt RK. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2007;297:1568–1576. doi: 10.1001/jama.297.14.1568. [DOI] [PubMed] [Google Scholar]
- 36.Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20:530–541. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- 37.Rubio D, Garcia-Castro J, Martín MC, de la Fuente R, Cigudosa JC, Lloyd AC, Bernad A. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–3039. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 38.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- 39.Spees JL, Gregory CA, Singh H, Tucker HA, Peister A, Lynch P, Hsu SC, Smith J, Prockop DJ. Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol Ther. 2004;9:747–756. doi: 10.1016/j.ymthe.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Horwitz EM, Gordon PL, Koo WK, Marx JC, JC MD, McNall RY, Muul L, Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]