Abstract
Background and Objectives
Irradiated wound healing is a highly complex and dynamic process. The latest technology making a huge difference in this process is stem cell therapy. The goal of this study was to evaluate the use of bone marrow-derived mesenchymal stem cells (BM-MSCs) or human amniotic epithelial cells (HAECs) in the healing of irradiated wounds.
Methods and Results
Forty five male albino rats were subjected to whole body 6 gray gamma radiations. One day post irradiation, full-thickness incisional wound was created in the tibial skin. The rats were randomly equally divided into three groups. The incisions of the first group (gp I) were injected intra-dermally with saline before stitching and those of both the second (gp II) and the third groups (gp III) were intradermally injected with BM-MSCs and HAECs before stitching respectively. Animals were sacrificed after the third, seventh and fourteenth days postoperative. The healing process was assessed histopathologically. CXCL-5, SDF-1 and Transforming growth factor-beta 1 (TGF-β1) expression were also detected in biopsies from all wounds. Expression of TGF-β1 in gp I was more than the other groups leading to severe inflammation, deficient healed dermis and delayed reepithelialization. SDF-1 expression was high in gp II while CXCL-5 expression was high in gp III causing accelerated wound healing. BM-MSCs showed a great effect on the quality of the dermis, while superiority of the epithelium and its appendages were achieved in HAECs group.
Conclusions
Using BM-MSCs and HAECs could be used safely in case of irradiated wounds.
Keywords: Wound healing, BM-MSCs, HAECs
Introduction
Wound healing is a highly complex and dynamic process and remains a major challenge in modern medicine. The optimal healing of a cutaneous wound requires a well-orchestrated integration of the complex biological and molecular events of cell migration and proliferation as well as of extracellular matrix deposition and remodeling. Even under optimal conditions, the healing process leads to fibrosis or a scar. One of the recent technology making a huge difference in the wound healing process is stem cell therapy, which offers a novel approach to many diseases (1, 2).
Mesenchymal stem cells (MSCs), originating from bone marrow, adipose tissue, umbilical cord blood and many other tissues, are multipotent stem cells that have proliferative and self-renewal potential and that can differentiate into multi-lineage cell types, including osteoblasts, chondrocytes, adipocytes, myocytes, cardiomyocytes, neurons and epithelial cells (3, 4).
Bone marrow-derived mesenchymal stem cells (BMMSCs) are self renewing and expandable stem cells. BM-MSCs engrafted in wound can accelerate wound healing as they assume an epithelial-like phenotype (5). A subset of adult human BM-MSCs could differentiate into sweat glands. BM-MSCs when intravenously injected into full-thickness rat skin wounds, they appeared in the hair follicles, sebaceous glands, blood vessels and dermis of these wounds. Therefore, differentiation and trans-differentiation of BM-MSCs are involved in wound healing in skin and its appendages (6). BM-MSCs have also low immunogenesity and immunoregulatory action by suppressing the functions of immune cells, including T cells. Thus these allogeneic stem cells might be able to accelerate wound healing like autologous ones (7).
In spite of these data, BM-MSC transplantation requires harvesting a large amount of bone marrow under general anesthesia, which may lead to severe complications. Thus the easy and risk-free collection process raises few ethical concerns (8).
Knowing that the ability to heal wounds relies on the rapid migration and proliferation of epithelial cells located in the basal layer of the epidermis adjacent to the area of tissue damage attract the attention to the role of human amniotic epithelial cells (HAECs) in the wound healing. It has long been proposed that HAECs could harbor the potential to differentiate into a wide variety of different organs including heart, liver and brain (9). Human amniotic fluid stem cells have an immune tolerance and/or immunosuppression effect, similar to that reported for other stem cells and they can be easily obtained (10).
Radiotherapy has been utilized for over 100 years (11). It is used to cure certain cancers, such as cancer of the retina, central nervous system, skin, oropharynx and larynx, and lymphoma. Radiotherapy is also used as an adjuvant treatment, in addition to surgical resection (and chemotherapy, when appropriate) for cancers of the lung, breast, testis (seminoma) and soft tissues (sarcoma). In some patients, radiotherapy has a palliative role for pain relief and preservation of the skeleton following bone metastases (12).
Although radiation therapy is used to kill cancerous cells, it also damages healthy cells, leading to many acute and long-term side effects. The long-term effects of radiotherapy include skin atrophy, soft tissue fibrosis and micro vascular damage, leading to a higher risk of developing problematic, non healing wounds which are unamenable to surgical repair. Advances in the delivery of radiotherapy have aimed to minimize this damage by reducing the radiation effect on healthy tissue while maximizing the effect on cancerous tissue. However, it is inevitable that some normal tissue will be affected, after radiotherapy. The skin is particularly affected by radiation damage, as it is in the path of all external radiotherapy (13, 14).
Wound healing problems in these patients are often underestimated and are partly due to abnormally functioning fibroblasts that cause lack of collagen synthesis and/or deposition of defective collagen (15). This study was designed to improve the wound healing of previously irradiated skin. Two different types of stem cells; BM-MSCs and HAECs were used to have the challenge.
Materials and Methods
Isolation and culture of BM-MSCs
Bone marrow was harvested by flushing the tibiae and femurs of 6-week-old male white albino rats with Dulbecco's modified Eagle's medium (DMEM, GIBCO/BRL) supplemented with 10% fetal bovine serum (GIBCO/BRL). Nucleated cells were isolated with a density gradient (Ficoll/Paque [Pharmacia]) and resuspended in complete culture medium supplemented with 1% penicillin- streptomycin (GIBCO/BRL). Cells were incubated at 37℃ in 5% humidified CO2 for 12∼14 days as primary culture or upon formation of large colonies. When large colonies developed (80∼90% confluence), cultures were washed twice with phosphate buffer saline (PBS) and the cells were trypsinized with 0.25% trypsin in 1mM EDTA (GIBCO/BRL) for 5 min at 37℃. After centrifugation, cells were resuspended with serum-supplemented medium and incubated in 50 cm2 culture flask (Falcon). The resulting cultures were referred to as first-passage cultures (16). MSCs in culture were characterized by their adhesiveness and fusiform shape (17).
Isolation and culture of HAECs
Amniotic fluids (10 ml each) were obtained from four women undergoing caesarean section at a gestational age of 38 to 39 weeks. Amniotic fluids were centrifuged and supernatants discarded. Cell pellets were resuspended with Chang Medium (α-MEM, 15% embryonic stem cell-fetal bovine serum (Gibco-Invitrogen, Grand Island, NY, USA) with 18% Chang B and 2% Chang C (Irvine Scientific, Irvine, CA, USA) in a petri dish. Cells were incubated within a 37℃/5% CO2 incubator. Non-adherent cells were discarded after one week. Adherent cells were passed for expansion on reaching 80% confluence, and culture medium was replaced every three days (10).
Animals
Forty five male albino rats, with average body weights 130∼150 g, were selected for the study. The animals were housed in separate cages, under normal temperature, pressure, humidity, good ventilation and illumination conditions during the whole period of experimentation. All animals were fed with semi purified diet and water ad libitum for one week before the start of the experiment. Rats which were inactive or showed signs of infection were discarded before treatment.
The experimental protocol used was approved by the department of Animal care, Cairo University that adhered to the European Communities Council guiding principles for the care and use of Laboratory animals.
Radiation exposure
Animal irradiation was performed in the National Centre for Radiation Research and Technology (NCRRT), Cairo, Egypt using 137 Cesium Gamma Cell 40 giving a dose rate of 0.806 gray/min at the time of experiment. All the animals were subjected to whole body 6 gray gamma radiations, taken back and kept in a quarantine to be operated on the second day.
Surgical procedures and grouping
One day post irradiation, Ketamine (ketamine [as HCL] 50 mg/ml; EIPICO, Egypt) 50 mg/kg body weight, and xylazine (xylazine, M.H. Reg. No. 1373/99 Vet; ADWIA, Egypt) 20 mg were administered intramuscularly in a 1:1 ratio. In addition, 2% lidocaine (Mepecaine, Alexandria Co. for Pharmaceuticals, Egypt) was injected locally at the surgical sites. After sedation, the region of the tibiae of each animal was shaved and surgically prepared with consecutive applications of 10% providone iodine scrub, and 70% isopropanol. The surgical site of 2 cm was outlined with a sterile surgical marker as a template. Full-thickness incisional wounds were created. The rats were randomly equally divided into three groups. The incisions of the first group (gp I) were intra-dermally injected with saline before stitching; those of the second group (gp II) were intra- dermally injected with BM-MSCs while of the third group (gp III) were intra-dermally injected with HAECs before stitching. After the surgery, each animal received intramuscular PAN-Terramycin (PAN-Terramycin antibiotic [oxytetracycline HCl] Pfizer, Egypt) antibiotic at a dose of 1 cm3/10 kg to protect against post-operative infection and analgesic at 0.05 mgm/kg for three successive days. One week after the operation, the sutures were removed.
Animal sacrifice and specimen preparation
The rats of each group were further randomly subdivided into three subgroups (5 rats from each group) according to the date of sacrifice, 3, 7 and 14 days after incision. The subgroups were assigned into A, B and C respectively. The animals were sacrificed by cervical dislocation and the tibial skin was then dissected carefully.
Total RNA isolation
Tissue was homogenized by homogenizer (ART-MICCRA- Germany) and total RNA was isolated with RNA assay. Mini Kit (Qiagen) and further analyzed for quantity and quality with Beckman dual spectrophotometer (USA). The RNA integrity and the GAPDH- RNA (house keeping gene) ratio were used as the quality control.
Real time PCR (qRT-PCR)
The mRNA expression level was quantified by qRTPCR (real time PCR). 1,000 ng of the total RNA from each sample were used for cDNA synthesis by reverse transcription using High capacity cDNA Reverse Transcriptase Kit (Applied Biosystem, USA). The cDNA was subsequently amplified with the Syber Green I PCR Master Kit (Fermentas) in a 48-well plate using the Step One Instrument (Applied Biosystem, USA) as follows: 10 minutes at 95℃ for enzyme activation followed by 40 cycles of 15 seconds at 95℃, 20 seconds at 55℃ and 30 seconds at 72℃ for the amplification step. Changes in the expression of each target gene (CXCL-5, SDF-1 and Transforming growth factor beta-1 [TGFβ-1]) were measured relative to the mean critical threshold (CT) values of GAPDH housekeeping gene by the ΔCt method. 1 μM of both primers specific for each target gene was used. Primers sequence and annealing temperature specific for each target gene were demonstrated in Table 1.
Table 1.
Primers sequence and annealing temperature specific for each target gene
| Target gene | Primer sequence: 5'-3' | Annealing | Reference |
|---|---|---|---|
| CXCL-5 | Forward: TTCATGAGAAGGCAATGCTG | 53℃ | 18 |
| Reverse: CCCAGGCTCAGACGTAAGAA | |||
| SDF-1 | Forward: GAGAGCCACATCGCCAGAG' | 53℃ | 18 |
| Reverse: TTTCGGGTCAATGCACACTTG | |||
| TGFβ1 | Forward: GCGACGAAGAGTACTACGCC | 57℃ | 19 |
| Reverse: CTCCATTGCTGAGACGTCAA | |||
Histopathological analysis
Specimens for histopathological analysis were fixed in 10% calcium formol for 48 h, followed by dehydration in ascending grades of alcohol clearing in xylene and embedding in paraffin wax. Sections of 5 μ thick were stained with hematoxylin-eosin for routine examination.
Results
CXCL-5, SDF-1 and TGFβ1 expression
Gp III (HAECs group) showed the highest CXCL-5 expression followed by gp II (BM-MSCs group) while the least was detected in gp I (irradiated group). The expression mode was nearly the same; the highest expression at the seventh followed by the third then the fourteenth days postoperative. On the other hand, SDF-1 expression was the highest in gp II (BM-MSCs group), followed by gp III (HAECs group). The lowest cytokines expression was also found in gp I (irradiated group). Over expression of TGF-β1 was recorded in gp I (irradiated group) more than the other groups. It was also noticed that gp I showed different mode of expression; the highest at the third followed by the seventh then the fourteenth day (Fig. 1, Tables 2∼4).
Fig. 1. Graphical representation of CXCL-5 (A), SDF-1 (B) and TGF-β1 (C) expression in different groups through the healing process. 'a' represent significance difference between groups (p<0.05) as compared with irradiated group (control group).
Table 2.
CXCL-5 expression in all groups in different dates
| CXCL-5 | ||||
|---|---|---|---|---|
| HAECs | BM-MSCs | Irradiated | ||
| 3 Days | Mean | 0.92 | 0.92 | 0.371 |
| SD | 0.001247219 | 0.081649658 | 0.049935514 | |
| 7 Days | Mean | 1.23 | 0.841 | 0.416 |
| SD | 0.001247219 | 0.004189935 | 0.004966555 | |
| 14 Days | Mean | 0.6213 | 0.301 | 0.189 |
| SD | 0.012918548 | 0.008164966 | 0.000471405 | |
Table 4.
TGF-β1 expression in all groups in different dates
| TGF-β1 | ||||
|---|---|---|---|---|
| HAECs | BM-MSCs | Irradiated | ||
| 3 Days | Mean | 0.15 | 0.426 | 1.03 |
| SD | 0.008164966 | 0.000816497 | 0.0476189 | |
| 7 Days | Mean | 0.266666667 | 0.578333333 | 0.881 |
| SD | 0.001247219 | 0.004496913 | 0.008165 | |
| 14 Days | Mean | 0.144666667 | 0.335 | 0.441 |
| SD | 0.001699673 | 0.004966555 | 0.0008165 | |
Histological results
The wounds of gp I A (irradiated group 3 days postoperative) deprived from epithelial continuity. The wound gap was filled with granulation tissue that was infiltrated with inflammatory cells. Degenerated areas were numerous while fibroblasts and newly formed blood vessels were hardly detected. The adjacent connective tissue appeared ischemic and infiltrated with inflammatory cells (Fig. 2). The granulation tissue of the gp II A (BM-MSCs group 3 days postoperative) on the other hand, appeared more cellular than the previous group. Young fibroblasts and newly formed blood vessels were also more obvious as well as decreased inflammatory cell infiltrations (Fig. 3). In comparison to the previous groups, gp III A (HAECs group 3 days postoperative) showed similar findings to gp I A except for the presence of young fibroblasts and newly formed blood vessels that invaded the granulation tissue (Fig. 4). Dilated blood vessels were noticed in the adjacent connective tissue of both gps IIA & IIIA (Fig. 5).
Fig. 2. A photomicrograph of gp I A (irradiated group 3 days postoperative) shows degenerated granulation tissue filling the wound gap (G), inflammatory cells (arrows) and extravasated red blood cells (arrowheads) (H&E, ×200).

Fig. 3. A photomicrograph of gp II A (BMSCs group 3 days postoperative) shows granulation tissue filling the wound gap (G), newly formed blood vessels (V), inflammatory cells (arrows) and young fibroblasts (arrowheads) (H&E, ×200).

Fig. 4. A photomicrograph of gp III A (HAECs group 3 days postoperative) shows granulation tissue filling the wound gap (G), newly formed blood vessels (V), inflammatory cells (arrows) (H&E, ×200).
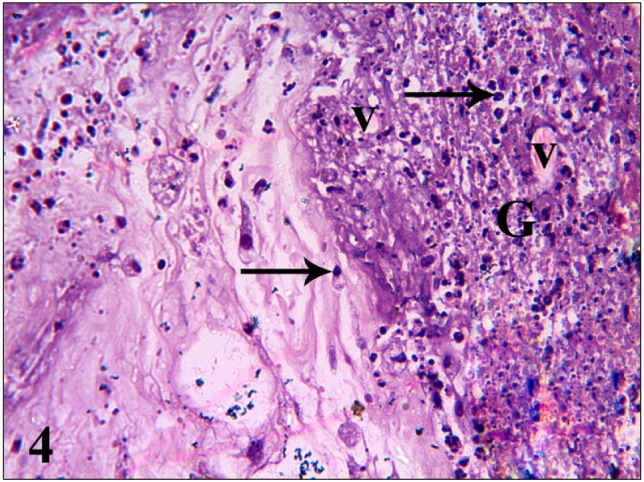
Fig. 5. A photomicrograph of gp III A (HAECs group 3 days postoperative) shows dilated blood vessels in the adjacent connective tissue (V) and inflammatory cells (arrows) (H&E, ×200).

Table 3.
SDF-1 expression in all groups in different dates
| SDF-1 | ||||
|---|---|---|---|---|
| HAECs | BM-MSCs | Irradiated | ||
| 3 Days | Mean | 1.21666667 | 1.3 | 0.582 |
| SD | 0.021602469 | 0.081241752 | 0.008164966 | |
| 7 Days | Mean | 1.741 | 1.7 | 0.628 |
| SD | 0.006236096 | 0.000816497 | 0.004496913 | |
| 14 Days | Mean | 0.679 | 0.9 | 0.496 |
| SD | 0.011775681 | 0.142731294 | 0.006599663 | |
After seven days of incision, the wound of gp I B (irradiated group 7 days postoperative) was still deprived from epithelium in spite of persistent suture materials. The marginal epithelium was necrotic (Fig. 6). The wound gap was filled with connective tissue that was fatty degenerated and infiltrated by inflammatory cells. The newly formed collagen bundles were thin and irregularly arranged (Fig. 7). Gp II B (BM-MSCs group 7 days postoperative) showed epithelial continuity with ill developed rete pegs. The collagen bundles of the underlying connective tissue appeared dense, well oriented and infiltrated with fewer inflammatory cells compared with the previous group (Fig. 8). The healed epithelium and underneath connective tissue appeared the same in gp III B (HAECs group 7 days postoperative) compared to gp II B except for the superficial area of the dermis where thin irregularly arranged collagen bundles were exist. However, the specialized structure of the skin seemed to be histologically normal in this group (Fig. 9).
Fig. 6. A photomicrograph of gp I B (irradiated group 7 days postoperative) shows marginal epithelium (asterisks) and inflammatory cells (arrows) (H&E, ×200).

Fig. 7. A photomicrograph of the connective tissues that fill the wound gap of gp I B (irradiated group 7 days postoperative) shows fat cells (F), thin collagen bundles (thick arrows), extravasated red blood cells (arrowheads) and inflammatory cells (thin arrows) (H&E, ×200).

Fig. 8. A photomicrograph of gp II B (BMSCs group 7 days postoperative) shows thin healed epithelium (arrowheads), dense collagen bundles (thick arrows), few inflammatory cells (thin arrows), sebaceous glands and sweat glands related to the unwounded area (S) (H&E, ×200).

Fig. 9. A photomicrograph of gp III B (HAECs group 7 days postoperative) shows thin healed epithelium (arrowheads), thin irregularly arranged collagen bundles (thick arrows), few inflammatory cells (thin arrows), sebaceous glands and sweat glands related to the healed wound (S) (H&E, ×200).

All groups showed healed wounds at the fourteenth day postoperative and were covered by relatively thick epithelium with flattened epithelial ridges compared with the previous date. Yet, inflammatory cells infiltrate was only spotted in gp I C (irradiated group 14 days postoperative). These inflammatory cells were severely condensed in the superficial layer of the dermis as well as in the epithelial layers in the form of clear cells. Most of the epithelial cells were vacuolated and failed to perform the cornification process properly. Besides the inflammatory cells infiltrate, the dermis showed fatty degeneration (Fig. 10). Compared with the previous group, the epithelium of gp II C (BMMSCs group 14 days postoperative) showed keratin layer, less epithelial vacuolization and fewer clear cells. The collagen bundles of this group appeared thick, tightly packed but infiltrated by few inflammatory cells. Absence of skin appendages were also noticed (Fig. 11). Gp III C (HAECs group 14 days postoperative) revealed irregular epithelial surface, thick keratin layer and well organized skin appendages. The collagen bundles were irregularly arranged and separated by degenerated areas (Fig. 12).
Fig. 10. A photomicrograph of gp I C (irradiated group 14 days postoperative) shows healed epithelium (arrowheads), thin irregularly arranged collagen bundles (thick arrows), inflammatory cells (thin arrows) and fat cells (F) (H&E, ×200).

Fig. 11. A photomicrograph of gp II C (BMSCs group 14 days postoperative) shows healed epithelium (arrowheads), dense collagen bundles with properly arranged fibroblasts (asterisks) (H&E, ×200).

Fig. 12. A photomicrograph of gp III C (HAECs group 14 days postoperative) shows healed epithelium (arrowheads), thick irregularly arranged collagen bundles (thick arrows), degenerated areas (D), sebaceous glands and sweat glands related to the healed wound (S) (H&E, ×200).

Discussion
Combined cancer treatment using both irradiation and surgery has changed the clinical approach to the local management of several different solid tumors. This combined therapy has resulted in improved functional and/or cosmetic outcomes in breast carcinoma, soft tissue sarcoma, rectal carcinoma and head and neck cancers. This improvement raised from the facts that this combined therapy permits the preservation of functional muscle, nerve and vascular tissue with local tumor control rates equal to or better than radical surgery alone. The use of irradiation prior to surgery was preferred to decrease the late complications of combined management both by decreasing the total radiation dose and decreasing the volume of tissue treated with radiation thus decreasing the late effects of radiation on normal tissues, which may contribute to improved patient function (20, 21).
The major detriment to use radiation prior to surgery is the risk of wound healing complications. Surgery in a previously irradiated field has been reported to result in complications in up to one half of patients (22). Any measures likely to improve wound healing in previously irradiated tissue could potentially be applied to a wide range of cancer patients. Therefore, the ultimate goal of this study was to develop new techniques that can be used in the clinical setting to improve wound healing in previously irradiated skin. This was done by intra-dermal injection of either BM-MSCs or HAECs into the irradiated incision.
Most therapeutic applications of MSCs to wound/ischemic targets dictate those exogenous populations are delivered using either systemic or direct/topical approaches (23). Systemic delivery mimics the route of endogenous MSCs via the circulatory system with final homing to target sites. This may either delay their transit or reduce the number of cells that finally appear at the target sites (24). An alternate method for the delivery MSCs to wound/ischemic sites is through direct or topical delivery (25). This method is fundamentally different from systemic delivery in that applied MSCs either migrate into the wound bed via non-vascular routes or release bioactive factors from a bandage or other type of carrier at the surface of the wound. A limitation of direct/topical delivery is the accessibility of the target site. Skin is an example of a highly accessible target site in which large surface area wounds and chronic non-healing wounds are amenable to topical MSC therapy (25, 26). In this study, the wounds in irradiated rats were ischemic (27), thus intra-dermal injection of the used stem cells were the way of choice.
Wound healing is a complex process involving a number of interdependent stages including hemostasis, inflammation, proliferation, and remodeling (28). Different cell types, complex signaling events and numerous growth factors are involved in each stage of wound healing (29). Interaction of ionizing radiation with wounded tissue creates a situation where normal response to injury will be disrupted, leading to a protracted recovery period. Irradiation has been reported to produce multiple negative effects on wound healing processes: it inhibits inflammatory reactions, connective tissue proliferation, maturation of granulation tissue, secretion of collagen, and neovascularization (30). These above mentioned data were similar to the histological findings of this study except for the inhibition of inflammatory reactions. In the present research, over exuberant inflammatory events was detected in gp I starting from the first day of inspection up till the end of the study. This increased inflammatory reaction retarded wound healing and could be an early response to ionizing radiation that enhanced the expression of molecules associated with TGF-β1 signaling pathway (31). TGF-β1 has been shown to have a pro-inflammatory effect (32). However, inclusion of BM-MSCs and HAECs in gps II and III, that showed lower expression of TGF-β1 at the third day postoperative, reduced this inflammatory infiltration and hasten repair (33). This anti-inflammatory effect of this cell-based therapy was also documented by Shumakov et al. (34) and Lataillade et al. (35) in their researches of BM-MSCs applications on the surface of deep burn wounds in rats and on severe buttock radiation burn of human respectively.
Expression of TGF-β1 is known to affect nearly every aspect of wound repair not only the inflammatory stage (36). In the proliferative phase, in response to TGF-β1 secreted by the recruited inflammatory cells, fibroblasts situated inside the wound transform into myofibroblasts. These myofibroblasts are less capable of proliferation, but are responsible for wound contraction and healing (37). TGF-β1 has also a role in granulation tissue remodeling by regulating enzymes responsible for collagen bundle deposition. In irradiated tissues, fibroblasts have been shown to generate a disorganized deposition of collagen bundles due to deficient TGF-β1 expression (15). The results of this study revealed improper dermal tissue with low TGF-β1 expression in gp I at the seventh day postoperatively. Gps II and III at this time exhibited relatively higher TGF-β1 expression that regulate fibroblastic activity producing more organized collagen bundles. BM-MSCs were also found to differentiate into myofibroblast like cells (38). Cascade TGF-β1 expression was noticed in all groups which could be a normal respond during repair (39).
Furthermore, the wound healing process was affected by SDF-1 expression. SDF-1 acts in an autocrine manner to activate and stimulate migration of human mesenchymal stem cells (18) and showed the least expression in gp I. Intra-dermal injection of BMSCs and HAECs in this study led to increased SDF-1 expression in gp II (BM-MSCs group) followed by gp III (HAECs group). This high SDF-1 expression improved greatly the granulation tissue with the upper hand to BM-MSCs group. The improvement of the healing process was continued till the fourteenth day where dermal rebuilding and remodeling were obvious (40).
As mentioned before, expression of TGF-β1 as a response to ionizing radiation is known to affect nearly every aspect of wound repair, reepithelialization rate appeared to be also affected. Reepithelialization rate was the slowest in gp I which reflects the role of TGF-β1 as a growth inhibitor for most of the epidermal cells especially in full thickness wounds (41, 42). Confirmatory role of TGF-β1 on the epithelium proliferation, migration and differentiation was achieved from its low expression in gps II and III. Reepithelialization as well as skin appendages were well developed in gp III that showed the least TGF-β1 expression. In this research, gp II showed deficient in skin appendages regeneration which was opposite to previous research work (6, 43). Moreover, the rate of reepithelialization of the wounds in this study was affected by the increased CXCL-5 expression. Gps III and II showed the greatest expression to CXCL-5 in this experiment respectively which might clarify the earlier reepithelialization in these groups compared with gp I. This cytokine has been shown to be a potent stimulator of keratinocytes (18). In agreement with the results of this research, a study by Mishra et al. (44) there was a significant increased levels of CXCL5 and SDF-1 in the healing wounds treated with human mesenchymal stem cells as compared to normal control.
Neovascularization is a crucial step in the wound healing process. The formation of new blood vessels is necessary to sustain the newly formed granulation tissue and the survival of keratinocytes. In this study, the granulation tissue of gp I appeared ischemic (27). BM-MSCs -treated wounds and adjacent tissues (gp II) had enhanced capillary density, suggesting that BM-MSCs promote angiogenesis (5, 45). HAECs-treated wounds (gp III) showed also angiogenetic property but not as was recorded in gp II.
Because of the highly complex nature of irradiated wounds that result from destruction or loss of function of specific native cells, traditional medical therapies have been only moderately effective. The novel work reviewed here is highly promising, with the collective goal of identifying new therapeutic approaches to irradiated wound healing and can safely accelerate the transition of basic research findings into clinical advances in many areas of plastic and reconstructive surgery.
In conclusion, BM-MSCs and HAECs accelerated irradiated wound healing. BM-MSCs showed a greater effect on the quality of the connective tissue more than of HAECs. On the other hand, superiority of the epithelium and its appendages were achieved in HAECs group. Further studies thus are in need to investigate the possible effect of using both HAECs and BM-MSCs combined together on the healing process of the irradiated wound.
References
- 1.Gauglitz GG, Jeschke MG. Combined gene and stem cell therapy for cutaneous wound healing. Mol Pharm. 2011;8:1471–1479. doi: 10.1021/mp2001457. [DOI] [PubMed] [Google Scholar]
- 2.Nishino Y, Yamada Y, Ebisawa K, Nakamura S, Okabe K, Umemura E, Hara K, Ueda M. Stem cells from human exfoliated deciduous teeth (SHED) enhance wound healing and the possibility of novel cell therapy. Cytotherapy. 2011;13:598–605. doi: 10.3109/14653249.2010.542462. [DOI] [PubMed] [Google Scholar]
- 3.Badri L, Walker NM, Ohtsuka T, Wang Z, Delmar M, Flint A, Peters-Golden M, Toews GB, Pinsky DJ, Krebsbach PH, Lama VN. Epithelial interactions and local engraftment of lung-resident mesenchymal stem cells. Am J Respir Cell Mol Biol. 2011;45:809–816. doi: 10.1165/rcmb.2010-0446OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zanier ER, Montinaro M, Vigano M, Villa P, Fumagalli S, Pischiutta F, Longhi L, Leoni ML, Rebulla P, Stocchetti N, Lazzari L, De Simoni MG. Human umbilical cord blood mesenchymal stem cells protect mice brain after trauma. Crit Care Med. 2011;39:2501–2510. doi: 10.1097/CCM.0b013e31822629ba. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou Z, Zhang Y, Hao L, Wang F, Liu D, Su Y, Sun H. More insight into mesenchymal stem cells and their effects inside the body. Expert Opin Biol Ther. 2010;10:215–230. doi: 10.1517/14712590903456011. [DOI] [PubMed] [Google Scholar]
- 7.Griffin MD, Ritter T, Mahon BP. Immunological aspects of allogeneic mesenchymal stem cell therapies. Hum Gene Ther. 2010;21:1641–1655. doi: 10.1089/hum.2010.156. [DOI] [PubMed] [Google Scholar]
- 8.Si YL, Zhao YL, Hao HJ, Fu XB, Han WD. MSCs: biological characteristics, clinical applications and their outstanding concerns. Ageing Res Rev. 2011;10:93–103. doi: 10.1016/j.arr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Sandler TW. Langman’s medical embryology. William and Wilkins; Baltimore: 1995. pp. 71–80. [Google Scholar]
- 10.Kim BS, Chun SY, Lee JK, Lim HJ, Bae JS, Chung HY, Atala A, Soker S, Yoo JJ, Kwon TG. Human amniotic fluid stem cell injection therapy for urethral sphincter regeneration in an animal model. BMC Med. 2012;10:94. doi: 10.1186/1741-7015-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.del Regato JA. One hundred years of radiation oncology. In: Tobias JS, Thomas PRM, ed. Current radiation oncology. Vol. 2. Arnold and New York: Oxford University Press Inc.; London, Sydney, Auckland: 1996. [Google Scholar]
- 12.Ferris FD, Bezjak A, Rosenthal SG. The palliative uses of radiation therapy in surgical oncology patients. Surg Oncol Clin N Am. 2001;10:185–201. [PubMed] [Google Scholar]
- 13.Gu Q,, Wang D, Gao Y, Zhou J, Peng R, Cui Y, Xia G, Qing Q, Yang H, Liu J, Zhao M. Expression of MMP1 in surgical and radiation-impaired wound healing and its effects on the healing process. J Environ Pathol Toxicol Oncol. 2002;21:71–78. [PubMed] [Google Scholar]
- 14.Dormand EL, Banwell PE, Goodacre TE. Radiotherapy and wound healing. Int Wound J. 2005;2:112–127. doi: 10.1111/j.1742-4801.2005.00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson LB, Jorgensen LN, Adawi D, Blomqvist P, Asklöf GB, Gottrup F, Jeppsson B. The effect of preoperative radiotherapy on systemic collagen deposition and postoperative infective complications in rectal cancer patients. Dis Colon Rectum. 2005;48:1573–1580. doi: 10.1007/s10350-005-0066-0. [DOI] [PubMed] [Google Scholar]
- 16.Alhadlaq A, Mao JJ. Mesenchymal stem cells: isolation and therapeutics. Stem Cells Dev. 2004;13:436–448. doi: 10.1089/scd.2004.13.436. [DOI] [PubMed] [Google Scholar]
- 17.Rochefort GY, Vaudin P, Bonnet N, Pages JC, Domenech J, Charbord P, Eder V. Influence of hypoxia on the domiciliation of mesenchymal stem cells after infusion into rats: possibilities of targeting pulmonary artery remodeling via cells therapies? Respir Res. 2005;6:125. doi: 10.1186/1465-9921-6-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishra PJ, Mishra PJ, Banerjee D. Cell-free derivatives from mesenchymal stem cells are effective in wound therapy. World J Stem Cells. 2012;4:35–43. doi: 10.4252/wjsc.v4.i5.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lafontaine L, Chaudhry P, Lafleur MJ, Van Themsche C, Soares MJ, Asselin E. Transforming growth factor Beta regulates proliferation and invasion of rat placental cell lines. Biol Reprod. 2011;84:553–559. doi: 10.1095/biolreprod.110.086348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spiro IJ, Rosenberg AE, Springfield D, Suit H. Combined surgery and radiation therapy for limb preservation in soft tissue sarcoma of the extremity: the Massachusetts General Hospital experience. Cancer Invest. 1995;13:86–95. doi: 10.3109/07357909509024899. [DOI] [PubMed] [Google Scholar]
- 21.Ngan SY. Radiotherapy in soft tissue sarcoma of the extremities. Acta Orthop Scand Suppl. 1997;273:112–116. doi: 10.1080/17453674.1997.11744714. [DOI] [PubMed] [Google Scholar]
- 22.Cheng EY, Dusenbery KE, Winters MR, Thompson RC. Soft tissue sarcomas: preoperative versus postoperative radiotherapy. J Surg Oncol. 1996;61:90–99. doi: 10.1002/(SICI)1096-9098(199602)61:2<90::AID-JSO2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 23.Hamou C, Callaghan MJ, Thangarajah H, Chang E, Chang EI, Grogan RH, Paterno J, Vial IN, Jazayeri L, Gurtner GC. Mesenchymal stem cells can participate in ischemic neovascularization. Plast Reconstr Surg. 2009;123(2 Suppl):45S–55S. doi: 10.1097/PRS.0b013e318191be4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, Kouttab N, Shrayer D, Carson P. Autologous bone marrow- derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13:1299–1312. doi: 10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]
- 26.Hanson SE, Bentz ML, Hematti P. Mesenchymal stem cell therapy for nonhealing cutaneous wounds. Plast Reconstr Surg. 2010;125:510–516. doi: 10.1097/PRS.0b013e3181c722bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stone HB, Coleman CN, Anscher MS, McBride WH. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol. 2003;4:529–536. doi: 10.1016/s1470-2045(03)01191-4. [DOI] [PubMed] [Google Scholar]
- 28.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 29.Aarabi S, Bhatt KA, Shi Y, Paterno J, Chang EI, Loh SA, Holmes JW, Longaker MT, Yee H, Gurtner GC. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 2007;21:3250–3261. doi: 10.1096/fj.07-8218com. [DOI] [PubMed] [Google Scholar]
- 30.Gu Q, Wang D, Cui C, Gao Y, Xia G, Cui X. Effects of radiation on wound healing. J Environ Pathol Toxicol Oncol. 1998;17:117–123. [PubMed] [Google Scholar]
- 31.Mueller CK, Thorwarth M, Schultze-Mosgau S. Late changes in cutaneous gene expression patterns after adjuvant treatment of oral squamous cell carcinoma (OSCC) by radiation therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:694–699. doi: 10.1016/j.tripleo.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 32.Wang XJ, Han G, Owens P, Siddiqui Y, Li AG. Role of TGF beta-mediated inflammation in cutaneous wound healing. J Investig Dermatol Symp Proc. 2006;11:112–117. doi: 10.1038/sj.jidsymp.5650004. [DOI] [PubMed] [Google Scholar]
- 33.Choi TH, Tseng SC. In vivo and in vitro demonstration of epithelial cell-induced myofibroblast differentiation of keratocytes and an inhibitory effect by amniotic membrane. Cornea. 2001;20:197–204. doi: 10.1097/00003226-200103000-00019. [DOI] [PubMed] [Google Scholar]
- 34.Shumakov VI, Onishchenko NA, Rasulov MF, Krasheninnikov ME, Zaidenov VA. Mesenchymal bone marrow stem cells more effectively stimulate regeneration of deep burn wounds than embryonic fibroblasts. Bull Exp Biol Med. 2003;136:192–195. doi: 10.1023/a:1026387411627. [DOI] [PubMed] [Google Scholar]
- 35.Lataillade JJ, Doucet C, Bey E, Carsin H, Huet C, Clairand I, Bottollier-Depois JF, Chapel A, Ernou I, Gourven M, Boutin L, Hayden A, Carcamo C, Buglova E, Joussemet M, de Revel T, Gourmelon P. New approach to radiation burn treatment by dosimetry-guided surgery combined with autologous mesenchymal stem cell therapy. Regen Med. 2007;2:785–794. doi: 10.2217/17460751.2.5.785. [DOI] [PubMed] [Google Scholar]
- 36.Klass BR, Grobbelaar AO, Rolfe KJ. Transforming growth factor beta1 signalling, wound healing and repair: a multifunctional cytokine with clinical implications for wound repair, a delicate balance. Postgrad Med J. 2009;85:9–14. doi: 10.1136/pgmj.2008.069831. [DOI] [PubMed] [Google Scholar]
- 37.Helary C, Ovtracht L, Coulomb B, Godeau G, Giraud-Guille MM. Dense fibrillar collagen matrices: a model to study myofibroblast behaviour during wound healing. Biomaterials. 2006;27:4443–4452. doi: 10.1016/j.biomaterials.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Mishra PJ, Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, Ganesan S, Glod JW, Banerjee D. Carcinomaassociated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008;68:4331–4339. doi: 10.1158/0008-5472.CAN-08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Igarashi A, Okochi H, Bradham DM, Grotendorst GR. Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Mol Biol Cell. 1993;4:637–645. doi: 10.1091/mbc.4.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshikawa T, Mitsuno H, Nonaka I, Sen Y, Kawanishi K, Inada Y, Takakura Y, Okuchi K, Nonomura A. Wound therapy by marrow mesenchymal cell transplantation. Plast Reconstr Surg. 2008;121:860–877. doi: 10.1097/01.prs.0000299922.96006.24. [DOI] [PubMed] [Google Scholar]
- 41.Tredget EB, Demare J, Chandran G, Tredget EE, Yang L, Ghahary A. Transforming growth factor-beta and its effect on reepithelialization of partial-thickness ear wounds in transgenic mice. Wound Repair Regen. 2005;13:61–67. doi: 10.1111/j.1067-1927.2005.130108.x. [DOI] [PubMed] [Google Scholar]
- 42.Oshimori N, Fuchs E. Paracrine TGF-β signaling counterbalances BMP-mediated repression in hair follicle stem cell activation. Cell Stem Cell. 2012;10:63–75. doi: 10.1016/j.stem.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu Y, Zhao RC, Tredget EE. Concise review: bone marrow- derived stem/progenitor cells in cutaneous repair and regeneration. Stem Cells. 2010;28:905–915. doi: 10.1002/stem.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mishra PJ, Mishra PJ, Banerjee D. Cell-free derivatives from mesenchymal stem cells are effective in wound therapy. World J Stem Cells. 2012;4:35–43. doi: 10.4252/wjsc.v4.i5.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]



