Type 2 diabetes and the metabolic syndrome greatly increase the risk of cardiovascular disease, manifested as myocardial infarction and stroke. While it is well known that this risk is largely due to increased atherosclerosis, the cellular and molecular events within the artery wall responsible for the worsening of atherosclerosis associated with type 2 diabetes and the metabolic syndrome are less clear. These states are frequently associated with several cardiovascular risk factors, including dyslipidemia, hypertension, obesity, hyperglycemia and systemic insulin resistance, each of which may contribute to atherosclerosis.
Insulin resistance in now known to affect the vascular wall itself, in addition to the better studied insulin target tissues liver, skeletal muscle, and adipose tissue. Vascular endothelial cells, which play critical roles in atherosclerosis by allowing monocytes and other immune cells to enter the atherosclerotic lesion and by producing pro- and anti-atherogenic molecules, develop insulin resistance in both humans and mice concomitant with dyslipidemia and systemic insulin resistance.1 Endothelial cells can also contribute to systemic insulin resistance.2
Insulin has important biological effects in endothelial cells that impact atherosclerosis. Activation of the insulin receptor results in tyrosine phosphorylation of insulin receptor substrate (IRS) 1 and 2, and subsequent activation of phosphoinositide 3-kinase (PI3K) and the serine/threonine protein kinase Akt. Akt has several targets in endothelial cells, one of which is endothelial nitric oxide synthase (eNOS). Insulin-induced activation of the PI3K-Akt-eNOS pathway causes increased production of nitric oxide (NO), vasorelaxation,3 and suppressed expression of VCAM-1, an important adhesion molecule utilized by monocytes to invade the vessel wall. These actions of insulin are likely to be anti-atherosclerotic (Figure 1), because both VCAM-1-deficient mice and eNOS-deficient mice exhibit reduced atherosclerosis4-5 (although eNOS can exert pro-atherogenic effects if “uncoupled” to produce superoxide).
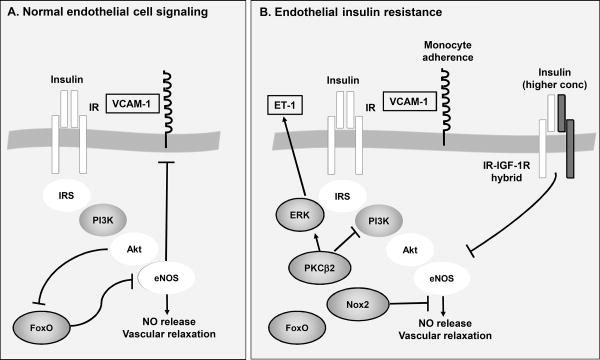
Figure 1. Endothelial insulin resistance and promotion of atherosclerosis.
A. Under normal conditions, insulin binds to the insulin receptor (IR) on endothelial cells and this initiates phosphorylation of insulin receptor substrate 1 and 2 (IRS) and downstream activation of PI3K, Akt and eNOS. Activation of eNOS causes increased production of NO, vasodilatation, and is believed to inhibit atherosclerosis. This pathway also results in suppression of VCAM-1 expression, a monocyte adhesion receptor known to promote atherosclerosis, and inhibition of FoxO nuclear translocation and activation of pro-atherogenic target genes. B. Endothelial insulin resistance is characterized by a reduced ability of insulin to activate the anti-atherosclerotic PI3K-Akt-eNOS pathway, mediated by increased expression of PKCβ2, Nox2 and perhaps an increased activation of insulin-IGF-1 hybrid receptors. Under these conditions, insulin signaling may be shifted to a more pro-atherogenic pathway mediated by ERK and downstream events, such as increased production of endothelin-1 (ET-1). Molecules framed by black are pro-atherogenic and molecules highlighted in white have been shown to exert anti-atherogenic effects in mouse models.
Furthermore, the effect of complete loss of insulin signaling in the endothelium was revealed by the development of endothelium-specific insulin receptor-deficient mice. These mice demonstrate impaired vasorelaxation, increased endothelial-leukocyte adhesion and accelerated atherosclerosis,6 consistent with the notion that the overall effect of insulin in the endothelium is anti-atherogenic, at least in mice. However, human studies and studies primarily on bovine endothelial cells have demonstrated that insulin also stimulates production of endothelin 1 (ET-1),7-8 which mediates pro-atherosclerotic effects.9 Insulin-induced ET-1 production is mediated by the serine/threonine kinase ERK independently of the PI3K-Akt-eNOS pathway.
How does the endothelium become insulin resistant? Previous studies have implicated the protein kinase C β2 isoform (PKCβ2),10 the transcription factor FoxO (another Akt target),11 and the NADPH oxidase Nox2 isoform12 as mediators of endothelial insulin resistance. Other studies suggest that formation of hybrid receptors consisting of one insulin hemireceptor and one IGF-1 hemireceptor reduce endothelial insulin sensitivity.13 All of these molecules inhibit the PI3K-Akt-eNOS pathway.
In this issue of Circulation Research, Li and colleagues nicely illustrate the role of endothelium-specific overexpression of PKCβ2 in inducing endothelial insulin resistance and promoting atherosclerosis.14 This group has previously shown that PKCβ2 is activated in aortas of insulin resistant rats.10 Using fat-fed Apoe-/- mice that overexpress PKCβ2 under control of the endothelial cell-specific VE-cadherin promoter they now demonstrate that PKCβ2 mediates insulin resistance in part by stimulating threonine phosphorylation of the PI3K p85α subunit, which in turn blunts insulin-stimulated Akt-eNOS activation (Figure 1). Another recent study demonstrated that PKCβ2 increases serine phosphorylation of IRS2, suggesting that PKCβ2 has multiple targets in the endothelial insulin signaling pathway.15 The authors also show that PKCβ2-overexpression results in increased endothelial-leukocyte adhesion via increased expression of VCAM-1, as well as loss of insulin-mediated inhibition of VCAM-1 expression.14 Accordingly, endothelial overexpression of PKCβ2 increased atherosclerosis, predominantly in the abdominal aorta, without affecting plasma lipids or blood pressure.14 The abdominal lesions in PKCβ2 overexpressing mice were larger and more advanced at the 12-week time-point studied, indicating that a more rapid initiation and/or progression of lesions might have been responsible for the phenotype. The finding that lesions in the aortic root and arch, which often develop earlier than those of the abdominal aorta, were no different in size after 12 weeks of fat-feeding might indicate that PKCβ2 overexpression primarily promotes lesion initiation.
The studies by Li and colleagues show that endothelial insulin resistance induced by PKCβ2 is likely to promote atherosclerosis,14 similar to findings on mice with endothelial-targeted deletion of FoxO.11 However, like FoxO,11 PKCβ2 clearly has pro-atherosclerotic effects in endothelial cells beyond inhibition of insulin signaling, in part by regulating basal levels of eNOS and ERK.14 The pro-atherosclerotic effects of endothelial PKCβ2 are consistent with recent studies on whole-body PKCβ2-deficient mice, which exhibit reduced atherosclerosis.16-17 It is not known to what extent endothelial deletion of PKCβ2 contributes to atherosclerosis in these whole-body knockout mice, but it is likely that non-insulin-dependent mechanisms are at play both in endothelial cells and myeloid cells.
It has been shown that hepatic insulin resistance does not affect all downstream arms of insulin signaling equally. Thus, whereas insulin-mediated suppression of gluconeogenesis is susceptible to insulin resistance, insulin-stimulated lipogenesis is not. This phenomenon is termed selective insulin resistance, and diverges downstream of Akt in hepatocytes.18 In the arterial wall, and in endothelial cells in particular, a similar phenomenon has been proposed in which the PI3K arm of insulin signaling becomes insulin resistant while the ERK arm is spared.19 Furthermore, this divergence in signaling has been hypothesized to explain insulin's dual effect on ET-1 and NO release; NO release being downstream of PI3K-Akt-eNOS, while ET-1 release is downstream of ERK8 (Figure 1). Selective endothelial insulin resistance has been proposed to contribute to endothelial dysfunction and augmented atherosclerosis in states of insulin resistance. In the study by Li et al.,14 insulin does not activate ERK unless PKCβ2 is overexpressed. It is therefore possible that ERK is preferentially active in insulin resistant endothelial cells. Furthermore, the ERK pathway is basally activated in states of endothelial insulin resistance, which could contribute to atherosclerosis.11,14 Thus, in insulin resistant states there might be a shift from the anti-atherosclerotic PI3K-Akt-eNOS pathway to a pro-atherosclerotic ERK pathway, which governs the biological effects not only of insulin, but of other molecules that activate these signaling pathways. The role of endothelial ERK in atherosclerosis needs further investigation.
In aggregate, recent research shows that several molecules act to inhibit the insulin PI3K-Akt-eNOS pathway in insulin resistant endothelial cells, and that endothelial insulin resistance is likely to promote atherosclerosis. The studies by Li et al.14 convincingly add endothelial PKCβ2 as a mediator of endothelial insulin resistance and atherosclerosis.
Acknowledgments
Sources of Funding: The authors are supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) under award numbers R01HL062887, P01HL092969, and R01HL097365 (KEB). JEK is supported in part by The Dick and Julia McAbee Endowed Fellowship in Diabetes Research Fellowship from the Diabetes Research Center (P30DK017047). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
This is a commentary on article Li Q, Park K, Li C, Rask-Madsen C, Mima A, Qi W, Mizutani K, Huang P, King GL. Induction of vascular insulin resistance and endothelin-1 expression and acceleration of atherosclerosis by the overexpression of protein kinase C-β isoform in the endothelium. Circ Res. 2013;113(4):418-27.
Footnotes
Disclosures: None
References
- 1.Mather KJ, Steinberg HO, Baron AD. Insulin resistance in the vasculature. J Clin Invest. 2013;123:1003–1004. doi: 10.1172/JCI67166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kubota T, Kubota N, Kadowaki T. The role of endothelial insulin signaling in the regulation of glucose metabolism. Rev Endocr Metab Disord. 2013;14:207–216. doi: 10.1007/s11154-013-9242-z. [DOI] [PubMed] [Google Scholar]
- 3.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev. 2007;28:463–491. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- 4.Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, Davis V, Gutierrez-Ramos JC, Connelly PW, Milstone DS. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. 2001;107:1255–1262. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ponnuswamy P, Schröttle A, Ostermeier E, Grüner S, Huang PL, Ertl G, Hoffmann U, Nieswandt B, Kuhlencordt PJ. eNOS protects from atherosclerosis despite relevant superoxide production by the enzyme in apoE mice. PLoS One. 2012;7:e30193. doi: 10.1371/journal.pone.0030193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rask-Madsen C, Li Q, Freund B, et al. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell Metab. 2010;11:379–89. doi: 10.1016/j.cmet.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardillo C, Nambi SS, Kilcoyne CM, Choucair WK, Katz A, Quon MJ, Panza JA. Insulin stimulates both endothelin and nitric oxide activity in the human forearm. Circulation. 1999;100:820–825. doi: 10.1161/01.cir.100.8.820. [DOI] [PubMed] [Google Scholar]
- 8.Formoso G, Chen H, Kim JA, Montagnani M, Consoli A, Quon MJ. Dehydroepiandrosterone mimics acute actions of insulin to stimulate production of both nitric oxide and endothelin 1 via distinct phosphatidylinositol 3-kinase- and mitogen-activated protein kinase-dependent pathways in vascular endothelium. Mol Endocrinol. 2006;20:1153–1163. doi: 10.1210/me.2005-0266. [DOI] [PubMed] [Google Scholar]
- 9.Simeone SM, Li MW, Paradis P, Schiffrin EL. Vascular gene expression in mice overexpressing human endothelin-1 targeted to the endothelium. Physiol Genomics. 2011;43:148–160. doi: 10.1152/physiolgenomics.00218.2009. [DOI] [PubMed] [Google Scholar]
- 10.Naruse K, Rask-Madsen C, Takahara N, Ha SW, Suzuma K, Way KJ, Jacobs JR, Clermont AC, Ueki K, Ohshiro Y, Zhang J, Goldfine AB, King GL. Activation of vascular protein kinase C-β inhibits Akt-dependent endothelial nitric oxide synthase function in obesity-associated insulin resistance. Diabetes. 2006;55:691–698. doi: 10.2337/diabetes.55.03.06.db05-0771. [DOI] [PubMed] [Google Scholar]
- 11.Tsuchiya K, Tanaka J, Shuiqing Y, Welch CL, DePinho RA, Tabas I, Tall AR, Goldberg IJ, Accili D. FoxOs integrate pleiotropic actions of insulin in vascular endothelium to protect mice from atherosclerosis. Cell Metab. 2012;15:372–381. doi: 10.1016/j.cmet.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sukumar P, Viswambharan H, Imrie H, et al. Nox2 NADPH oxidase has a critical role in insulin resistance-related endothelial cell dysfunction. Diabetes. 2013;62:2130–2134. doi: 10.2337/db12-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbas A, Imrie H, Viswambharan H, et al. The insulin-like growth factor-1 receptor is a negative regulator of nitric oxide bioavailability and insulin sensitivity in the endothelium. Diabetes. 2011;60:2169–2178. doi: 10.2337/db11-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Q Li, Park K, Li C, Rask-Madsen C, Mima A, Qi W, Mizutani K, Huang PL, King GL. Induction of vascular insulin resistance, endothelin-1 expression, and acceleration of atherosclerosis by the overexpression of protein kinase C β isoform in the endothelium. Circ Res. 2013 Jun 11; doi: 10.1161/CIRCRESAHA.113.301074. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park K, Li Q, Rask-Madsen C, Mima A, Mizutani K, Winnay J, Maeda Y, D'Aquino K, White MF, Feener EP, King GL. Serine phosphorylation sites on IRS2 by angiotensin II and PKC activated to induce selective insulin resistance in endothelial cells. Mol Cell Biol. 2013 Jun 17; doi: 10.1128/MCB.00506-13. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harja E, Chang JS, Lu Y, Leitges M, Zou YS, Schmidt AM, Yan SF. Mice deficient in PKCβ and apolipoprotein E display decreased atherosclerosis. FASEB J. 2009;23:1081–1091. doi: 10.1096/fj.08-120345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kong L, Shen X, Lin L, Leitges M, Rosario R, Zou YS, Yan SF. PKCβ promotes vascular inflammation and acceleration of atherosclerosis in diabetic ApoE null mice. Arterioscler Thromb Vasc Biol. 2013 Jun 13; doi: 10.1161/ATVBAHA.112.301113. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Jiang ZY, Lin YW, Clemont A, Feener EP, Hein KD, Igarashi M, Yamauchi T, White MF, King GL. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest. 1999;104:447–457. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]