Abstract
Background and Objectives
Hydroxyapatite has biocompatibility and bioactivity and similar to bone of in human body. The purpose of this study is to evaluate osteogenic differentiation of bone marrow stem cell (BMSC) in PLGA Scaffold added various ratio of hydroxyapatite (HAp).
Methods and Results
PLGA and PLGA/HAp scaffold were prepared using solvent casting/salt-leaching method. BMSC was seeded on the PLGA and PLGA/HAp scaffold and the samples were cultured in 37℃ incubator with 5% CO2 for 28 days. Alkaline phosphatase (ALP) was carried out to evaluate alkaline phosphatase activity at 1, 3, 7, 10 and 14 days. Alizarin Red S stating was performed to identify calcium in scaffold at 1, 7, 14, 21 and 28 days. Compressive strength was measured to evaluate mechanical property of scaffold. To confirm cell viability, MTT was carried out at 1, 3, 7, 14 and 28 days. RT-PCR was performed to verify specific marker expression of osteoblast and stem cell at 7, 14, 21 and 28 days.
Conclusions
Osteogenic differentiation of BMSC was confirmed through ALP, RT-PCR, and alizarin red S staining in this study. These results suggest that HAp helps osteogenic differentiation of BMSC.
Keywords: Hydroxyapatite, Poly(lactic-co-glycolic acid), Scaffold, Bone marrow stem cell, Osteogenic differentiation
Introduction
In tissue engineering field, mixture of cells, scaffolds, and bioactive substance is used for transplantation or grafting to regenerate or recovery (1-5). The scaffold among three components plays important roles, which are maintaining the transplanted cells and helping functions effectively (6-12). Therefore, the methods for fabricating the scaffold with various structure and form have been developed. Artificial bone transplantation as a final requiring medical technology in irreparable situation through accident or disease is increasing by advanced country. To transplant artificial bone, material or device should non-toxic during performance of function in the body (13, 14). HAp (Ca10(PO4)6(OH)2) is known as the highest biocompatible substitution material among developed artificial bones and accounts for 70% of bone constituent in human body (15, 16).
HAp as an alkaline calcium phosphate has excellent biocompatibility and bioactivity because that similar to bone and minerals component of tooth in human body (17-22). In other words, HAp is the most widely used biomaterials in coating of hard tissue and metal implant because that has non-toxic and promote osteogenic differentiation induction (23-25). As stated above, calcium phosphate similar to bone chemically as a substitution material of bone which has been tried to coating at bio-metallic material and invested biocompatibility because that has advantage that the time fixated on bone is fast (26-28). Poly(lactic- co-glycolic acid) (PLGA) as a copolymer of PLA and PGA have been used in medical field because it has good biodegradation and mechanical strength and it was approved from FDA. And, PLGA has advantage that can be control biodegradation by ratio of PLA and PGA, but there is a problem about an attachment of cells on PLGA due to hydrophobic property. To resolve the problem, some methods adding natural polymers have been tried in tissue engineering. Osteogenic differentiation of BMSC cultured in scaffold prepared by mixing PLGA and HAp was evaluated by alkaline phosphatase (ALP), alizarin red S red staining, MTT, RT-PCR, SEM as an assay, in this study.
Materials and Methods
Materials
HAp (hydroxyapatite) was purchased from CGbio Co. (Korea). PLGA (lactide/glycolide: 75/25, molecular weight: 90,000 g/mole) was bought from Boehringer Ingelheim Chem. Co. (Germany). Methylene chloride and all reagents were used a high performance liquid chromatography (HPCL) grade.
Isolation of bone marrow stem cell (BMSC)
BMSC was isolated from leg of female white rabbit (4 weeks, White New Zealand). After remove hair of rabbit, a leg bone was collected using surgical tools. And, bone marrow was collected using 18-gauge syringe on clean bench after cutting bone. Monocyte was isolated from collected bone marrow using centrifugal separator.
Cell culture
BMSCs were cultured in Minimum Essential Medium-α (Gibco Co., Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Gibco Co.). The media was replaced every 2 days. 0.05% trypsin solution (Gibco Co.) was used to detach a cell from T-flask floor surface when passaging the BMSC. The BMSC was used at passage 2 in this study. 5×104 cells of BMSC were cultured on PLGA and PLGA/HAp scaffolds for 28 days. After seeding the cell, the media was replaced every two days.
Preparation of PLGA and PLGA/HAp scaffolds
PLGA and PLGA/HAp scaffold were prepared using solvent casting/salt-leaching method as shown in Fig. 1. To prepare the PLGA/HAp scaffolds by various ratio of HAp, the scaffolds were prepared through ratio like Table 1. Manufacturing process of the scaffolds is as follow. After dissolving PLGA of 1 g in methylene chloride of 4 mL, added HAp of ratio of 0, 10, 20, 40 and 60% in there, and added NaCl with 250∼355 μm size of 9 g. And, the mixture was put in silicon mold (diameter: 7 mm, height: 3 mm), then compressed the silicon mold under 60 kgf/cm2 for 24 hours. To remove the salt, the scaffold immersed in distilled water for 48 hours and the distilled water was changed every 6 hours. Finally, the scaffolds removed the salt was lyophilized under 8mTorr and −80℃ for 24 hours.
Fig. 1. The manufacturing methods of PLGA and PLGA/HAp scaffold.
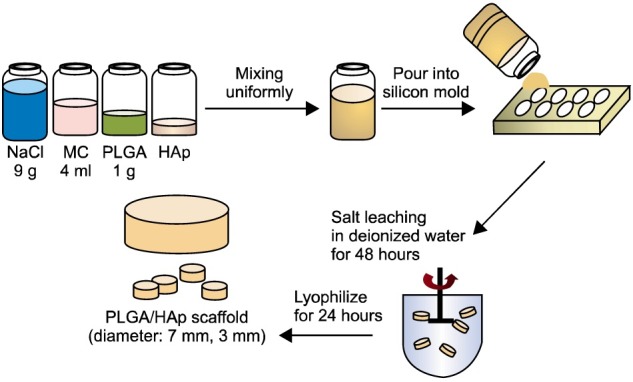
Table 1.
The ratio of PLGA and HAp in scaffold
| PLGA | HAp | ||||
|---|---|---|---|---|---|
| 0 g | 0.1 g | 0.2 g | 0.4 g | 0.6 g | |
| 1 g | Type 1 | Type 2 | Type 3 | Type 4 | Type 5 |
| (0%) | (10%) | (20%) | (40%) | (60%) | |
Compressive strength of scaffolds
To confirm mechanical property of scaffolds, compressive strength test was carried out using texture analyzer (TMS-Pro, Sterling, VA, USA). Height of each scaffold was measured to evaluate compressive strength of scaffold and half height of the scaffold was compressed under speed of 20 mm/sec and force of 0.5 N.
Scanning Electron Microscope (SEM)
The morphology of PLGA and PLGA/HAp scaffold was observed using SEM (LV-SEM, S-3000N, Hitachi Co., Tokyo, Japan). To observe the scaffold using SEM, the scaffold was fixed on the carbon fiber tape and then coated by platinum-palladium for 180 sec under argon gas. The sample was measured in voltage of 10.0 kV and 20.0 kV.
Cell viability
MTT assay was carried out to determine adhesion and viability of cell in PLGA and PLGA/HAp scaffold containing various content of HAp. The cell was cultured on prepared scaffold for 1, 3, 7, 10, 14 and 28 days. The scaffold with culture media of 1 ml containing MTT solution (Sigma Aldrich, St. Louis, MO, USA) of 100 μl was placed in 37℃ incubator with 0.5% CO2 for 4 hours. After incubation, the media was removed and refilled dimethyl sulfoxide (Sigma Co.) of 1 ml and then placed in incubator overnight. And, the optical density was measured at wavelength of 570 nm using absorbance readers (BioTek, Winooski, VT, USA).
Alkaline phosphatase activity
Alkaline phosphatase assay (ALP) was performed to confirm osteogenic differentiation of BMSC cultured in PLGA and PLGA/HAp scaffold using TRAP and ALP Assay Kit (TaKaRa, Otsu, Japan) for 1, 3, 7, 10 and 14 days, respectively. To break a cell wall, extraction solution of 1 ml was put into scaffold seeded cell and then pNPP solution of 50 μl put into the sample in order to activate alkaline phosphatase in scaffold and the sample was placed in incubator for 1hour. After 1 hour, stop solution of 1 ml was put into the sample. And, the optical density was measured at wavelength of 570 nm using absorbance reader (BioTek).
Alizarin Red S staining
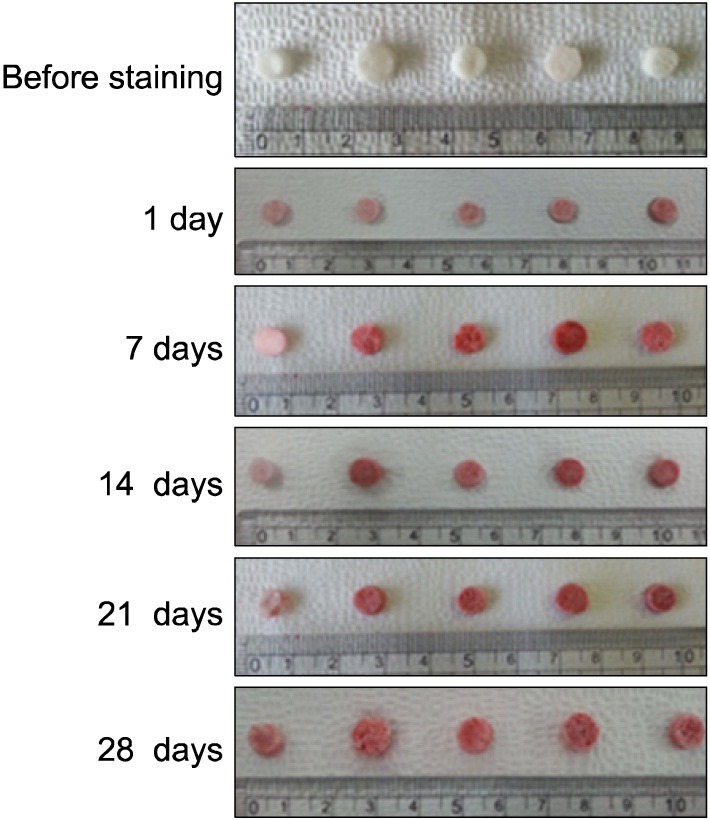
To evaluate the osteogenic differentiation of BMSC cultured in PLGA and PLGA/HAp scaffold, alizarin red S stating was carried out for 1day, 1, 2, 3 and 4 weeks. Media was removed in the scaffold seeded BMSC and washed the scaffold using PBS three times. Alizarin Red S staining solution of 50 μl was dropped on the washed scaffold and waited for 2 min 30 sec. After 2 min 30 sec, the scaffold was washed using PBS many times in order to remove remaining staining solution. And, the stained scaffold was photographed using camera (Canon Power shot G6, Tokyo, Japan).
RNA isolation and RT-PCR
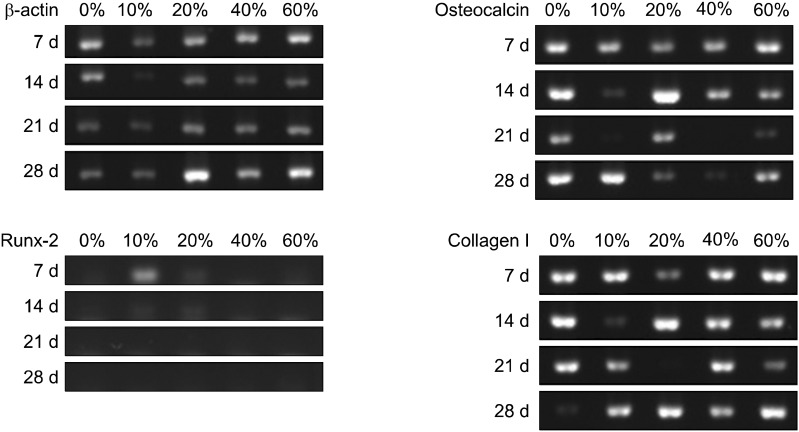
RT-PCR assay was carried out to confirm gene expression of BMSC in PLGA and PLGA/HAp scaffold. To confirm mRNA of BMSC cultured in PLGA and PLGA/HAp combined HAp in various ratios, the scaffolds was put into 24-well plate and were sterilized using 70% alcohol. And, cells of 5×104 density was seeded on the scaffolds and the cells were cultured for 1, 3, 7, 14 and 28 days in order to carry out RT-PCR. After cell culture, mRNA was isolated from cell using TRIzol (TaKaRa). The mRNA and each primer were put into PCR tube using One-step RT PCR DryMIX (Enzynomics, Daejeon, Korea). And, reverse transcription was performed and specific markers of the samples was amplified through PCR thermal cycler (TaKaRa). And then amplified DNA of 2 μl was put into 1.2% (w/v) agarose gel and carried out electrophoresis under voltage of 100 V for 30 min. β-actin was used as a house keeping gene and collagen type I, osteocalcin and runx-2 and were used as specific marker of osteoblast and stem cell. Relative expression value was visualized by SYBR green fluorescent (SYBRTM Green I Nucleic Acid Gel Stain, Cambrex, UK) and the image was observed using ultraviolet irradiation apparatus (Vilber Lourmat ETX-20.M, Marne-la-Vallée, France).
Results
Compressive strength
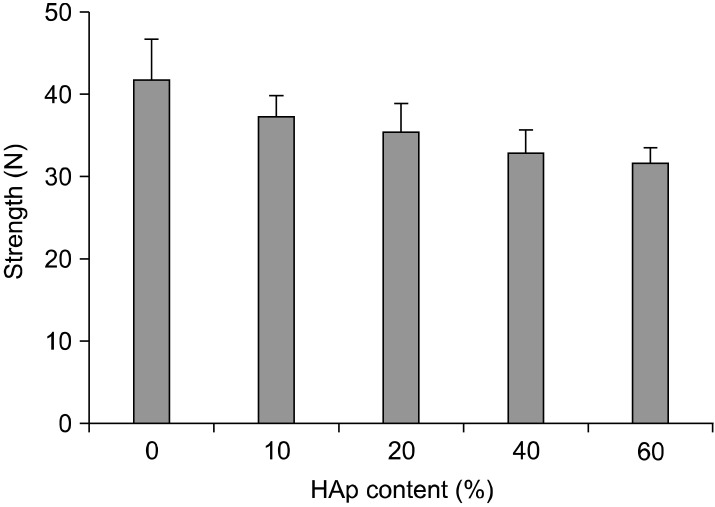
Compressive strength of PLGA and PLGA/HAp scaffolds was measured to confirm mechanical property as a substitution material of bone. Fig. 2 is compressive strength result of PLGA and PLGA/HAp scaffold. The compressive strength of PLGA scaffold was the highest and content of HAp in PLGA/HAp scaffold increases, the compressive strength was decrease. It is showed that HAp weakens the compressive strength of PLGA scaffold.
Fig. 2. Compressive strength of PLGA and PLGA/HAp scaffold.
Cell viability
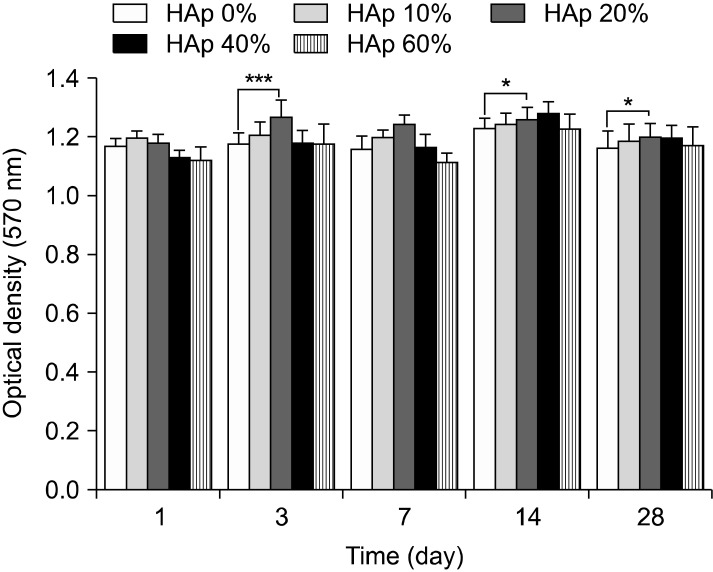
MTT assay carried out to confirm cell viability. MTT result found no significant differences as shown in Fig. 3. There is no significant differences overall, but cell viability cultured on PLGA scaffold was lower than cell viability cultured on PLGA/HAp scaffolds. Especially, cell viability cultured on scaffold containing 20% HAp was slightly higher than other groups.
Fig. 3. The proliferation and viability of BMSC cultured in PLGA and PLGA/HAp scaffold for 1, 3, 7, 14 and 28 days (***p<0.001, *p<0.05).
Morphology of scaffold
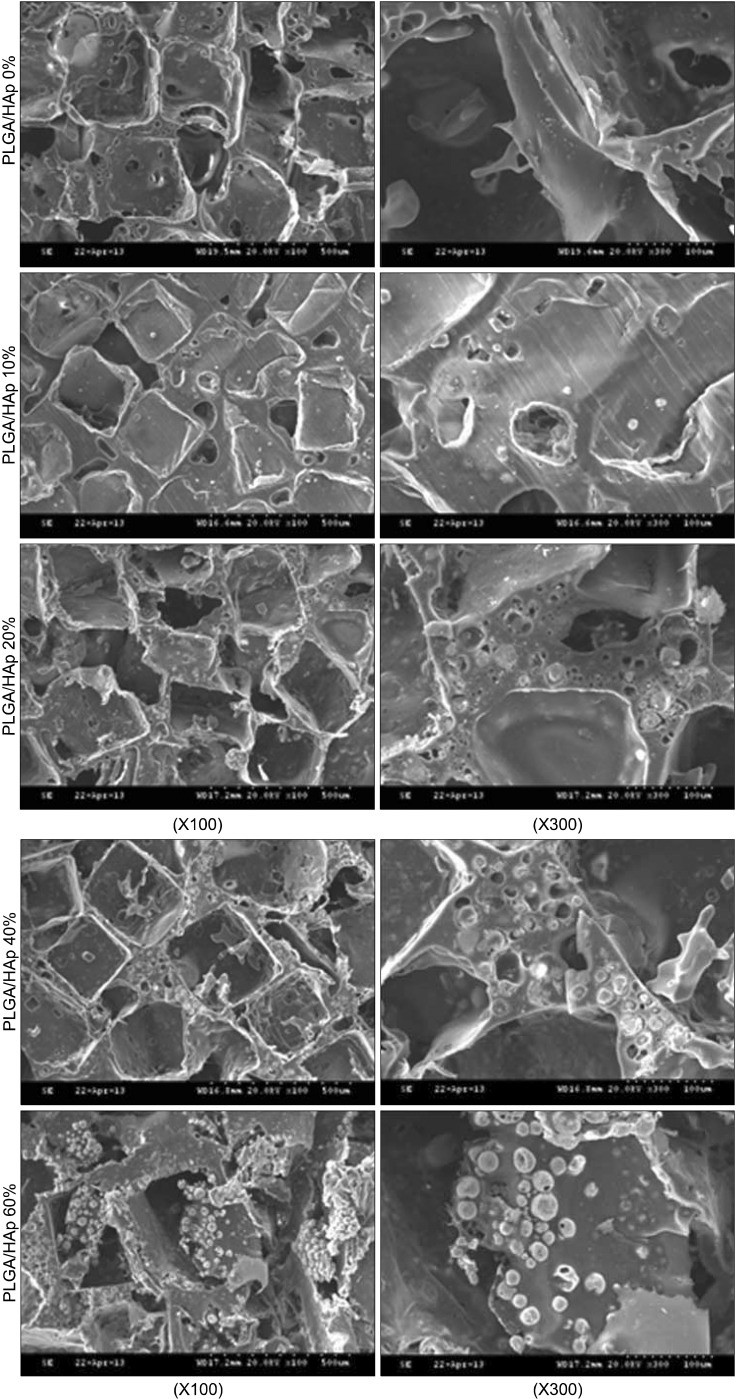
SEM was carried out to observe morphology of PLGA and PLGA/HAp scaffold. SEM image of the scaffolds was appeared in Fig. 4. The HAp was spread out more widely on PLGA containing high content of HAp as shown in Fig. 4. And, HAp was stuck between pore walls of scaffold.
Fig. 4. SEM image of PLGA and PLGA/HAp scaffold.
Alkaline phosphatase activity
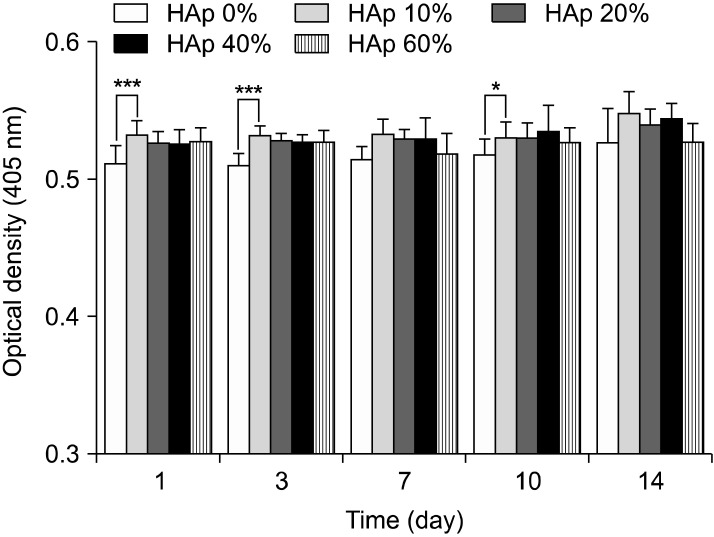
Fig. 5 showed alkaline phosphatase assay result. As shown in Fig. 5, ALP result also found no significant differences overall. However, alkaline phosphatase activity of BMSC cultured on scaffold containing 10% HAp was higher than other groups, but not that much of a difference.
Fig. 5. Alkaline phosphatase activity in PLGA and PLGA/HAp scaffold for 1, 3, 7, 10 and 14 days (***p<0.001, *p<0.05).
Alizarin Red S staining
To confirm calcium in scaffold during cell culture, alizarin red S staining was performed for 1, 7, 14, 21 and 28 days. Alizarin Red S staining result showed in Fig. 6. The scaffold cultured cells has white color before staining and the scaffold had turned red color after stating as shown in Fig. 6. And. the scaffold stained at 1day has more white color than other groups (7, 14, 21 and 28 days). As time goes by, the staining color became slightly richer color than before.
Fig. 6. Alizarin Red S staining of PLGA and PLGA/HAp scaffold for 1, 7, 14, 21 and 28 days.
Reverse transcription-polymers chain reaction (RT-PCR)
RT-PCR was carried out to verify specific marker expression of osteoblast. β-actin was measured as a house keeping gene and collagen type I and osteocalcin were measured as a specific marker of osteoblast. And, runx-2 was measured as a specific marker of stem cell. Expression of β-actin appeared at all groups and expression of osteocalcin appeared to be low at 21 days as shown in Fig. 7. Also, expression of runx-2 appeared at initial stage like 7 and 14 days and collagen type I appeared at almost all groups.
Fig. 7. Gene expression of BMSC cultured in PLGA and PLGA scaffold for 7, 14, 21 and 28 days.
Discussion
In this study, PLGA scaffold containing various ratio of HAp was prepared to evaluate osteogenic differentiation of BMSC. After preparing the PLGA and PLGA/HAp scaffold, BMSCs were cultured on the PLGA and PLGA/HAp scaffold for 28 days in vitro. Compressive strength of prepared scaffold was measured to confirm mechanical property in connection with bone. Bone is the hardest tissue in human body, therefore scaffold for bone needs hardness. When comparing the PLGA and PLGA/HAp scaffold, compressive strength of scaffolds was decreased as HAp content increases (29). Compression strength of scaffolds containing HAp of 0, 10, 20, 40 and 60% was about 41, 38, 36, 33 and 31 N, respectively. And, it was related to SEM image. HAp was stuck between pore walls as shown in Fig. 4. It is considered that the compressive strength of scaffolds containing large amount of HAp was weak because HAp as a ceramic has weak strength. In MTT result, cell viability was high on scaffold containing 20% HAp, but not that much of a difference. It is indicated that 20% HAp help cell viability a little, but that not effective for BMSC proliferation (30). ALP as an abundant hydrolase enzyme in osteoblast shown at initial stage was carried out to evaluate alkaline phosphatase activity for 1, 3, 7, 10 and 14 days (31). Also, there are significant differences in ALP result. However, the result showed steady alkaline phosphatase activity as time goes by. Especially, alkaline phosphatase activity was higher in scaffold containing HAp than in PLGA scaffold (32, 33). Most of all, alkaline phosphatase phosphatase activity was the highest in scaffold containing 10% HApnsider that HAp influenced osteogenic differentiation of BMSC. This result is similar to MTT result and therefore large amount like HAp of 40% and 60% was not effective for cell viability and differentiation. RT-PCR was performed to identify gene expression of osteogenic differentiaton of BMSC cultured in PLGA and PLGA/HAp scaffold. β-actin as a house keeping gene appeared in all group as shown in Fig. 6. And, osteocalcin as a specific marker of osteoblast appeared in almost all group. When normalizing the expression of β-actin and osteocalcin, expression of osteocalcin was a little higher at the scaffold containing 10% HAp than other group. It is similar to ALP result and osteogenic differentiation of BMSC was the most effective on the scaffold containing 10% HAp. Runx-2 as a specific marker of stem cell appeared at 7 days and that didn’t appeared after 14 days. It is consider that differentiation of BMSC occurred because expression of runx-2 was has been decreasing. Collagen type I is also specific marker of osteoblast, and expression of collagen type I appeared at almost all group. The expression of collagen type I don’t have uniform result value, but it was high in PLGA/HAp scaffold containing 10% HAp when normalizing expression of β-actin and collagen type I (data was not attached). In RT-PCR result, osteogenic differentiation of BMSC was also good on the scaffold containing 10% HAp. As a result, it is suggested that osteogenic differentiation of BMSC was the best on scaffold containing 10% HAp, and large amount of HAp in scaffold wasn’t effective for viability and differentiation of BMSC based on above result. Therefore, adding some HAp to scaffold may help osteogenic differentiation BMSC (34).
In summary, osteogenic differentiation of BMSC was great in 10% HAp among 0, 10, 20, 40 and 60% of HAp through ALP result and RT-PCR. Cell viability was good in 20% HAp. Finally, the results of this study suggest that HAp of 10% and 20% was better than other groups (40% and 60%) for bone tissue engineering.
Potential conflict of interest
The authors have no conflicting financial interest.
Acknowledgments
This research was supported by WCU (R31-20029) and Bio-industry Technology Development Program (112007- 05-1-SB010), by Bio-industry Technology Development Program (112007-05-1-SB010), Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea.
References
- 1.Kahlig A, Hansmann J, Groeber F, Schwarz T, Weyhmuller J, Illig A, Kleinhans C, Walles H. In silico approaches for the identification of optimal culture condition for tissue engineered bone substitutes. Curr Anal Chem. 2013;9:16–28. [Google Scholar]
- 2.Park CH, Rios HF, Jin Q, Sugai JV, Padial-Molina M, Taut AD, Flanagan CL, Hollister SJ, Giannobile WV. Tissue engineering bone-ligament complexes using fiber-guiding scaffolds. Biomaterials. 2012;33:137–145. doi: 10.1016/j.biomaterials.2011.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naveena N, Venugopal J, Rajeswari R, Sundarrajan S, Sridhar R, Shayanti M, Narayanan S, Ramakrishna S. Biomimetic composites and stem cells interaction for bone and cartilage tissue regeneration. J Mater Chem. 2012;22:5239–5253. [Google Scholar]
- 4.Gu BK, Kim MS, Kim SJ, Park YH. The effect of electrical stimulation on nerve cells using conducting polymer. Inter J Tissue Reg. 2011;2:83–87. [Google Scholar]
- 5.Kim TH, Ko JH, Kim SJ, Park YH. Implantable medical textiles. Inter J Tissue Reg. 2011;2:1–12. [Google Scholar]
- 6.Yu XH, Xia ZM, Wang LP, Peng F, Jiang X, Huang JP, Rowe D, Wei M. Controlling the structural organization of regenerated bone by tailoring tissue engineering scaffold architecture. J Mater Chem. 2012;22:9721–9730. [Google Scholar]
- 7.Sun F, Zhou H, Lee J. Various preparation methods of highly porous hydroxyapatite/polymer nanoscale biocomposites for bone regeneration. Acta Biomater. 2011;7:3813–3828. doi: 10.1016/j.actbio.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Clyne AM. Thermal processing of tissue engineering scaffolds. J Heat Trans T Asme. 2011;133 [Google Scholar]
- 9.Smith IO, Ma PX. Cell and biomolecule delivery for regenerative medicine. Sci Technol Adv Mat. 2010;11 doi: 10.1088/1468-6996/11/1/014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramachandran K, Gouma PI. Electrospinning for bone tissue engineering. Recent Pat Nanotechnol. 2008;2:1–7. doi: 10.2174/187221008783478608. [DOI] [PubMed] [Google Scholar]
- 11.Moutos FT, Guilak F. Composite scaffolds for cartilage tissue engineering. Biorheology. 2008;45:501–512. [PMC free article] [PubMed] [Google Scholar]
- 12.Yang DH, Park HN, Lee JB, Heo DN, Bae MS, Kwon IK. Effect of YIGSR/PEG and IKVAV/PEG immobilized PU films on the proliferation and differentiation of PC-12 cells. Inter J Tissue Reg. 2011;2:119–124. [Google Scholar]
- 13.Jaiswal AK, Kadam SS, Soni VP, Bellare JR. Improved functionalization of electrospun PLLA/gelatin scaffold by alternate soaking method for bone tissue engineering. Appl Surf Sci. 2013;268:477–488. [Google Scholar]
- 14.Joshy MIA, Elayaraja K, Sakthivel N, Chandra VS, Shanthini GM, Kalkura SN. Freeze dried cross linking free biodegradable composites with microstructures for tissue engineering and drug delivery application. Mat Sci Eng C-Mater. 2013;33:466–474. doi: 10.1016/j.msec.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Wu Q, Zhang XL, Wu B, Huang W. Effects of microwave sintering on the properties of porous hydroxyapatite scaffolds. Ceram Int. 2013;39:2389–2395. [Google Scholar]
- 16.Jiang J, Hao W, Li Y, Yao J, Shao Z, Li H, Yang J, Chen S. Hydroxyapatite/regenerated silk fibroin scaffold-enhanced osteoinductivity and osteoconductivity of bone marrow-derived mesenchymal stromal cells. Biotechnol Lett. 2013;35:657–661. doi: 10.1007/s10529-012-1121-2. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Li RX, Fan YB, Liu H, Guo Y, Wang L, Shi CH, Zhu D, Zhang XZ. Microarchitectural and mechanical characterization of chitosan/hydroxyapatite/demineralized bone matrix composite scaffold. J Porous Mat. 2012;19:251–259. [Google Scholar]
- 18.Sun F, Koh K, Ryu SC, Han DW, Lee J. Biocompatibility of nanoscale hydroxyapatite-embedded chitosan films. B Korean Chem Soc. 2012;33:3950–3956. [Google Scholar]
- 19.Pallela R, Venkatesan J, Janapala VR, Kim SK. Biophysicochemical evaluation of chitosan-hydroxyapatite-marine sponge collagen composite for bone tissue engineering. J Biomed Mater Res A. 2012;100A:486–495. doi: 10.1002/jbm.a.33292. [DOI] [PubMed] [Google Scholar]
- 20.Neshati Z, Bahrami AR, Eshtiagh-Hosseini H, Matin MM, Housaindokht MR, Tabari T, Edalatmanesh MA. Evaluating the biodegradability of Gelatin/Siloxane/Hydroxyapatite (GSHyd) complex in vivo and its ability for adhesion and proliferation of rat bone marrow mesenchymal stem cells. Cytotechnology. 2012;64:485–495. doi: 10.1007/s10616-012-9426-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nazarpak MH, Pourasgari F, Aghajamali M, Sarbolouki M. Preparation and evaluation of porous alginate/hydroxyapatite composite scaffold coated with a biodegradable triblock copolymer. Asian Biomed. 2012;6:753–758. [Google Scholar]
- 22.Liu L, Wang Y, Guo S, Wang Z, Wang W. Porous polycaprolactone/nanohydroxyapatite tissue engineering scaffolds fabricated by combining NaCl and PEG as co-porogens: structure, property, and chondrocyte-scaffold interaction in vitro. J Biomed Mater Res B Appl Biomater. 2012;100:956–966. doi: 10.1002/jbm.b.32658. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Nan K, Shi S, Chen H. Preparation and characterization of nano-hydroxyapatite/chitosan cross-linking composite membrane intended for tissue engineering. Int J Biol Macromol. 2012;50:43–49. doi: 10.1016/j.ijbiomac.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 24.Leung LH, Naguib HE. Characterization of the viscoelastic properties of poly(epsilon-caprolactone)-hydroxyapatite microcomposite and nanocomposite scaffolds. Polym Eng Sci. 2012;52:1649–1660. [Google Scholar]
- 25.Jin HH, Kim DH, Kim TW, Shin KK, Jung JS, Park HC, Yoon SY. In vivo evaluation of porous hydroxyapatite/ chitosan-alginate composite scaffolds for bone tissue engineering. Int J Biol Macromol. 2012;51:1079–1085. doi: 10.1016/j.ijbiomac.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 26.Jun SH, Lee EJ, Jang TS, Kim HE, Jang JH, Koh YH. Bone morphogenic protein-2 (BMP-2) loaded hybrid coating on porous hydroxyapatite scaffolds for bone tissue engineering. J Mater Sci Mater Med. 2013;24:773–782. doi: 10.1007/s10856-012-4822-0. [DOI] [PubMed] [Google Scholar]
- 27.Zo SM, Singh D, Kumar A, Cho YW, Oh TH, Han SS. Chitosan-hydroxyapatite macroporous matrix for bone tissue engineering. Curr Sci India. 2012;103:1438–1446. [Google Scholar]
- 28.Zhu WM, Lu W, Zhang XJ, Cai ZM, Liu HF, Peng LQ, Li H, Han Y, Fen WZ, Wang DP, Zeng YJ. Nano-hydroxyapatite/fibrin glue/recombinant human osteogenic protein- 1 artificial bone for repair of bone defect in an animal model. Micro Nano Lett. 2012;7:467–471. [Google Scholar]
- 29.Li B, Huang LN, Wang XB, Ma JH, Xie F. Biodegradation and compressive strength of phosphorylated chitosan/chitosan/hydroxyapatite bio-composites. Mater Design. 2011;32:4543–4547. [Google Scholar]
- 30.Ngiam M, Liao S, Patil AJ, Cheng Z, Chan CK, Ramakrishna S. The fabrication of nano-hydroxyapatite on PLGA and PLGA/collagen nanofibrous composite scaffolds and their effects in osteoblastic behavior for bone tissue engineering. Bone. 2009;45:4–16. doi: 10.1016/j.bone.2009.03.674. [DOI] [PubMed] [Google Scholar]
- 31.Vines JB, Lim DJ, Anderson JM, Jun HW. Hydroxyapatite nanoparticle reinforced peptide amphiphile nanomatrix enhances the osteogenic differentiation of mesenchymal stem cells by compositional ratios. Acta Biomater. 2012;8:4053–4063. doi: 10.1016/j.actbio.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jang JH, Castano O, Kim HW. Electrospun materials as potential platforms for bone tissue engineering. Adv Drug Deliv Rev. 2009;61:1065–1083. doi: 10.1016/j.addr.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Kim HW, Song JH, Kim HE. Nanofiber generation of gelatin- hydroxyapatite biomimetics for gulded tissue regeneration. Adv Funct Mater. 2005;15:1988–1994. [Google Scholar]
- 34.Budiraharjo R, Neoh KG, Kang ET. Hydroxyapatite-coated carboxymethyl chitosan scaffolds for promoting osteoblast and stem cell differentiation. J Colloid Interface Sci. 2012;366:224–232. doi: 10.1016/j.jcis.2011.09.072. [DOI] [PubMed] [Google Scholar]