Abstract
Background
Preclinical data indicate that dual HER2 inhibition overcomes trastuzumab resistance and that use of an HER2 inhibitor with an anti-angiogenic agent may augment responses.
Patients and methods
We conducted a dose-escalation, phase I study of a combination of trastuzumab, lapatinib and bevacizumab. The subset of patients with metastatic breast cancer was analyzed for safety and response.
Results
Twenty-six patients with metastatic breast cancer (median = 7 prior systemic therapies) (all with prior trastuzumab; 23 with prior lapatinib; one with prior bevacizumab) received treatment on a range of dose levels. The most common treatment-related grade 2 or higher toxicities were diarrhea (n = 11, 42%) and skin rash (n = 2, 8%). The recommended phase 2 dose was determined to be the full Food and Drug Administration (FDA) approved doses for all the three agents (trastuzumab 8 mg/kg loading dose, 6 mg/kg maintenance dose, intravenously every 3 weeks; lapatinib 1250 mg daily, bevacizumab 15 mg/kg intravenously every 3 weeks). The overall rate of stable disease (SD) ≥6 months and partial or complete remission (PR/CR) was 50% (five patients with SD ≥6 months; seven PRs (including one unconfirmed); one CR). The rate of SD ≥6 months/PR/CR was not compromised in patients who had previously received study drugs, those with brain metastases, and patients treated at lower dose levels.
Conclusions
The combination of trastuzumab, lapatinib and bevacizumab was well-tolerated at maximally approved doses of each drug, and its activity in heavily pretreated patients with metastatic breast cancer suggests that it warrants further investigation.
ClinTrials.gov ID
Keywords: breast cancer, her2, bevacizumab, trastuzumab, lapatinib
introduction
HER2 and EGFR play fundamental roles in cancer pathophysiology [1]. HER2 overexpression is present in 25%–30% of invasive breast cancers [2], and is associated with decreased survival [3]. Trastuzumab, a monoclonal antibody directed against the extracellular domain of HER2, is approved for the treatment of HER2-positive breast cancer and improves overall survival [4]. In addition to HER2 expression, EGFR amplification is present in ∼6% of primary breast cancers [5]. Lapatinib, a dual kinase inhibitor, is approved for the treatment of metastatic HER2-positive breast cancer after progression on prior trastuzumab treatment [6].
HER2 overexpression is associated with vascular endothelial growth factor (VEGF) upregulation, which plays an important role in breast cancer development and metastases [7]. Bevacizumab, a recombinant monoclonal antibody to VEGF, has demonstrated improved response rate and progression-free survival in combination with paclitaxel, but has not improved overall survival, in patients with metastatic breast cancer [8]. Although targeting HER2, EGFR or VEGF alone does not provide adequate tumor control in many patients [9, 10], preclinical studies suggest potential for increased efficacy with combination therapy [11–13]. Furthermore, clinical trials of various doublet combinations of trastuzumab, lapatinib and bevacizumab suggest increased efficacy and that combined anti-HER2 and anti-VEGF treatment may overcome resistance to anti-HER2 monotherapy [14–18]. Here, we report the results of administering a dual HER2/EGFR inhibitor (lapatinib) and an HER2 antibody (trastuzumab) together with an anti-angiogenic agent (bevacizumab) in 26 patients with heavily pretreated breast cancer.
methods
study design and patients
The study was conducted at The University of Texas MD Anderson Cancer Center (MDACC) in accordance with Institutional Review Board guidelines. The breast cancer cohort reported herein included all patients with breast cancer who started therapy between 21 February 2008 and 28 October 2011 as part of a dose-escalation study conducted in patients with advanced cancer. The dose-escalation portion of the study determined the recommended phase II dose (RP2D) (Table 1). A cycle was 21 days. Patients with breast cancer reported herein were treated at variable dose levels, depending on the time of study entry (Table 1). Patients had metastatic or advanced breast cancer not amendable to established forms of therapy, an Eastern Cooperative Oncology Group (ECOG) performance status 0–2 [19], and adequate hematologic, hepatic and renal function. Further details on the exclusion criteria and molecular testing are available in supplementary methods, available at Annals of Oncology online.
Table 1.
Treatment-related grade 2–4 adverse events
| Dose level | 1 n = 0 |
2 n = 1 |
3 n = 0 |
4 n = 1 |
5 n = 1 |
6 n = 1 |
7 n = 3 |
8 n = 2 |
9 n = 2 |
10 n = 2 |
11 n = 3 |
12a n = 10 |
Total n = 26 |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bevacizumab dose, mg/kg IV q3w | 2.5 | 2.5 | 5 | 5 | 5 | 7.5 | 7.5 | 7.5 | 10 | 10 | 10 | 15 | ||
| Trastuzumab dose, mg/kg IV q3wb | 2,1 | 2,1 | 2,1 | 4,2 | 4,2 | 4,2 | 6,4 | 6,4 | 6,4 | 8,6 | 8,6 | 8,6 | ||
| Lapatinib dose, mg po daily | 250 | 500 | 500 | 500 | 750 | 750 | 750 | 1000 | 1000 | 1000 | 1250 | 1250 | ||
| Fatigue | ||||||||||||||
| Grade 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 (4%) | |
| Skin rash | ||||||||||||||
| Grade 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 2 (8%) | |
| Hypertension | ||||||||||||||
| Grade 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1(4%) | |
| Nausea | ||||||||||||||
| Grade 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1(4%) | |
| Anorexia | ||||||||||||||
| Grade 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1(4%) | |
| Diarrhea | ||||||||||||||
| Grade 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 6 | 7 (26.9%) | |
| Grade 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1(DLT) | 1 | 0 | 0 | 1(DLT) | 1(DLT) | 4 (15%) | |
| Mucositis | ||||||||||||||
| Grade 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 (4%) | |
| Skin fissure | ||||||||||||||
| Grade 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 (4%) | |
| Elevated bilirubin | ||||||||||||||
| Grade 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 (4%) | |
aRecommended phase II dose [22]. This includes full approved doses of each drug.
bTrastuzumab dose shown as loading dose, maintenance dose.
DLT, dose-limiting toxicity; IV, intravenous; po, orally; q3w, every 3 weeks.
safety
Clinically significant adverse events were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. Clinical history, physical examination, hematology, blood chemistry and urinalysis were carried out at baseline and at regular intervals while receiving treatment.
evaluation of efficacy
Treatment efficacy was evaluated by diagnostic imaging per Response Evaluation Criteria in Solid Tumors (RECIST) 1.0 [20]. Radiologic assessments were conducted at baseline and about every 8 weeks thereafter. Patients with known brain metastases before enrollment underwent brain imaging at baseline and at regular intervals.
molecular testing
Molecular testing was carried out in the Clinical Laboratory Improvement Amendments approved MDACC laboratory, including estrogen and progesterone receptor by standard immunohistochemistry (IHC) [21], HER2 amplification by fluorescent in situ hybridization, HER2 expression by IHC (AB8 Neomarkers primary antibody (1:3000 dilution, Labvision, Fremont, California)) and EGFR and PIK3CA mutation analysis.
statistical analysis
Spearman's correlation, chi-square or Fisher's exact test, and an independent samples t-test were used to analyze correlations, dichotomous variables and mean differences, respectively.
results
demographics
Twenty-six patients with metastatic breast cancer were enrolled (Table 2). Most patients were heavily pretreated, with a median of seven prior systemic therapies in the adjuvant or metastatic setting (range 2–17). Histological features, molecular/receptor characteristics and prior therapies are described in Table 2.
Table 2.
Patient demographics
| Characteristics (n = 26) | |
|---|---|
| Age (years) | |
| Median | 56 |
| Range | 28–72 |
| Gender, n (%) | |
| Women | 26 (100%) |
| Histologies, n (%) | |
| Invasive ductal | 20 (77%) |
| Mixed ductal and lobular | 2 (8%) |
| Poorly differentiated carcinoma | 1 (4%) |
| Unspecified carcinoma | 1 (4%) |
| Unspecified adenocarcinoma | 1 (4%) |
| Micropapillary carcinoma | 1 (4%) |
| No. of prior systemic therapiesa | |
| Median | 7 |
| Range | 2–17 |
| Prior trastuzumab | 26 |
| Prior trastuzumab (but no prior bevacizumab or lapatinib) | 2 |
| Prior trastuzumab and lapatinib (sequential) | 13 |
| Prior trastuzumab and lapatinib (sequential) and ARRY-380 | 3 |
| Prior trastuzumab and lapatinib (concurrent) | 5 |
| Prior trastuzumab and lapatinib (concurrent) and ARRY-380 | 2 |
| Prior trastuzumab and bevacizumab | 1 |
| Months since the last exposure to trastuzuamb or lapatinib | |
| Median | 2 |
| Range | 1–9 |
| Estrogen and/or progesterone receptor expression, n (%) | |
| Positive | 12 (46%) |
| Negative | 14 (54%) |
| EGFR mutations, n (%) | |
| Positive | 0 (0%) |
| Negative | 12 (46%) |
| Unknown | 14 (54%) |
| HER2 amplification | |
| Positive | 26 (100%) |
| Negative | 0 (0%) |
| ECOG performance status, n (%) | |
| 0 | 5 (19%) |
| 1 | 21 (81%) |
aIncludes both adjuvant and metastatic regimens.
ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor 1; HER2, human epidermal growth factor receptor 2.
adverse events
Twelve patients (46%) experienced no drug-related toxicity higher than grade 1. The most common treatment-related grade 2 or higher adverse events were diarrhea (n = 11, 42%) and rash (n = 2, 8%) (Table 1). Three patients experienced dose-limiting toxicity (DLT) due to diarrhea (n = 3, dose levels 7, 11 and 12). Seven patients required dose reduction for toxicity, including for diarrhea (n = 4), comorbid rash and elevated bilirubin (n = 1), comorbid diarrhea and skin fissures (n = 1), and comorbid diarrhea and mucositis (n = 1). Two patients (8%) withdrew due to toxicity, including diarrhea (n = 1) and fatigue (n = 1). No deaths resulted from adverse events.
responses
In total, SD ≥6 months, PR or CR was achieved in 13 patients (50%). The overall confirmed response rate was 27% (PR + CR) (Figure 1). One patient (4%) achieved a CR, but was noted to have brain metastases at 11 months (after non-specific complaints of memory loss); she did not have any evidence of systemic recurrence. It was unclear whether these brain metastases were new since no prior scans of the brain had been carried out. Her treatment was continued after stereotactic brain radiation, and she remains on treatment at 34+ months. This patient was the only individual on the study with micropapillary histology. No other patients received concomitant radiation or surgery while on study. Seven patients (27%) achieved a PR (one was an unconfirmed PR). The duration on study for six patients with confirmed PR was 4, 5, 6, 8+, 9 and 12, months.
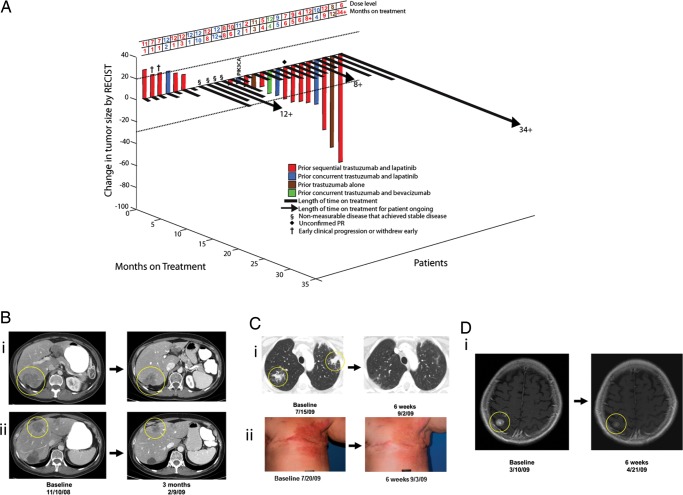
Figure 1.
(A) 3DWaterfall plot showing best response as determined by Response Evaluation Criteria in Solid Tumors (RECIST) and the time on study in all 26 breast cancer patients treated. The patient who withdrew before first restaging due to grade 3 diarrhea and any patients with clinical progression or new lesions are arbitrarily depicted as 21% increase by RECIST and are considered treatment failures. Patients with non-measurable disease that achieved best response of stable disease (SD) are depicted in the figure as +0.5%. None of the patients had prior cetuximab or erlotinib. The patient with a PIK3CA mutation is noted as such. The dose level for each patient and the number of months the patient received treatment on study are shown in the table above the waterfall plot. Patients still on treatment have a ‘+’ after the number of months and are indicated with an arrow (>) on the black bar for that patient. (B) Patient with partial response (36% decrease, seven prior systemic therapies) on treatment for 6 months. (i) and (ii) computed tomography (CT) scans showing decrease in size of liver metastases. (C) Patient with partial response (86% decrease, three prior systemic therapies), (i) CT scans showing decreased size of lung metastases, (ii) response in skin metastases. (D) Patient with brain metastases only and partial response (30% decrease, six prior systemic therapies), CT scans showing the decrease in size of brain metastases.
prior HER2 inhibitor, EGFR inhibitor or VEGF inhibitor therapy and response
Prior trastuzumab and lapatinib, even if given concurrently, did not preclude SD ≥6 months/PR/CR. One of the two patients (50%) who received prior trastuzumab and no prior bevacizumab or lapatinib, achieved a PR. Of the 16 patients who received prior sequential trastuzumab and lapatinib, 9 (56%) achieved SD ≥6 months/PR/CR. Of the seven patients who had previously received concurrent trastuzumab and lapatinib, three (43%) achieved SD ≥6 months/PR/CR. The patient who had received prior concurrent trastuzumab and bevacizumab achieved SD for 4 months. Of the 13 patients with SD ≥6 months/PR/CR, 9 (69%) had received prior sequential trastuzumab and lapatinib, three (23%) had received prior concurrent trastuzumab and lapatinib and one (8%) had prior trastuzumab and no lapatinib nor any prior bevacizumab (Table 3 and Figure 1).
Table 3.
Patient characteristics for those who achieved stable disease (SD) of at least 6 months, partial response or complete response
| Case # | Histology | Best Response % | Treatment duration (months) | Estrogen Receptor positive | Progesterone receptor positive | EGFR mutation | HER2 Amplification | PIK3CA mutation | Prior trastuzumab | Prior bevacizumab | Prior lapatinib | Prior ARRY-380 | Brain metastases | Dose Level | Diarrhea Grade |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 64 | Papillary carcinoma | −100 | 34+a | No | No | ND | Yes | ND | Yes | No | Yes | No | No | 6 | 0 |
| 103 | Invasive ductal carcinoma | −86 | 12 | No | No | ND | Yes | ND | Yes | No | No | No | No | 8 | 0 |
| 210 | Carcinoma | −67 | 9 | Yes | Yes | No | Yes | ND | Yes | No | Yes | No | Yes | 12 | 2 |
| 230 | Invasive ductal carcinoma | −41 | 4 | No | No | ND | Yes | ND | Yesb | No | Yesb | No | Yes | 10 | 2 |
| 236 | Invasive ductal carcinoma | −38 | 8+ | No | No | ND | Yes | ND | Yes | No | Yes | No | No | 12 | 2 |
| 121 | Invasive ductal carcinoma | −35 | 5 | Yes | Yes | No | Yes | ND | Yes | No | Yes | No | Yes | 9 | 0 |
| 49 | Invasive ductal carcinoma | −36 | 6 | No | No | No | Yes | ND | Yes | No | Yes | No | No | 4 | 0 |
| 68 | Invasive ductal carcinoma | −30c | 6 | No | No | No | Yes | ND | Yes | No | Yes | No | Yes | 7 | 0 |
| 127 | Invasive ductal carcinoma | −5 | 6 | No | No | No | Yes | ND | Yes | No | Yes | No | No | 10 | 1 |
| 227 | Ductal and lobular carcinoma | 0d | 12+ | Yes | Yes | ND | Yes | ND | Yesb | No | Yesb | No | Yes | 12 | 2 |
| 170 | Invasive ductal carcinoma | 0d | 10 | No | No | No | Yes | ND | Yesb | No | Yesb | No | No | 12 | 1 |
| 109 | Poorly differentiated carcinoma | 0d | 8 | Yes | Yes | ND | Yes | ND | Yes | No | Yes | Yes | Yes | 8 | 3 |
| 203 | Invasive ductal carcinoma | 0d | 8 | No | No | No | Yes | ND | Yes | No | Yes | No | No | 12 | 2 |
aPatient 64 was discovered to have brain metastases at 11 months but did not have any evidence of systemic recurrence. Her brain metastases were treated with radiation, and her treatment was continued. She remains on treatment at 34+ months without systemic recurrence.
bIndicates patients who received prior study drugs concurrently.
cIndicates an unconfirmed PR.
dPatients 109, 170, 203 and 227 had non-measurable disease by RECIST guidelines.
EGFR, epidermal growth factor receptor 1; HER2, human epidermal growth factor receptor 2; ND, not done; PIK3CA, phosphoinositide-3-kinase, catalytic, alpha polypeptide; +, indicates ongoing therapy.
Among patients who previously received concurrent trastuzumab and lapatinib, the time from the prior exposure until starting the current clinical trial was 1, 1, 3, 4, 6 and 7 months, respectively. The prior concurrent trastuzumab and lapatinib treatment was discontinued for progressive disease in all patients, except for one who discontinued treatment because of diarrhea. The duration of prior trastuzumab and lapatinib was similar to the duration of treatment on the current clinical trial in two cases, and one patient (#227) was on the current trial for 4+ months longer than the prior treatment with concurrent trastuzumab and lapatinib. She continues receiving treatment on this trial without progression.
Among the five patients who had received prior HER2 kinase inhibitor ARRY-380, two had also received prior sequential trastuzumab and lapatinib, and three had received prior concurrent trastuzumab and lapatinib. One of these five patients, who was previously treated with ARRY-380 and had also received prior sequential trastuzumab and lapatinib, achieved SD of 8 months.
brain metastases and response
Of the 10 patients with brain metastases, 6 (60%) achieved SD ≥6 months/PR/CR (Table 3), with the longest duration being 12+ months. Therefore, the presence of brain metastases did not preclude SD ≥6 months/PR/CR. Only one patient attained measurable decrease in brain metastases; in the other five patients the brain lesions remained stable. Of the four patients with progressing brain metastases at the time of enrollment, one achieved an unconfirmed PR and received treatment for 6 months. None of the patients experienced adverse events related to brain metastases.
dosing and response
Of the 15 patients on dose levels 10–12 (Table 1, Figure 1), 7 (47%) achieved SD ≥6 months/PR/CR. For the patients treated at dose levels 1–9, 6 of 11 (55%) achieved SD ≥6 months/PR/CR (P = 1.00, Table 1 and Figure 1). The only patient who achieved CR was treated at dose level 6 and remains on treatment at 34+ months. Therefore, there was no obvious dose–response correlation, although the number of patients was small. The treatment duration was 7.55 (SD = 9.37) and 4.93 (SD = 3.63) months for patients who received lower (levels 1–9) and higher (levels 10–12) dose levels, respectively. There was a non-significant trend toward longer treatment duration for individuals given low doses versus high doses (t(24) = .9884, P = 0.33).
molecular aberrations and responses
Achievement of SD ≥6 months/PR/CR was observed in 4 of the 12 patients (33%) positive for estrogen or progesterone receptors, versus 9 of 14 patients (64%) negative for both estrogen and progesterone receptors (P = 0.24).
All 26 patients in this study (100%) had HER2 amplification. Of the 12 patients tested, all were EGFR wild type, and 7 (58%) achieved SD ≥6 months/PR/CR.
Of the two patients tested, only one had a PIK3CA mutation (H1047R), and she received treatment for only 2.3 months before progressing.
histology and response
The patient with the best response to treatment (CR by RECIST) was the only patient in the study with micropapillary histology. Of the six patients with non-ductal histology, four (67%) achieved SD ≥6 months/PR/CR. Nine of the 20 patients (45%) with tumors with ductal histology achieved SD ≥6 months/PR/CR.
toxicity and response
Diarrhea was the most frequently observed adverse event (Table 1), with 11 patients (42%) experiencing grade 2 or higher diarrhea. Of the 11 patients with grade 2 or higher diarrhea, 6 (55%) achieved SD ≥6 months/PR/CR. Of the 15 patients with grade 1 or no diarrhea, 7 (47%) achieved SD ≥6 months/PR/CR (P = 1.00).
discussion
We report the results of the cohort of patients with metastatic breast cancer, treated on a phase I dose-escalation trial of combination bevacizumab, trastuzumab and lapatinib. This combination of drugs was well tolerated, and the recommended phase 2 dose was determined to be the full FDA-approved doses for all the three drugs [22]. In contrast to previous studies in which trastuzumab was combined with lapatinib 1000 mg daily [14, 15], our study demonstrated that combination with lapatinib 1250 mg daily was well tolerated. The prevalence and severity of diarrhea, the most commonly observed adverse effect on this study, were similar to what has been reported previously in lapatinib monotherapy studies [23]. This regimen demonstrated antitumor activity with 13 patients (50%) who had a best overall response of SD ≥6 months (n = 5), PR (n = 7) (one PR was unconfirmed), or CR (n = 1). Antitumor activity was observed even in patients who were heavily pretreated, who had received prior trastuzumab and/or lapatinib, those with brain metastases and patients treated at lower dose levels. Of interest, the best response was in the only individual on study with micropapillary histology. Invasive micropapillary cancer of the breast is a distinct and aggressive HER2+ variant of breast cancer with high relapse rates and short disease-free intervals [24]. This patient continues on study at 34+ months.
Remarkably, patients who had failed prior concurrent or sequential trastuzumab and lapatinib achieved SD ≥6 months/PR/CR. In fact, overcoming resistance to prior concurrent trastuzumab and lapatinib and achieving a longer treatment duration with the combination of trastuzumab, lapatinib and bevacizumab was demonstrated, which may suggest that the contribution of bevacizumab to this treatment combination is significant.
The presence of brain metastases did not compromise the rate of SD ≥6 months/PR/CR, suggesting that patients with HER2+ breast cancer and brain metastases can safely receive this regimen and that it has activity. None of the patients experienced intracranial hemorrhage as a result of the treatment. Although brain metastasis involvement has been an exclusion criterion in many breast cancer trials that include bevacizumab [25], recent studies suggest that brain metastases do not preclude safe treatment with bevacizumab [26], and our study further supports the safety of bevacizumab in the presence of brain metastases.
Low dose levels did not preclude response, which is in accordance with the previous literature [27]. No maximum tolerated dose was found in this study, and a non-significant trend of longer treatment duration was observed in patients receiving low dose levels. As such, while the R2PD includes full FDA recommended doses, lower doses may also be considered.
One limitation of our study was the inability to identify a relevant biomarker for response. In the AVEREL phase III trial of bevacizumab with trastuzumab in combination with docetaxel, analysis suggested that high VEGF-A levels were associated with better response in the bevacizumab arm, but not significantly so [28]. In contrast, other trials have failed to identify correlation between VEGF-A level and efficacy, and further studies are needed to validate VEGF-A isoforms and other prospective biomarkers [29].
In conclusion, the results presented here demonstrate that dual inhibition of HER2 with trastuzumab and lapatinib, combined with the VEGF antibody bevacizumab, is well tolerated, allowing full doses of all the three drugs in patients with HER2+ breast cancer. SD ≥6 months/PR/CR was achieved in 50% of this heavily pretreated patient population, suggesting that this regimen merits further investigation, perhaps in a randomized trial in comparison with combination of trastuzumab and lapatinib, with biomarker correlates to identify patient subgroups which may be more likely to benefit from bevacizumab.
funding
This research was supported by a K12 Paul Calabresi Career Development Award for Clinical Oncology, CA088084 from the National Institutes of Health and the National Cancer Institute. GF received research funding and travel reimbursement for conference attendance from GlaxoSmithKline. RK received research funding and has received honoraria from GlaxoSmithKline, Genentech and Roche.
disclosure
All the remaining authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
We thank the patients and their families. We also thank Adrienne Howard for regulatory protocol assistance and Dr Lawrence Cormack and Joann Aaron for assistance in the preparation of Figure 1.
references
- 1.Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clin Cancer Res. 2006;12:5268–5272. doi: 10.1158/1078-0432.CCR-05-1554. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 3.Prowell TM, Armstrong DK. Selecting endocrine therapy for breast cancer: what role does HER-2/neu status play? Semin Oncol. 2006;33:681–687. doi: 10.1053/j.seminoncol.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 4.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 5.Bhargava R, Gerald WL, Li AR, et al. EGFR gene amplification in breast cancer: correlation with epidermal growth factor receptor mRNA and protein expression and HER-2 status and absence of EGFR-activating mutations. Mod Pathol. 2005;18:1027–1033. doi: 10.1038/modpathol.3800438. [DOI] [PubMed] [Google Scholar]
- 6.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 7.Konecny GE, Meng YG, Untch M, et al. Association between HER-2/neu and vascular endothelial growth factor expression predicts clinical outcome in primary breast cancer patients. Clin Cancer Res. 2004;10:1706–1716. doi: 10.1158/1078-0432.ccr-0951-3. [DOI] [PubMed] [Google Scholar]
- 8.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 9.Luu T, Chung C, Somlo G. Combining emerging agents in advanced breast cancer. Oncologist. 2011;16:760–771. doi: 10.1634/theoncologist.2010-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roodink I, Leenders WP. Targeted therapies of cancer: angiogenesis inhibition seems not enough. Cancer Lett. 2010;299:1–10. doi: 10.1016/j.canlet.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Konecny GE, Pegram MD, Venkatesan N, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66:1630–1639. doi: 10.1158/0008-5472.CAN-05-1182. [DOI] [PubMed] [Google Scholar]
- 12.Scaltriti M, Verma C, Guzman M, et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene. 2009;28:803–814. doi: 10.1038/onc.2008.432. [DOI] [PubMed] [Google Scholar]
- 13.Xia W, Gerard CM, Liu L, et al. Combining lapatinib (GW572016), a small molecule inhibitor of ErbB1 and ErbB2 tyrosine kinases, with therapeutic anti-ErbB2 antibodies enhances apoptosis of ErbB2-overexpressing breast cancer cells. Oncogene. 2005;24:6213–6221. doi: 10.1038/sj.onc.1208774. [DOI] [PubMed] [Google Scholar]
- 14.Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28:1124–1130. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 15.Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379(9816):633–640. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurvitz S, Pegram M, Lin L, et al. Final results of a phase II trial evaluating trastuzumab and bevacizumab as first-line treatment of HER2-amplified advanced breast cancer. Thirty-Second Annual Cancer Therapy & Research Center-American Association for Cancer Research San Antonio Breast Cancer Symposium 2009; San Antonio, TX: American Association for Cancer Research; 2009. [Google Scholar]
- 17.Rugo HS, Franco S, Munster P, et al. A phase II evaluation of lapatinib (L) and bevacizumab (B) in HER2+ metastatic breast cancer (MBC). American Society of Clinical Oncology Annual Meeting 2008; Chicago, IL: American Society of Clinical Oncology; 2008. [Google Scholar]
- 18.Rugo HS, Jo Chien A, Franco SX, et al. A phase II study of lapatinib and bevacizumab as treatment for HER2-overexpressing metastatic breast cancer. Breast Cancer Res Treat. 2011;134:13–20. doi: 10.1007/s10549-011-1918-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 20.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 21.Hammond ME, Hayes DF, Wolff AC. Clinical notice for American Society of Clinical Oncology—College of American Pathologists guideline recommendations on ER/PgR and HER2 testing in breast cancer. J Clin Oncol. 2011;29:e458. doi: 10.1200/JCO.2011.35.2245. (17 September 2013, date last accessed) [DOI] [PubMed] [Google Scholar]
- 22.Falchook GS, Wheler JJ, Naing A, et al. A phase I study of bevacizumab in combination with sunitinib, sorafenib, and erlotinib plus cetuximab, and trastuzumab plus lapatinib. American Society of Clinical Oncology Annual Meeting 2010; Chicago, IL: American Society of Clinical Oncology; 2010. [Google Scholar]
- 23.Burris HA., III Dual kinase inhibition in the treatment of breast cancer: initial experience with the EGFR/ErbB-2 inhibitor lapatinib. Oncologist. 2004;9(Suppl 3):10–15. doi: 10.1634/theoncologist.9-suppl_3-10. [DOI] [PubMed] [Google Scholar]
- 24.Pettinato G, Manivel CJ, Panico L, et al. Invasive micropapillary carcinoma of the breast: clinicopathologic study of 62 cases of a poorly recognized variant with highly aggressive behavior. Am J Clin Pathol. 2004;121:857–866. doi: 10.1309/XTJ7-VHB4-9UD7-8X60. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton EP, Blackwell KL. Safety of bevacizumab in patients with metastatic breast cancer. Oncology. 2011;80:314–325. doi: 10.1159/000328757. [DOI] [PubMed] [Google Scholar]
- 26.Socinski MA, Langer CJ, Huang JE, et al. Safety of bevacizumab in patients with non-small-cell lung cancer and brain metastases. J Clin Oncol. 2009;27:5255–5261. doi: 10.1200/JCO.2009.22.0616. [DOI] [PubMed] [Google Scholar]
- 27.Jain RK, Lee JJ, Hong D, et al. Phase I oncology studies: evidence that in the era of targeted therapies patients on lower doses do not fare worse. Clin Cancer Res. 2010;16:1289–1297. doi: 10.1158/1078-0432.CCR-09-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gianni L, Romieu GH, Lichinister M, et al. AVEREL: a randomized phase III trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer. J Clin Oncol. 2013;31:1719–1725. doi: 10.1200/JCO.2012.44.7912. [DOI] [PubMed] [Google Scholar]
- 29.Lambrechts D, Lenz HJ, de Haas S, et al. Markers of response for the antiangiogenic agent bevacizumab. J Clin Oncol. 2013;31:1219–1230. doi: 10.1200/JCO.2012.46.2762. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.