Abstract
To better understand the neurobiology of methamphetamine (METH) dependence and the cognitive impairments induced by METH use, we compared the effects of extended (12 h) and limited (1 h) access to METH self-administration on locomotor activity and object place recognition, and on extracellular dopamine levels in the nucleus accumbens and caudate-putamen. Rats were trained to self-administer intravenous METH (0.05 mg/kg). One group had progressively extended access up to 12-h sessions. The other group had limited-access 1-h sessions. Microdialysis experiments were conducted during a 12-h and 1-h session, in which the effects of a single METH injection (self-administered, 0.05 mg/kg, i.v.) on extracellular dopamine levels were assessed in the nucleus accumbens and caudate-putamen compared with a drug-naive group. The day after the last 12-h session and the following day experimental groups were assessed for their locomotor activities and in a place recognition procedure, respectively. The microdialysis results revealed tolerance to the METH-induced increases in extracellular dopamine only in the nucleus accumbens, but not in the caudate-putamen in the extended-access group compared with the control and limited-access groups. These effects may be associated with the increased lever-pressing and drug-seeking observed during the first hour of drug exposure in the extended-access group. This increase in drug-seeking leads to higher METH intake and may result in more severe consequences in other structures responsible for the behavioral deficits (memory and locomotor activity) observed in the extended-access group, but not in the limited-access group.
Keywords: addiction, animal model, behavioral neurosciences, cognition, tolerance
Introduction
According to the 2011 report from the United Nations on drugs and crime, methamphetamine (METH) was ranked as the second most commonly abused drug in the world, surpassing both heroin and cocaine (United Nations Office on Drugs and Crime, 2011). Prolonged and intensive use of this highly addictive drug can induce persistent cognitive and social impairments, psychosis, and schizophrenia-like symptoms (Homer et al., 2008; Duarte et al., 2012; Gururajan et al., 2012). However, evidence suggests that these effects depend on the dose and duration of use, and may not occur after recreational use of the drug (Simon et al., 2000; Hart et al., 2012). Because the experimental examination of high-dose METH exposure in human subjects is limited by ethical considerations (Hart et al., 2012), further investigations of the effects of METH in sensitive preclinical models is important to accurately study the consequences of intensive METH abuse.
In animals, contingent and non-contingent METH administration have been used to investigate the neural mechanisms that mediate the behavioral effects of METH (Kuczenski et al., 2009; Hadamitzky et al., 2011; Reichel et al., 2011, 2012). Non-contingent METH administration models can be useful to mimic human pharmacokinetics, the pharmacodynamics of the drug and some long-term consequences of METH abuse (O'Neil et al., 2006; Kuczenski et al., 2007), but such investigations do not bridge the gap between the neurochemical impairments seen in humans and behavioral abnormalities, such as compulsive drug-seeking, induced by the drug. The motivational and reinforcing aspects of drug intake can be studied using contingent administration. The intravenous self-administration model showed that rats with extended access to the drug for up to 6 h increased their drug intake (Kitamura et al., 2006; Hadamitzky et al., 2011), and had persistent cognitive impairments in novel object and novel object-in-place recognition tasks (Reichel et al., 2012) compared with subjects with limited-access (1 h). Furthermore, extended-access METH self-administration accurately mimics the pharmacokinetic pattern of METH abuse observed in humans (Hadamitzky et al., 2011). Therefore, extended-access METH self-administration is a useful experimental approach to the study of the neurobiological and behavioral consequences of METH abuse.
The present study had several objectives: (i) to provide a potential link between the behavioral consequences of METH seeking and taking, and the neurobiological modifications that occur in the nucleus accumbens and caudate-putamen; (ii) to compare how extended-access vs limited-access to METH differentially affects these neurochemical effects of METH exposure, and how both limited- and extended-access subjects respond to a METH challenge compared with a drug-naive control group; and (iii) to identify potential differential behavioral dysfunction (i.e. locomotor activity and place recognition) that may occur in extended- and limited-access rats.
Materials and methods
Subjects
Adult male Sprague–Dawley rats (350–450 g) were purchased from Harlan (Gilroy, CA, USA). The animals were housed two per cage with ad libitum access to food and tap water. The animals stayed in the experimental room during the entire experiment in either their home cages or experimental chambers. The experimental room temperature (20–22 °C) and humidity (55 ± 5%) were controlled. To perform the experiments during the active phase of the animals’ circadian cycle, the room was maintained on a reverse 12 h/12 h light/dark cycle (lights on 08:00–20:00 h). The testing chambers and experimental room were equipped with white lights (20:00–08:00 h) and red lights (08:00–20:00 h). The facilities and experimental procedures were approved by the Institutional Animal Care and Use Committee, and were in accordance with the National Institutes of Health and Association for the Assessment and Accreditation of Laboratory Animal Care guidelines.
Surgery
After 1 week of acclimation, the animals were anesthetized using an isoflurane/oxygen mixture (1–3% isoflurane), and catheters were implanted into the right jugular vein as previously described (Hadamitzky et al., 2011). Two microdialysis guide cannulae (directed at the dorsal caudate-putamen and nucleus accumbens) were implanted on the top of the animal's skull. The dialysis probe was then inserted to allow approximately 18 h of equilibration prior to the experimental manipulation. The tips of the dialysis probes were directed as follows: for the caudate-putamen, at a site 0.4 mm posterior to bregma, 3.0 mm lateral to the midline and 8.0 mm from the skull surface. For the nucleus accumbens, at a site 1.5 mm anterior to bregma, 0.8 mm lateral to the midline and 9.0 mm from the skull surface. For a diagram of similar probe placements, see Kuczenski et al. (1991).
The animals were divided into three groups: one control group that received no METH; one extended-access METH group; and one limited-access METH group (see below for description of limited and extended METH access).
METH self-administration
The animals had 7 days to recover from surgery before training began in 1-h sessions of intravenous METH self-administration. The Plexiglas chambers used for the experiment were custom-made (30 × 30 × 38 cm) and placed within ventilated, sound-attenuating boxes. One wall of the chambers (with the exception of the control group's chambers) contained two retractable levers: one inactive lever and one active lever. Pressing the active lever resulted in an infusion of METH at a dose of 0.05 mg/kg in a volume of 0.05 mL over a period of 1.5 s (fixed-ratio 1) paired with a 20-s cue light located between the two levers that signaled a timeout period. At the end of the session, the levers were automatically retracted, and the data for each test session were stored on computers using MED-PC IV software (Med Associates, St Albans, VT, USA). The rat's performance was considered stable when the rat pressed the active lever at least 10 times over each of three consecutive days.
After reaching the criterion for stable intravenous METH self-administration, the extended-access METH group began with 5 days of 1 h access, followed by five sessions of 3 h, five sessions of 6 h and 20 sessions of 12 h. Each set of five sessions was followed by 2 days of no exposure to the drug. Each 12-h session was separated by 1 day of no drug exposure. The limited-access METH group had access to 1 h METH self-administration on all days on which the extended-access group had access to METH self-administration.
During the experiment, the animals either stayed in the testing chamber and had access to METH, or were housed two per cage in home cages located in the experimental room without access to METH. In addition, during the first 3 h in the chambers, the animals had access to water but not food; barriers that blocked access to food were removed after that time. A control group had the same duration of exposure to the experimental chambers as the extended-access METH group, but these subjects were not connected to the METH delivery system and did not self-administer saline. Thus, these animals were drug-free throughout the experiment. The animals’ body weights were monitored daily.
Body weight
Although METH intake in the extended-access group did induce significant body weight loss immediately after each of the longer sessions, most if not all of the lost weight was regained during the subsequent drug-free interval (data not shown). As a consequence, over the course of the experiment, changes in body weight in this group reflected a failure of these animals to gain weight, an effect that is consistent with that seen in our previous studies. For example, weights across groups were similar throughout the initial phases of the drug treatment, but there was a weight gain difference between groups from the beginning of the 12-h sessions to the last 12-h sessions of 24.3 ± 5.2 g, 17.8 ± 4.8 g and −8.3 ± 9.2 g for control groups, limited-access groups and extended-access groups, respectively.
At the time of testing, these animals did not exhibit symptoms of impaired health, such as flaccidity or evidence of dehydration. Furthermore, extended-access animals were tested at 12 h for locomotor activity and at 36 h for place recognition after the end of METH self-administration, by which time most of the acute loss in body weight had recovered. The average body weights at the different time points, after the self-administration session, before locomotor activity and before place recognition for the control group were: 438 ± 5 g, 437 ± 5 g and 439 ± 5 g, respectively; for the limited-access group: 441 ± 5 g, 444 ± 6 g and 445 ± 6 g, respectively; and for the extended-access group: 397 ± 6 g, 401 ± 6 g and 408 ± 6 g, respectively. Statistical analysis two-way anova between three treatments and within 3 days revealed a main effect of ‘Treatment’ (F2,44 = 14.62, P < 0.05) and ‘Day’ (F2,88 = 25.38, P < 0.05), and a significant ‘Treatment × Time’ interaction (F4,88 = 10.01, P < 0.05). Further post hoc analyses using Bonferroni corrections showed that there was a significant difference in weight between the extended-access group and the other groups on all days (P < 0.05); however, the extended-access group's weight after the self-administration session was significantly different from the weights of the same group on all other days (P < 0.05). Thus, it does not appear that the behavioral deficits observed in these tasks were due to compromised health.
Microdialysis experiment
In our typical microdialysis studies, animals are connected to dialysis tubing in the afternoon on the day prior to sample collection to allow for equilibration of the probe within brain tissue. However, preliminary studies revealed that maintaining intravenously self-administering animals within the experimental chamber overnight disrupts lever-pressing when the levers are introduced into the chamber the following morning, suggesting that continuous exposure to the contextual cues of the experimental chambers without access to METH affects subsequent drug-seeking and drug-taking behavior once the levers are once again extended and METH access is provided. To avoid this potential complication, we constructed opaque Plexiglas chambers that fit inside the experimental chamber, and thus changed the contextual cues and obscured the retracted lever mechanisms. At the end of each intravenous self-administration session during the week prior to the microdialysis study, the experimental animals were placed into the opaque chamber within the experimental chamber where they remained until the beginning of the next intravenous self-administration session.
On the day prior to the experimental day (15:00–16:00 h), each rat was lightly anesthetized with isoflurane and placed in the opaque inner chamber. The dialysis probe was then inserted to allow approximately 18 h of equilibration prior to the experimental manipulation. Concentric microdialysis probes were constructed of Spectra/Por hollow fiber (MW cut-off 6000, 250 μm outer diameter) according to the method of Robinson & Whishaw (1988), with modifications (Kuczenski & Segal, 1989). The length of the active probe membrane was 3 mm for caudate-putamen probes and 2 mm for nucleus accumbens probes. The probes were perfused with artificial cerebrospinal fluid (in mm: NaCl, 147; CaCl2, 1.2; MgCl2, 0.9; KCl, 4.0) delivered by a microinfusion pump (1.5 μL/min) via 50 cm of Micro-line ethyl vinyl acetate tubing connected to a fluid swivel. Dialysate was collected through glass capillary tubing into vials that contained 20 μL of 25% methanol and 0.2 m sodium citrate, pH 3.8. Under these conditions, dialysate dopamine and metabolites were stable throughout the collection and analysis interval. The samples were collected outside the experimental chamber to avoid disturbing the animal. At the end of the experiment, each animal was perfused with formalin for histological verification of probe placements.
On the experimental day, three samples were collected at 20-min intervals to establish baseline dialysate levels. During the last baseline sample collection, the animals were removed from the inner opaque box and introduced to the self-administration chamber. For the sample test, the animals were allowed to press the lever only once before the lever was retracted, giving both the experimental animals and their yoked control animals only one dose of METH. Twenty minutes after the initial lever press, the lever was re-introduced into the chamber and remained active for the next 12 h. To assess dopamine levels in both the nucleus accumbens and caudate-putamen, the animals were randomized into two groups: each group was dialysed on the first day in one brain region; and on the second day in the second brain region.
Samples were assayed for dopamine, 3,4-dihydroxyphenylacetic acid, homovanillic acid and serotonin. High-performance liquid chromatography with electrochemical detection consisted of a 100 × 4.6 mm ODS-C18 3 μm column (Regis) maintained at 40 °C. The mobile phase (0.05 m citric acid, 7% methanol, 0.1 mm Na2EDTA and 0.2 mm octane sulfonate, adjusted to pH 4.0–4.5) was delivered at 0.6–0.8 mL/min using a Waters model 510 pump. Amines were detected using a Waters 460 detector with a glassy carbon electrode maintained at +0.65 V relative to an Ag/AgCl reference electrode. Concentrations were estimated from peak heights using a Waters Maxima 820 data station.
Place recognition task
The place recognition task was performed 2 days after the last METH self-administration session in the extended- and limited-access groups. All testing was performed in two adjacent identical plastic chambers (55 × 40 × 30 cm). A clear Perspex lid was placed on top of both chambers to prevent the rats from escaping, but allowed air circulation. A camera was fixed above the two boxes to record all behaviors. Both chambers had crosses marked on the bottom of the chambers at the four corner locations where objects were to be presented. The room was lit by a single, centrally placed overhead red light. The objects were chosen based on the criteria of being easily cleaned and not easily gnawed by the rats. The objects were sufficiently heavy so the rats could not push them over. During the task, six copies of each object were used to minimize the presence of odor cues.
Locomotor activity and place recognition task
On the day prior to place recognition testing, each group was habituated to the behavioral chamber for 10 min. During this time, locomotor activity and rearing were recorded throughout the session. After habituation, animals from each group were returned to their home cage and tested for place recognition the next day in the same open field apparatus.
Trials consisted of one ‘Sample 1 phase’ (acquisition), one ‘Sample 2/Test 1 phase’ (retrieval and new acquisition) and one ‘Test 2 phase’ (retrieval; Fig. 1). The phases were separated by a 90-min delay. In all of the phases, the rat began from the same location in the arena. Identical copies of the same object were used in each phase of the trial to prevent the presence of odor cues.
Fig. 1.
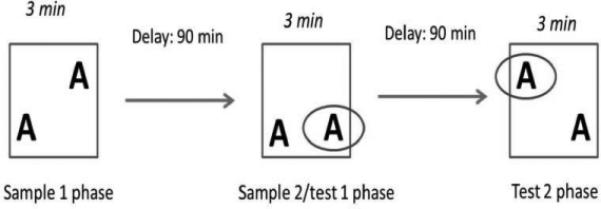
Schematic representation of the experimental design of the place recognition task. Rats explored an open field with two similar objects and were tested twice after a 90-min interval for their ability to remember the previous object location.
Sample 1 phase
The Sample 1 phase was conducted simultaneously in both chambers, with the two objects placed in the lower left corner and upper right corner, respectively (Fig. 1). The rats were allowed to freely explore for 3 min. The rats were then placed in their holding cage for 90 min while the arenas were cleaned.
Sample 2/Test 1 phase
The objects were rearranged, with one in the lower left corner and one in the lower right corner. The rats were allowed to freely explore for 3 min, after which time they were placed in their holding cage for another delay period of 90 min before the next test phase (Fig. 1). In this configuration, both objects were familiar, but the object in the lower right corner had never been encountered in this specific location.
Test 2 phase
The objects were rearranged, with one object in the upper left corner and one object in the lower right corner. The rats were allowed to freely explore for 3 min. Each object was familiar to the rat, but the object in the upper left corner had never been encountered in this specific location (Fig. 1).
The locomotor activity and place recognition tasks were recorded throughout each test by a camera on top of the boxes, and the videos were analysed later by an experimenter who was blind to the animals’ treatment.
Statistical analysis
For the microdialysis study, the results are expressed as the percentage of median baseline dopamine levels, and were analysed using two-way anova, with ‘Time’ (baseline and single hit) as the within-subjects factor and ‘Treatment’ (access to METH) as the between-subjects factor. Statistically significant interactions were followed by the Dunnett post hoc test. Locomotor activity and rearing data are expressed as the time in seconds and number of times the animals reared, respectively. A one-way anova, with ‘Treatment’ as the between-subjects factor, was used to analyse the data, followed by the Bonferroni post hoc test. For the place recognition task, during the Sample 1 phase (i.e. first exposure to the objects), the time spent exploring the objects in both locations (left + right) was calculated. For the Test phase, the scores are expressed as the following ratio: (time spent exploring the novel place − time spent exploring the familiar place)/(time spent exploring the novel place + time spent exploring the familiar place). For each rat, the score was calculated as the average score obtained during the Sample 2/Test 1 and Test 2 phases. A score of zero indicates no discrimination between the novel and familiar places of the object. During the Sample phase, a two-way anova was performed, with ‘Location’ (left and right) as the within-subjects factor and ‘Treatment’ as the between-subjects factor, followed by the Bonferroni post hoc test. For the test phase, a one-way anova was performed, with ‘Treatment’ as the between-group factor, followed by the Bonferroni post hoc test. Furthermore, for the test phase one-sample t-tests were also carried out to establish whether individual groups’ performance differed from chance levels (meaning that the time spent exploring the novel object and time spent exploring the familiar object are identical, and therefore the ratio = 0). All statistical analyses were conducted using SPSS software (PASW statistics 18).
The sample size for control, limited-access and extended-access groups in each experiment can be found in the figure legend corresponding to the results of each experiment.
Variation in the number of subjects from behavioral to microdialysis experiments was due to the fact that some subjects reached and nibbled the microdialysis probe tubing overnight, and thus no data could be collected from these rats: for the nucleus accumbens microdialysis experiment two rats in the control group, four rats in the limited-access group and none in the extended-access group had destroyed probes; for the caudate-putamen microdialysis experiment three rats in the control group, five rats in the limited-access group and one rat in the extended-access group destroyed probes. Concerning the control groups, seven animals that spent the same amount of time as the extended-access group in a chamber without access to METH were added to the 12 yoked animals that received two contingent injections within 2 days, and one animal with one contingent injection of 0.05 mg/kg METH (see the microdialysis experimental section for details) 2 weeks prior to the behavioral tests, constituting a total of 20 control animals (Fig. 2).
Fig. 2.
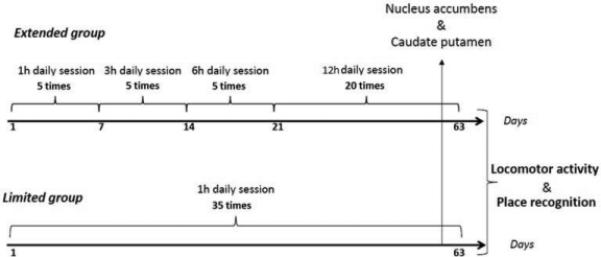
Schematic representation of the experimental design. The length of the study was 65 days in total, and included 63 days of self-administration and 2 days of behavioral tests.
Results
Self-administration
Figure 3 shows the total number of active lever presses in the limited-access group (black circles) and extended-access group (black squares) in each session, and the active lever presses during the first hour in the extended-access group (gray squares). METH intake is represented on the right abscissa. The limited-access group maintained a constant level of METH intake throughout the experiment after the training sessions, whereas the extended-access group exhibited an increase in METH intake during the first hour, as we have previously observed (Hadamitzky et al., 2011). The statistical analysis that compared the first hour of exposure in both groups revealed main effects of ‘Treatment’ (F1,25 = 49.56, P < 0.05) and ‘Time’ (F34,850 = 5.50, P < 0.05), and a significant ‘Treatment × Time’ interaction (F34,850 = 7.03, P < 0.05). Further post hoc analyses of first hour lever presses for each session revealed a difference in METH intake between the extended-access group and limited-access group that became statistically significant from the eighth session onward (P < 0.05).
Fig. 3.
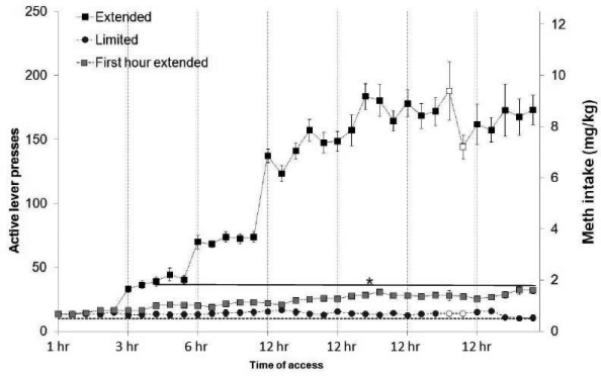
Active lever presses (right abscissa) and METH intake (left abscissa) in the extended-access (n = 15) and limited-access (n = 12) groups. During the first hour, beginning in session 8, the extended-access group exhibited a significant increase in METH intake compared with the limited-access group (*P < 0.05). White symbols represent the microdialysis days. The data are expressed as mean ± SEM.
Microdialysis results
In both the nucleus accumbens and caudate-putamen, the analysis of raw median baseline values revealed no significant differences between groups. Specifically, a one-way anova between treatment groups on the raw median baseline values for the nucleus accumbens (F2,33 = 0.37, P > 0.05) and the caudate-putamen (F2,30 = 0.69, P > 0.05) revealed no statistically significant differences.
Figure 4 depicts the percentage of change in extracellular dopamine in the nucleus accumbens (Fig. 4A) and caudate-putamen (Fig. 4B) in response to a single lever press for METH in the extended-access animals compared with the yoked controls. The extended-access group exhibited a significantly attenuated dopamine response in the nucleus accumbens, but not in the caudate-putamen, compared with the control group. The statistical analysis of dopamine release after METH administration in the nucleus accumbens revealed significant main effects of ‘Time’ (F1,33 = 87.58, P < 0.05) and ‘Treatment’ (F2,33 = 4.15, P < 0.05), but no ‘Time × Treatment’ interaction (F2,33 = 2.93, P > 0.05). The post hoc analysis revealed a significant increase in extracellular dopamine in the three groups after METH injection (P < 0.005). In addition, the analysis revealed a significantly lower increase in extracellular dopamine release as a percentage of median baseline in the extended-access group compared with the control group (P < 0.05) and limited-access group (P < 0.05). In the caudate-putamen, the analysis revealed a significant main effect of ‘Time’ (F1,30 = 18.33, P < 0.05) but not of ‘Treatment’ (F2,30 = 0.16, P > 0.05), and no ‘Time × Treatment’ interaction (F1,30 = 0.31, P > 0.05). The post hoc analyses revealed a significant increase in dopamine in the three experimental groups (P < 0.05), but no differences were found between groups (P > 0.05) in baseline levels or levels after METH administration.
Fig. 4.
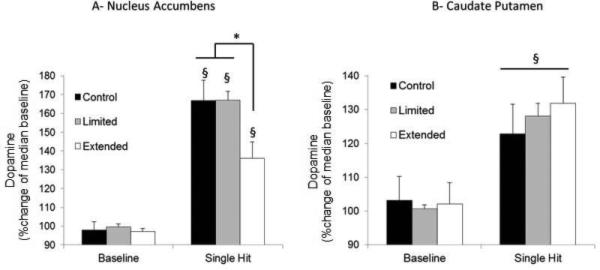
Extracellular dopamine released in (A) the nucleus accumbens in control (n = 13), limited-access (n = 8) and extended-access (n = 15) groups; and (B) in the caudate-putamen in control (n = 12), limited-access (n = 7) and extended-access (n = 14) groups. Results are expressed as changes in dopamine compared with the percentage of median baseline. The extended-access group exhibited tolerance to the effects of METH on dopamine release compared with the control group and the limited-access group in the (A) nucleus accumbens but not (B) the caudate-putamen (*P < 0.05, between groups; §P < 0.05, compared with baseline). The data are expressed as mean ± SEM. Baseline dopamine levels (nm) were: in the nucleus accumbens, 16.7 ± 2.5, 23.1 ± 5.1 and 16.6 ± 2.8; and in the caudate-putamen, 52.7 ± 8.0, 35.8 ± 4.1 and 59.2 ± 22.9 for control, limited-access and extended-access groups, respectively.
Locomotor activity
Figure 5 shows the time spent engaged in locomotor activity (Fig. 5A), and the number of rearings (Fig. 5B) in the limited- and extended-access groups during the 10 min of exposure to the novel arena compared with the control group. The extended-access group showed hypoactivity (i.e. less locomotor activity and fewer rearings) after METH withdrawal compared with the limited-access and control groups. For both locomotor activity and rearing, a main effect of ‘Treatment’ was found (F2,44 = 11.24, P < 0.05 and F2,44 = 13.29, P < 0.05, respectively). The post hoc analysis showed that rats in the extended-access group were hypoactive with regard to both locomotor activity and rearings compared with the control and limited-access groups (P < 0.01). It is important to note that analysis of the behavioral results for the subgroup of animals that had completed the microdialysis experiments showed similar results to those of the entire group that participated in the locomotor activity (F2,33 = 9.92, P < 0.05) and rearing (F2,33 = 11.55, P < 0.05) experiment, with significant differences between the extended-access group compared with either of the two control groups (P < 0.05).
Fig. 5.
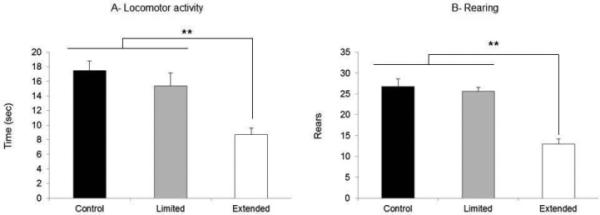
(A) Locomotor activity (in seconds) and (B) number of rearings after METH withdrawal in the control (n = 20), limited-access (n = 12) and extended-access (n = 15) groups. The extended-access group exhibited (A) less locomotion and (B) less rearing compared with the limited-access and control groups (**P < 0.05). The data are expressed as mean ± SEM.
Place recognition
In all experimental groups during the Sample phase, no place preference was observed between the left and right sides of the boxes. No differences were found in the time spent exploring the objects based on object location. However, the overall time spent exploring the objects was significantly greater in the control group compared with the extended-access group. The statistical analysis showed no effect of ‘Location’ (P > 0.05), a main effect of ‘Treatment’ (F2,44 = 3.52, P < 0.05), and no interaction. The post hoc analyses revealed no differences in the time spent exploring the two objects in the two different locations among groups (P > 0.05).
During the Test phase, the control and limited-access groups spent more time exploring the object in the new location, whereas the extended-access group did not spend more time exploring the object in the new location. The statistical analysis revealed an effect of ‘Treatment’ (F2,44 = 10.40, P < 0.05). The post hoc analyses indicated that the ratio for the extended-access group was significantly lower than the ratio for the control group (P < 0.005). The ratios of the control and limited-access groups were significantly greater than zero (control group: t19 = 9.36, P < 0.005; limited-access group: t11 = 3.84, P < 0.005), which was not the case for the extended-access group (t14 = 0.91, P > 0.05). Furthermore, analysis of the behavioral data for the animals that had completed the microdialysis experiments showed similar results to those of the entire group that participated in the place recognition experiment (F2,33 = 8.42, P < 0.05), with significant differences between the extended-access group and either of the two control groups (P < 0.05).
Discussion
The results of the present study are consistent with the interpretation that extended-access METH self-administration leads to neurochemical and behavioral adaptations that are distinct from the effects of limited-access METH self-administration. These results include: (i) a region-specific attenuation of the dopamine response to METH exposure compared with drug-naive rats and rats that only had limited access to METH; and (ii) significant behavioral differences between the limited- and extended-access groups, such as hypolocomotion and cognitive deficits. Overall, these results are consistent with results from human studies that discriminated between recreational abusers, in which few cognitive deficits were observed compared with intensive METH abusers who exhibited cognitive deficits (Simon et al., 2000; Hart et al., 2012).
Tolerance to METH-induced increase in extracellular dopamine in the nucleus accumbens, but not the caudate-putamen in extended-access but not limited-access rats
Dopamine has been shown to play an essential role in reward processing (Wise & Rompre, 1989). The mesolimbic pathway connects dopaminergic neurons in the ventral tegmental area to the nucleus accumbens and appears to be critical for reward seeking (Kesley et al., 1989). Nigrostriatal dopaminergic neurons project from the substantia nigra to the caudate-putamen and are involved in extrapyramidal motor function. The present study revealed an attenuated dopamine response to METH in the nucleus accumbens, but not the caudate-putamen, in rats that had extended, but not limited, access to METH (Fig. 4A and B). Paralleling these neurochemical changes, the extended-access, but not the limited-access, group exhibited a significant increase in the number of lever presses emitted during their first hour of exposure to METH. This effect could be mediated by the development of tolerance to the rewarding effects of METH. The escalation of drug intake in the extended-access group (Fig. 3) mimics the profile of METH abuse in humans (Simon et al., 2002), and clearly highlights differences in drug-seeking and drug-taking between the two access conditions that may be modulated by neuronal changes in the nucleus accumbens. These results appear to be consistent with the observed increase in METH intake in humans, which also suggests tolerance to the drug (Li et al., 2010), but opposite to the results of some studies that suggested sensitization to METH or cocaine in the nucleus accumbens associated with self-administration of the stimulant (Lominac et al., 2002; Ahmed et al., 2003; Vezina, 2004). It is conceivable that the stimulant administration protocol (contingent vs non-contingent challenge drug administration), the time of exposure to the drug (a maximum of 6 h in the study of Ahmed et al., 2003) and the nature of the stimulant itself (cocaine vs METH) may have played an important role in the discrepant findings. Further studies will be necessary to identify the relationships among these various factors, and to more accurately assess whether an attenuated nucleus accumbens response contributes to augmented lever-pressing in extended-access animals.
Interestingly, no differences in baseline dopamine levels (raw data) were observed among groups in either of the two structures, suggesting no depletion of afferent dopaminergic neurons and likely no neurotoxicity induced by extended-access METH self-administration. Consistent with this suggestion, we observed no evidence of neurotoxicity after similar METH exposure patterns (Lacan et al., 2013).
Cognitive deficits after extended, but not limited, access to METH
Previous studies in rodents demonstrated memory deficits after METH self-administration (Rogers et al., 2008; Reichel & See, 2010; Reichel et al., 2011, 2012) and chronic non-contingent treatment (Marshall et al., 2007; Herring et al., 2008). Our data suggest that extended, but not limited, access induced spatial memory deficits in the place recognition task (Fig. 6B). This finding is similar to previous findings that indicated memory deficits in the object recognition procedure (Reichel et al., 2011). Place recognition has been shown to be hippocampus-dependent, whereas variations in spontaneous recognition tasks, such as object or object-in-place recognition, appear to involve different brain areas that are primarily located in the temporal lobe and prefrontal cortex. Object recognition procedures appear to be perirhinal-dependent. Perirhinal neurons respond maximally to the first presentation of visual stimuli but less to the second presentation (Aggleton & Brown, 1999). Rats with perirhinal lesions spent a similar amount of time exploring both objects (Hannesson et al., 2004; Norman & Eacott, 2005). In rodents and non-human primates, object-in-place recognition, in contrast to object recognition, has been shown to be mostly dependent on an intact medial prefrontal cortex (Kim et al., 2011; Spanswick & Dyck, 2012). In contrast to the place recognition test, these two previous tasks do not require an intact hippocampus (Langston & Wood, 2010). Place recognition has been shown to rely on an intact hippocampus (Lee et al., 2005; Gilbert & Kesner, 2006), and particularly the CA3 region (Gilbert & Brushfield, 2009).
Fig. 6.
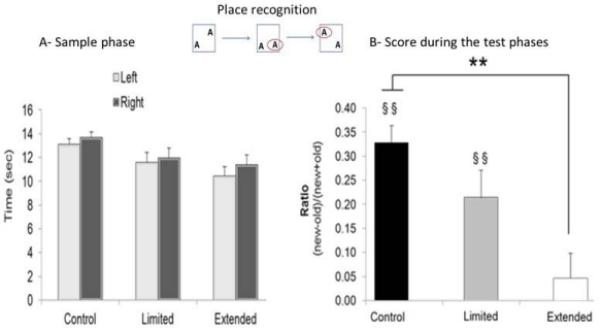
For each group: control (n = 20), limited-access (n = 12) and extended-access (n = 15) groups, (A) represents the average time spent exploring objects by location in each group during the Sample phase. (B) Average score discrimination expressed as a ratio by group during the Test phase: (time spent exploring the novel place − time spent exploring the familiar place)/(time spent exploring the novel place + time spent exploring the familiar place). The control and limited-access groups performed above zero (ratio = 0 indicates no place discrimination; §§P < 0.005), and both were significantly different from the extended-access group (**P < 0.005). The data are expressed as mean ± SEM.
Prolonged METH exposure has been shown to induce shrinkage and degeneration of pyramidal cell layers in the hippocampal CA3 region (Kuczenski et al., 2007). However, Kuczenski and colleagues used higher METH doses than those self-administered by the rats in the present study. Nevertheless, extended, but not limited, access to METH may have affected hippocampus (CA3 region) plasticity, leading to cognitive spatial impairment in the place recognition task. The place recognition task is a non-rewarded task that is based on the rat's spontaneous exploration of novelty (Ennaceur & Delacour, 1988). An effect of tolerance to METH in the nucleus accumbens region cannot be linked to this observed cognitive deficit. Finally, even if overall object exploration in the extended-access group was lower than in the control group, the amount of exploration of the objects and places was sufficiently high to exclude a possible effect of disinterest in the objects or places in the extended-access group. However, this lower exploration of the objects appears to be linked to the reduction of locomotor activity seen during the habituation stage on the previous day (Fig. 5). In addition, a previous study showed that hypolocomotion does not confound memory performance in the object recognition procedure (Le Cozannet et al., 2010).
Overall, the present results are consistent with the human literature on METH that showed little or no memory impairments in recreational abusers but more persistent cognitive changes in high-dose METH abusers (Simon et al., 2000; Hart et al., 2012).
Hypolocomotion after extended access to METH
Both human and rodent studies have shown that METH withdrawal induces psychomotor retardation associated with a depressive state and lethargy (Newton et al., 2004; Hoefer et al., 2006). The locomotor activity task used in the present study was conducted under red-light conditions to minimize the anxiogenic effects of a bright light (Bertoglio & Carobrez, 2002). Other studies (Robinson & Camp, 1987; Wallace et al., 1999) found a decrease in spontaneous locomotion after METH or d-amphetamine treatment using high-dose or neurotoxic regimens. However, the mechanisms that underlie this behavior have been difficult to identify. Based on the results of the microdialysis study, extracellular dopamine levels in the caudate-putamen and nucleus accumbens were not different among groups under baseline conditions, suggesting that this hypolocomotion is not mediated by dopaminergic depletion or neurotoxicity. This specific behavior may reflect a compensatory response to repeated METH administration. Other catecholamines, such as norepinephrine, that have been shown to decrease in the hypothalamus, medial prefrontal cortex and nucleus accumbens during amphetamine withdrawal (Vogel et al., 1985; Paulson et al., 1991; Kuczenski et al., 2009) may also be involved in the observed behavioral changes. Norepinephrine is responsible for the homeostasis of these brain regions and appears to play an important role in locomotor activity (Chruściel & Herman, 1969; Tyler & Tessel, 1980; Fishman et al., 1983). Interestingly, hypolocomotion was only observed in the extended- but not limited-access METH groups. This finding parallels results from human studies that reported more intense withdrawal symptoms, such as depression and lethargy, in METH abusers, depending on their history of drug exposure (McGregor et al., 2005; Cruickshank & Dyer, 2009).
In summary, the present results indicate that tolerance develops to the METH-induced increase in extracellular dopamine in the nucleus accumbens but not in the caudate-putamen, with extended-access METH self-administration only but not with limited-access METH self-administration. These results may be directly linked to an increase in lever presses during the METH sessions observed in the extended-access group but not the limited-access group. Furthermore, during METH withdrawal, a place recognition deficit and hypolocomotor activity were observed in the extended-access group but not the limited-access group. The place recognition deficit may be linked to an effect of METH exposure in the hippocampus. Hypolocomotor activity cannot be directly linked to dopamine because no differences in baseline dopamine levels were found compared with the control group. Dysregulation of norepinephrine might be responsible for this impairment. Thus, extended access to METH, rather than limited access, may be more useful for modeling the neurobiological changes associated with METH abuse.
Acknowledgement
This work was supported by National Institutes of Health grant R01DA01568 to R.K.
References
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav. Brain Sci. 1999;22:425–444. [PubMed] [Google Scholar]
- Ahmed SH, Lin D, Koob GF, Parsons LH. Escalation of cocaine self-administration does not depend on altered cocaine-induced nucleus accumbens dopamine levels. J Neurochem. 2003;86:102–113. doi: 10.1046/j.1471-4159.2003.01833.x. [DOI] [PubMed] [Google Scholar]
- Bertoglio LJ, Carobrez AP. Behavioral profile of rats submitted to session 1-session 2 in the elevated plus-maze during diurnal/nocturnal phases and under different illumination conditions. Behav. Brain Res. 2002;132:135–143. doi: 10.1016/s0166-4328(01)00396-5. [DOI] [PubMed] [Google Scholar]
- Chruściel TL, Herman ZS. Effect of dopalanine on behaviour in mice depleted of norepinephrine or serotonin. Psychopharmacology. 1969;14:124–134. doi: 10.1007/BF00403685. [DOI] [PubMed] [Google Scholar]
- Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104:1085–1099. doi: 10.1111/j.1360-0443.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- Duarte NA, Woods SP, Rooney A, Atkinson JH, Grant I. Working memory deficits affect risky decision-making in methamphetamine users with attention-deficit/hyperactivity disorder. J. Psychiatr. Res. 2012;46:492–499. doi: 10.1016/j.jpsychires.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats: 1. Behavioral data. Behav. Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Fishman RHB, Feigenbaum JJ, Yanai J, Klawans HL. The relative importance of dopamine and norepinephrine in mediating locomotor activity. Prog. Neurobiol. 1983;20:55–88. doi: 10.1016/0301-0082(83)90010-2. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Brushfield AM. The role of the CA3 hippocampal subregion in spatial memory: a process oriented behavioral assessment. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33:774–781. doi: 10.1016/j.pnpbp.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP. The role of the dorsal CA3 hippocampal subregion in spatial working memory and pattern separation. Behav. Brain Res. 2006;169:142–149. doi: 10.1016/j.bbr.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Gururajan A, Manning EE, Klug M, Van Den Buuse M. Drugs of abuse and increased risk of psychosis development. Aust. N. Z. J. Psychiatry. 2012;46:1120–1135. doi: 10.1177/0004867412455232. [DOI] [PubMed] [Google Scholar]
- Hadamitzky M, Markou A, Kuczenski R. Extended access to methamphetamine self-administration affects sensorimotor gating in rats. Behav. Brain Res. 2011;217:386–390. doi: 10.1016/j.bbr.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannesson DK, Howland JG, Phillips AG. Interaction between perirhinal and medial prefrontal cortex is required for temporal order but not recognition memory for objects in rats. J. Neurosci. 2004;24:4596–4604. doi: 10.1523/JNEUROSCI.5517-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Marvin CB, Silver R, Smith EE. Is cognitive functioning impaired in methamphetamine users? A critical review. Neuropsychopharmacology. 2012;37:586–608. doi: 10.1038/npp.2011.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring NR, Schaefer TL, Gudelsky GA, Vorhees CV, Williams MT. Effect of (+)-methamphetamine on path integration learning, novel object recognition, and neurotoxicity in rats. Psychopharmacology. 2008;199:637–650. doi: 10.1007/s00213-008-1183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefer ME, Voskanian SJ, Koob GF, Pulvirenti L. Effects of terguride, ropinirole, and acetyl-L-carnitine on methamphetamine withdrawal in the rat. Pharmacol. Biochem. Behav. 2006;83:403–409. doi: 10.1016/j.pbb.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Homer BD, Solomon TM, Moeller RW, Mascia A, DeRaleau L, Halkitis PN. Methamphetamine abuse and impairment of social functioning: a review of the underlying neurophysiological causes and behavioral implications. Psychol. Bull. 2008;134:301–310. doi: 10.1037/0033-2909.134.2.301. [DOI] [PubMed] [Google Scholar]
- Kelsey JE, Carlezon WA, Jr., Falls WA. Lesions of the nucleus accumbens in rats reduce opiate reward but do not alter context-specific opiate tolerance. Behav. Neurosci. 1989;103:1327–1334. doi: 10.1037//0735-7044.103.6.1327. [DOI] [PubMed] [Google Scholar]
- Kim J, Delcasso S, Lee I. Neural correlates of object-in-place learning in hippocampus and prefrontal cortex. J. Neurosci. 2011;31:16991–17006. doi: 10.1523/JNEUROSCI.2859-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology. 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Everall IP, Crews L, Adame A, Grant I, Masliah E. Escalating dose-multiple binge methamphetamine exposure results in degeneration of the neocortex and limbic system in the rat. Exp. Neurol. 2007;207:42–51. doi: 10.1016/j.expneurol.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS, Aizenstein ML. Amphetamine, cocaine, and fencamfamine: relationship between locomotor and stereotypy response profiles and caudate and accumbens dopamine dynamics. J Neurosci. 1991;11:2703–2712. doi: 10.1523/JNEUROSCI.11-09-02703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS, Melega WP, Lacan G, McCunney SJ. Human methamphetamine pharmacokinetics simulated in the rat: behavioral and neurochemical effects of a 72-h binge. Neuropsychopharmacology. 2009;34:2430–2441. doi: 10.1038/npp.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal D. Concomitant characterization of behavioral and striatal neurotransmitter response to amphetamine using in vivo microdialysis. J. Neurosci. 1989;9:2051–2065. doi: 10.1523/JNEUROSCI.09-06-02051.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacan G, Hadamitzky M, Kuczenski R, Melega WP. Alterations in the striatal dopamine system after long term intravenous methamphetamine exposure: contingent and noncontingent administration. Synapse. 2013 doi: 10.1002/syn.21654. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston RF, Wood ER. Associative recognition and the hippocampus: differential effects of hippocampal lesions on object-place, object-context and object-place-context memory. Hippocampus. 2010;20:1139–1153. doi: 10.1002/hipo.20714. [DOI] [PubMed] [Google Scholar]
- Le Cozannet R, Fone KC, Moran PM. Phencyclidine withdrawal disrupts episodic-like memory in rats: reversal by donepezil but not clozapine. Int. J. Neuropsychopharmacol. 2010;8:1011–1020. doi: 10.1017/S1461145710000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Hunsaker MR, Kesner RP. The role of hippocampal subregions in detecting spatial novelty. Behav. Neurosci. 2005;119:145–153. doi: 10.1037/0735-7044.119.1.145. [DOI] [PubMed] [Google Scholar]
- Li L, Lopez JC, Galloway GP, Baggott MJ, Everhart T, Mendelson J. Estimating the intake of abused methamphetamines using experimenter-administered deuterium labeled R-methamphetamine: selection of the R-methamphetamine dose. Ther. Drug Monit. 2010;32:504–507. doi: 10.1097/FTD.0b013e3181db82f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lominac KD, Sacramento AD, Szumlinski KK, Kippin TE. Distinct neurochemical adaptations within the nucleus accumbens produced by a history of self-administered vs non-contingently administered intravenous methamphetamine. Neuropsychopharmacology. 2012;37:707–722. doi: 10.1038/npp.2011.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JF, Belcher AM, Feinstein EM, O'Dell SJ. Methamphetamine-induced neural and cognitive changes in rodents. Addiction. 2006;102:61–69. doi: 10.1111/j.1360-0443.2006.01780.x. [DOI] [PubMed] [Google Scholar]
- McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Wongtan T, White JM. The nature, time course and severity of methamphetamine withdrawal. Addiction. 2005;100:1320–1329. doi: 10.1111/j.1360-0443.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- Newton TF, Kalechstein AD, Duran S, Vansluis N, Ling W. Methamphetamine abstinence syndrome: preliminary findings. Am. J. Addict. 2004;13:248–255. doi: 10.1080/10550490490459915. [DOI] [PubMed] [Google Scholar]
- Norman G, Eacott MJ. Dissociable effects of lesions to the perirhinal cortex and the postrhinal cortex on memory for context and objects in rats. Behav Neurosci. 2005;119:557–566. doi: 10.1037/0735-7044.119.2.557. [DOI] [PubMed] [Google Scholar]
- O'Neil ML, Kuczenski R, Segal DS, Cho AK, Lacan G, Melega WP. Escalating dose pretreatment induces pharmacodynamic and not pharmacokinetic tolerance to a subsequent high-dose methamphetamine binge. Synapse. 2006;60:465–473. doi: 10.1002/syn.20320. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Camp DM, Robinson TE. Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology (Berl) 1991;103:480–492. doi: 10.1007/BF02244248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE. Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology. 2011;36:782–792. doi: 10.1038/npp.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel C, See R. Modafinil effects on reinstatement of methamphetamine seeking in a rat model of relapse. Psychopharmacology. 2010;210:337–346. doi: 10.1007/s00213-010-1828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Chan CH, Ghee SM, See RE. Sex differences in escalation of methamphetamine self-administration: cognitive and motivational consequences in rats. Psychopharmacology (Berl) 2012;223:371–380. doi: 10.1007/s00213-012-2727-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Camp DM. Long-lasting effects of escalating doses of d-amphetamine on brain monoamines, amphetamine-induced stereotyped behavior and spontaneous nocturnal locomotion. Pharmacol. Biochem. Behav. 1987;26:821–827. doi: 10.1016/0091-3057(87)90616-2. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Whishaw IQ. Normalization of extracellular dopamine in striatum following recovery from a partial unilateral 6-OHDA lesion of the substantia nigra: a microdialysis study in freely moving rats. Brain Res. 1988;450:209–224. doi: 10.1016/0006-8993(88)91560-0. [DOI] [PubMed] [Google Scholar]
- Rogers JL, De Santis S, See RE. Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology (Berl) 2008;199:615–624. doi: 10.1007/s00213-008-1187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SL, Domier C, Carnell J, Brethen P, Rawson R, Ling W. Cognitive impairment in individuals currently using methamphetamine. Am. J. Addict. 2000;9:222–231. doi: 10.1080/10550490050148053. [DOI] [PubMed] [Google Scholar]
- Simon SL, Richardson K, Dacey J, Glynn S, Domier CP, Rawson RA, Ling W. A comparison of patterns of methamphetamine and cocain use. J. Addict. Dis. 2002;21:35–44. doi: 10.1300/j069v21n01_04. [DOI] [PubMed] [Google Scholar]
- Spanswick SC, Dyck RH. Object/context specific memory deficits following medial frontal cortex damage in mice. PLoS One. 2012;7:e43698. doi: 10.1371/journal.pone.0043698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler TD, Tessel RE. Norepinephrine uptake inhibitors as biochemically and behaviorally selective antagonists of the locomotor stimulation induced by indirectly acting sympathomimetic aminetic amines in mice. Psychopharmacology (Berl) 1980;69:27–34. doi: 10.1007/BF00426517. [DOI] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci. Biobehav. Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Vogel WH, Miller J, Waxman H, Gottheil E. Biochemical and behavioral changes in rats during and after chronic d-amphetamine exposure. Drug Alcohol Depend. 1985;15:245–253. doi: 10.1016/0376-8716(85)90004-3. [DOI] [PubMed] [Google Scholar]
- Wallace TL, Gudelsky GA, Vorhees CV. Methamphetamine-induced neurotoxicity alters locomotor activity, stereotypic behavior, and stimulated dopamine release in the rat. J. Neurosci. 1999;19:9141–9148. doi: 10.1523/JNEUROSCI.19-20-09141.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu. Rev. Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime . World Drug Report 2011. United Nations, New York: 2011. [Google Scholar]


