Abstract
Background
Despite improvement with intensive multi-agent chemotherapy, 2-year progression-free survival (PFS) rates for adults with high-risk Burkitt's lymphoma (BL) remains <55%.
Patients and methods
We conducted a phase II trial for newly diagnosed classic BL utilizing liposomal doxorubicin (Adriamycin) in lieu of doxorubicin and incorporating intravenous rituximab (at 500 mg/m2 twice/cycle) into the CODOX-M/IVAC regimen. Correlative analyses included paired serum and cerebrospinal fluid (CSF) rituximab levels and close examination of cardiac function.
Results
Among 25 BL patients, the median age was 44 years (23–70) and 4 patients were HIV positive. There were 20 high-risk and 5 low-risk patients. At baseline, 40% of high-risk patients had bone marrow involvement, 35% had bulky disease and 15% had central nervous system involvement. The overall response rate was 100% (complete remission 92%). At 34-month median follow-up, the 2-year PFS and overall survival (OS) rates for all patients were 80% and 84%, respectively (low-risk: both 100%; high-risk: 76% and 81%, respectively). Furthermore, the 2-year PFS, OS, and disease-specific survival (DSS) rates for high-risk, HIV-negative patients were 84%, 89% and 100%, respectively. Adverse events (AEs) appeared to be consistent with prior CODOX-M/IVAC data, although there were several grade 3 cardiac events noted (all declined ejection fraction without clinical symptoms). The mean serum rituximab levels at 24 h after cycles 1 and 3 for patients without relapse were 258 and 306 μg/ml, respectively, versus 131 and 193 μg/ml, respectively, for patients with early progression (P = 0.002 and 0.002, respectively). The mean CSF rituximab levels for all patients were 0.11 and 0.24 μg/ml, respectively, at cycle 1 (24/72 h), which equated to serum:CSF ratios of 0.05% and 0.20%, respectively.
Conclusions
The integration of rituximab into CODOX-M/IVAC for adult BL was feasible and tolerable, while changes in cardiac function warrant continued examination. This regimen was associated with excellent survival rates for HIV-negative BL. Further investigation of the predictive value of serum rituximab is needed. Clinicaltrials.gov NCT00392990.
Keywords: burkitt's lymphoma, cancer, liposomal doxorubicin, non-Hodgkin's lymphoma, prognosis, rituximab
introduction
Cure rates for Burkitt's lymphoma (BL) have improved in part due to the incorporation of intensive multi-agent chemotherapy [1, 2]. Magrath et al. from the National Cancer Institute (NCI) developed a regimen that alternated two courses of multi-agent chemotherapy: cyclophosphamide (Baxter, Deerfield, Illinois, USA), vincristine (Hospira, Inc., Lake Forest, Illinois, USA), doxorubicin (Adriamycin) (Pharmacia, Milan, Italy), high-dose methotrexate (CODOX-M) and ifosfamide, etoposide and high-dose cytarabine (IVAC) [3].
The CODOX-M/IVAC regimen has been refined over the past decade, including omission of day 15 vincristine [4] and decreased dosing of intravenous high-dose methotrexate [5]. The 2-year progression-free survival (PFS) and OS rates in the latter study for adult BL patients were 64% and 67%, respectively, with PFS and OS rates for high-risk patients of 49% and 52%, respectively. Despite improvement in the survival of adult BL patients [4–6], there remains a need to improve outcomes.
Incorporation of the monoclonal antibody, rituximab, has resulted in significantly improved outcomes for several B-cell non-Hodgkin's lymphomas (NHL). BL is a B-cell lymphoma with high CD20 expression and thus intuitively should be responsive to rituximab therapy. Retrospective analyses, however, have shown mixed results with several reports questioning the impact of rituximab added to CODOX-M/IVAC [7–10]. We conducted a prospective multicenter phase II study for patients with untreated classic BL (including HIV+) adding intravenous rituximab to CODOX-M/IVAC therapy and substituting liposomal doxorubicin in lieu of standard anthracycline therapy.
methods
eligibility and treatment
This clinical trial was registered at clinicaltrials.gov (NCT00392990). The study was approved by each participating Institutional Review Board (IRB). Eligible patients were age ≥18 years (no upper limit), had biopsy-proven newly diagnosed classic BL and an ECOG performance status (PS) of 0–2. Patients may have received a maximum of one cycle of CHOP-like therapy before study entry (maximum drug doses: cyclophosphamide 1 000 mg/m2, doxorubicin 50 mg/m2 and vincristine 2 mg). Patients were assigned risk according to the 2008 Mead et al. definition (i.e., low-risk having all features: (i) normal LDH, (ii) ECOG PS of 0–1, (iii) Ann Arbor stage I/II and (iv) no mass >10 cm; all other patients were considered high-risk) [5]. Low-risk patients received three consecutive cycles of CODOX-M regimen (regimen A). High-risk patients received four alternating cycles of CODOX-M (regimen A) and IVAC (regimen B). Treatment details are included in supplementary Tables s1–3, available at Annals of Oncology online. Additional supportive care guidelines, including recommendations for HIV+ patients, may be found in supplementary Table S4, available at Annals of Oncology online.
statistical analysis
The primary objective was to evaluate the complete remission (CR) rate after completion of therapy. The two-stage design tested the null hypothesis of P ≤ 0.500 versus the alternative that P ≥ 0.750 (CR rate). The associated alpha was 0.046 and the beta/power was 0.80. Exploratory objectives included examination of cardiac adverse events (AEs) including serial assessments of ejection fraction (EF) before, during and following completion of all therapy. In addition, we investigated paired serum and CSF rituximab pharmacokinetics for the first 10 patients enrolled on study (supplementary Appendix S5, available at Annals of Oncology online for detailed methods).
results
patients
Twenty-five patients were enrolled and treated (Table 1). The median age was 44 years (23–70); there were 20 high-risk and 5 low-risk patients. Three high-risk and one low-risk patient were HIV+; all HIV patients had newly diagnosed HIV at the time of BL diagnosis and each were started on highly active anti-retroviral therapy (HAART) before chemotherapy. Further, the mean CD4 count at diagnosis for HIV+ patients was 158 cells/μl (67–314). Among all high-risk BL patients, other disease characteristics included: 15% CNS involvement; 35% bulky disease (i.e. >10 cm); and 40% bone marrow involvement.
Table 1.
Patient characteristics
| Characteristic | No. of patients (%) |
|---|---|
| Age (years) | |
| Median | 44 |
| Range | 23–70 |
| Gender | |
| Male | 22 (50) |
| Female | 3 (50) |
| HIV status | |
| Positive | 4 (16%) |
| Negative | 21 (84%) |
| Histology | |
| Classical BLa | 25 (100) |
| ECOG performance status (PS) | |
| Median | 1 |
| Range | 0–2 |
| CNS involvement | |
| Yesb | 3 (12%) |
| No | 22 (88%) |
| Elevated LDH | |
| Yes | 17 (68%) |
| No | 8 (32%) |
| Bulky disease (>10 cm) | |
| Yes | 7 (28%) |
| No | 18 (72%) |
| Bone marrow involvement | |
| Yes | 8 (32%) |
| No | 17 (68%) |
| Disease risk | |
| Low | 5 (20%) |
| High | 20 (80%) |
aIncluded one patient with ‘double hit’ lymphoma (MYC and BCL-2).
bIncluded two patients with cerebrospinal fluid (CSF) involvement and one patient with cranial nerve (ocular) infiltration.
No, number; BL, Burkitt's lymphoma; ECOG, Eastern Cooperative Oncology Group; HIV, human immunodeficiency virus; CNS, central nervous system; LDH, lactate dehydrogenase.
Therapy was completed at a median of 13 weeks (11–20) for high-risk patients and 10 weeks for low-risk patients (9–12).
outcomes
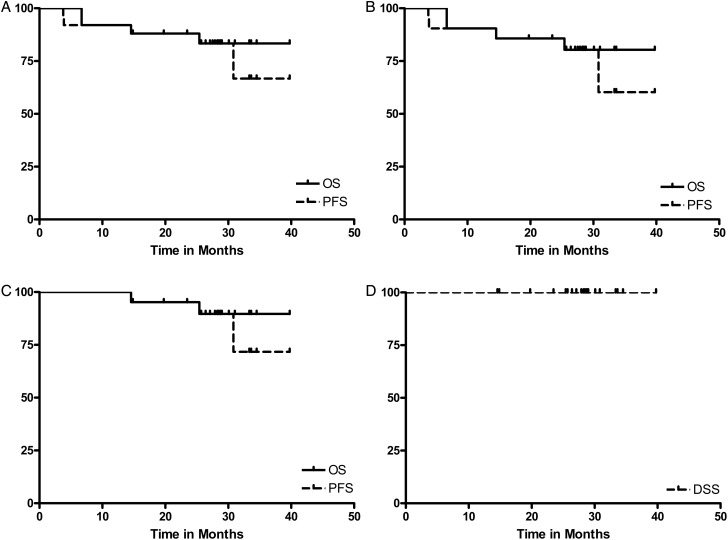
The overall response rate (ORR) after two cycles was 100% (CR 67%), while at completion of therapy, the ORR was 100% (CR 92%). At a median follow-up of 34 months (15–45 months), 2-year PFS and OS rates for all patients were 80% and 84%, respectively (Figure 1). Two-year PFS and OS for low-risk patients were 100% and 100%, respectively; the 2-year PFS and OS for high-risk patients were 76% and 81%, respectively. Further, the 2-year PFS, OS and disease-specific survival (DSS) rates for the 19 high-risk, HIV-negative patients were 84%, 89% and 100%, respectively (Figure 1).
Figure 1.
Survival of all BL patients and high-risk patients. The Kaplan–Meier 2-year (A) progression-free survival (PFS) and overall survival (OS) for the 25 BL patients were 80% and 84%, respectively. The 2-year PFS and OS for all low-risk patients were 100% and 100%, respectively, and (B) the 2-year PFS and OS for all high-risk patients were 76% and 81%, respectively. BL, Burkitt's lymphoma. The Kaplan–Meier (C) 2-year PFS and OS for the 19 high-risk, HIV-negative patients were 84% and 89%, while the (D) 2-year disease-specific survival (DSS) rate for these patients was 100%. HIV, human immunodeficiency virus; BL, Burkitt's lymphoma.
Among all, three patients experienced progressive disease and four patients died. Two of the three progressions occurred early (i.e. within 6 weeks of completion of therapy); both were HIV-positive men with high-risk disease. These two relapses were heralded by an abrupt >1000 log rise in their viral load by PCR. Despite attempts at salvage therapy, both patients died <3 months after initial progression. The third progression occurred at 31 months in a 64-year-old, HIV-negative female with high-risk disease. At relapse, a ‘double hit’ lymphoma was identified with concurrent BCL-2 and MYC over-expression. The patient is alive and currently undergoing salvage chemotherapy. The two other deaths were a 70-year-old HIV-negative man with high-risk disease who died from unknown causes at 17 months (in remission) and a 65-year old HIV-negative man with high-risk disease at 24 months due to myelodysplastic syndrome/acute leukemia, which was diagnosed 2.5 months after study entry; cytogenetics showed deletion of chromosome 5.
adverse events
Altogether, toxic effects appeared to be consistent with prior CODOX-M/IVAC series [3–5]. As noted in Table 2, grade 3/4 thrombocytopenia occurred in 68% of patients (60% grade 4) with 72% of patients experiencing grade 3/4 anemia (4% grade 4). The most frequent non-hematologic grade 3/4 toxicity was mucositis. Notably, mucositis resulted in intermittent non-compliance with HAART for HIV+ patients; however, there were no significant differences in AEs comparing HIV+ and negative patients (data not shown). Cardiac status was tracked closely in all patients. The median change in EF for all patients at baseline versus study end was −2% (−22% to +11%). There were two grade 2 and three grade 3 cardiac AEs that occurred. All of these events were depressed EF without clinical evidence of congestive heart failure. The grade 3 cardiac AEs occurred in 53-, 69- and 70-year-old men, all with HIV-negative, high-risk disease and the latter two patients had history of myocardial infarction.
Table 2.
Adverse events: grade 3 and 4*
| Event | No. G3 | No. G4 |
|---|---|---|
| Hematologic | ||
| Anemia | 16 (68%) | 1 (4%) |
| Neutropenia | 7 (28%) | 8 (32%) |
| Thrombocytopenia | 2 (8%) | 15 (60%) |
| Non-Hematologic | ||
| Mucositis | 9 (36%) | 3 (12%) |
| Infection | 9 (36%) | 0 |
| Elevated transaminases | 8 (32%) | 0 |
| Fever (neutropenic) | 7 (28%) | 0 |
| Low phosphate | 6 (24%) | 0 |
| Sodium abnormalities | 6 (24%) | 0 |
| Hyperglycemia | 4 (16%) | 5 (20%) |
| Hypoalbuminemia | 4 (16%) | 0 |
| Hypokalemia | 4 (16%) | 0 |
| Cardiac | 3 (12%) | 0 |
| Diarrhea | 2 (8%) | 0 |
| Elevated creatinine | 2 (8%) | 0 |
| Nausea/vomiting | 1 (4%) | 0 |
| Low blood pressure | 1 (4%) | 0 |
| Rash | 1 (4%) | 0 |
| Edema | 1 (4%) | 0 |
| Low magnesium | 1 (4%) | 0 |
*Cumulative (i.e. all patients, all cycles).
No, number; G3, Grade 3; G4, Grade 4.
rituximab pharmacokinetics
The mean CSF rituximab levels at 24 and 72 h of rituximab for cycle 1 were 0.11 and 0.24 µg/ml, respectively, and cycle 3 were 0.24 and 0.26 µg/ml, respectively. The mean (paired) serum rituximab levels for patients at 24 and 72 h for cycle 1 were 235 and 122 µg/ml, respectively, and at cycle 3 were 286 and 205 µg/ml, respectively. The serum:CSF rituximab ratios for patients at 24 and 72 h for cycle 1 were 0.04% and 0.20%, respectively, and the associated ratios for cycle 3 were 0.08% and 0.13%, respectively.
The mean serum and CSF rituximab levels for the two patients who experienced early relapse (and died) were compared with patients without relapse (Table 3). Interestingly, cycle 1, 24-h serum rituximab levels were significantly higher among patients without relapse compared with the two patients who relapsed/died (258 versus 131 µg/ml, respectively, P = 0.002) as were cycle 1, 72-h (139 µg/ml versus 45 µg/ml respectively, P = 0.004) and cycle 3, 24-h serum rituximab levels (306 versus 193 µg/ml, respectively, P = 0.002). Notably, rituximab PK levels did not differ based on marrow involvement, bulky disease or high-/low-risk disease (data not shown). Additionally, there were no clinical or laboratory prognostic factors that predicted survival.
Table 3.
Serum and CSF rituximab levels for patients with and without early progression
| Chemotherapy cycle and hours after rituximab infusion | Mean serum rituximab level (μg/ml) for patients w/o relapse | Serum rituximab levels (μg/ml) for two patients with early progression |
Mean CSF rituximab level (μg/ml) for patients w/o relapse | CSF rituximab levels (μg/ml) for two patients with early progression |
||
|---|---|---|---|---|---|---|
| Pt #1 | Pt #2 | Pt #1 | Pt #2 | |||
| C1/24 h | 258* | 171 | 91 | 0.104 | 0.27 | 0.23 |
| C1/72 h | 139* | 60 | 29 | 0.253 | 0.22 | 0.12 |
| C3/24 h | 306* | 162 | 224 | 0.246 | 0.27 | N/A |
| C3/72 h | 219 | 149 | 135 | 0.196 | 0.38 | 0.50 |
Abbreviations: CSF, cerebrospinal fluid; Pt, patient; C, cycle; w/o, without; N/A, not available.
*P < 0.005 when compared with the mean serum levels of the two patients with early progression.
discussion
BL is a rare form of cancer in adults with ∼200–250 new cases occurring each year in the United States, which accounts for <0.5% of all NHLs. Survival rates have improved significantly over the past 15–20 years primarily as a result of dose intensive systemic chemotherapy protocols. This was first shown to be a successful therapeutic strategy in pediatric BL patients [3, 11, 12]. In 1996, Magrath et al. reported excellent results in children and young adults with CODOX-M/IVAC [3]. In subsequent studies using CODOX-M/IVAC for older populations, the 2-year PFS rate for high-risk patients was 54% [4, 5]. We observed excellent outcomes here with the incorporation of high-dose rituximab and liposomal doxorubicin into CODOX-M/IVAC, including a 2-year PFS rate for high-risk, HIV-negative BL patients of 84%, which appears to be higher compared with prior published data. In addition, serum rituximab levels appeared to correlate with patient outcome, while cardiac toxicity here did not appear diminished compared with historical controls.
It is important to note that the initial results reported by Magrath et al. were in a younger population (i.e. median age of the 21 children and 20 adults on 89-C-41 study were 12 and 25 years, respectively) [3]. Survival rates for adult BL patients appeared to be comparable across the two contemporary Mead studies, while toxicity was reduced. LaCasce et al. treated 14 BL/BLL patients with a further modified version of CODOX-M/IVAC [6]. Two-year PFS and OS for high-risk patients were 60% and 60%, respectively. We observed 2-year PFS and OS rates of 80% and 84%, respectively, with rates for low-risk patients of 100% and 100%, respectively, and for high-risk patients of 76% and 81%, respectively. Moreover, the 2-year PFS, OS and DSS rates for high-risk, HIV-negative patients were 84%, 89% and 100%, respectively. Among the 19 high-risk, HIV-negative patients, there were only two deaths, both from non-relapse causes; the only additional relapse was in a patient with apparent ‘double hit’ NHL who relapsed slightly <3 years after initial therapy. The double-hit was not identified at original diagnosis; this may have represented clonal evolution or may have been related to the degree of necrosis seen in the original tissue specimen that affected sensitivity of FISH analysis. Altogether, the survival rates seen here appear >20%–25% higher compared with historical controls. A potential explanation for this improvement may be to rituximab.
There have been recent conflicting data regarding an added benefit of rituximab to the CODOX-M/IVAC regimen. Barnes et al. retrospectively examined 80 patients treated with CODOX-M/IVAC with or without rituximab; they found a numeric, but statistically non-significant improvement in survival (i.e. 3-year PFS 74% versus 61%, respectively). A recent retrospective population-based data analysis showed that rituximab was not associated with improved OS on multivariate analysis [7]. Conversely, Thomas et al. showed in a phase II study that rituximab added to hyperCVAD/MA appeared to be associated with a significant improvement in 3-year PFS and OS (i.e. 80% and 89%, respectively) compared with hyperCVAD/MA without rituximab (i.e. 52% and 53%, respectively) [13]. Further, an Italian group recently documented favorable outcomes adding rituximab to CODOX-M/IVAC with 4-year PFS of 92% in 15 BL patients [14]. Moreover, a phase III randomized study reported in abstract form showed an improved event-free survival and OS for patients who received rituximab combined with chemotherapy [15]. The optimal dosing of rituximab in BL is not known; we utilized a higher dose given the propensity of CNS involvement by BL and the hypothesis that higher dosing would result in more drug crossing the blood brain barrier (BBB). Of note, there is extremely limited data regarding the amount of rituximab that cross the BBB.
The serum:CSF rituximab ratios in the current study appeared consistent with prior data from Rubenstein et al. (2 primary CNS patients treated with rituximab (375 mg/m2) had serum:CSF ratios of ∼0.12) [16]. There is also prior data that serum rituximab concentrations may correlate with outcome [17, 18]. The COG recently examined rituximab serum levels in BL pediatric and adolescents [19] where levels did not correlate with outcome. Mean serum levels here ranged from 139–306 μg/ml, while 24-h serum rituximab levels at cycles 1 and 3 appeared higher among patients without relapse compared with the two patients with early relapse. However, the sample size here was small and these results are only speculative; in addition, the most optimal timing for rituximab PK data should be at the trough prior to the next dose.
The optimal treatment of BL for HIV+ patients is not known. In the post-HAART era, intensive chemotherapy has been shown to be feasible and safe. Cortes et al. treated 13 HIV+ BL patients with hyperCVAD/MA showing a 100% ORR (92% CR). Further, when matching characteristics to an HIV-negative BL population, tolerability and outcome were similar compared with the HIV+ population. Wang et al. treated 14 HIV+ adults with BL (8 with standard CODOX-M/IVAC and 6 with other regimens). The 2-year survival for CODOX-M/IVAC-treated HIV+ patients was 60%, and they showed that HIV status did not adversely affect outcomes. Of the four HIV+ patients treated on the current study, two experienced early progression and died. It is not clear if there was a clinical significance to the abrupt rise in HIV viral load that heralded relapse in these two patients; the rise in viral load may have been related to intermittent use of HAART, which was accentuated by chemotherapy-related mucositis. There are recent encouraging data utilizing dose-adjusted EPOCH with rituximab therapy in HIV+ BL. These investigators advocate holding/stopping HAART during EPOCH therapy.
Finally, the overall toxicity profile of the current protocol appeared to be similar compared with prior CODOX-M/IVAC data. In an attempt to mitigate cardiac toxicity, we used liposomal doxorubicin in lieu of standard doxorubicin. We tracked EF more frequently than standard of care (i.e. baseline, mid-treatment, and completion of treatment). The median change of EF from baseline to study completion was −2%, while there were two grade 2 and three grade 3 cardiac AEs. Interestingly, all cardiac AEs were as a result of declined EF in asymptomatic patients; there were no cases of clinical heart failure seen. There are no prior data that have examined cardiac function in BL patients treated with intensive chemotherapy. Further, it is not known whether the increased rates of cardiac AEs seen here were as a result of close cardiac assessment versus a true signal of cardiac toxicity. More and closer examination of acute and long-term cardiac function in BL patients is warranted, which may include sensitive cardiac markers such as NT-proBNP.
In summary, we identified that the integration of high-dose rituximab into the CODOX-M/IVAC regimen for adult BL is feasible and associated with mostly similar tolerability compared with prior reports. The use of liposomal doxorubicin did not appear to decrease acute cardiac toxicity. Altogether, this regimen was associated with excellent survival rates, especially for HIV-negative BL. Further investigation of the predictive value of serum rituximab concentration is warranted.
funding
Supported in part through K23 CA109613-A1 to AME.
disclosure
LIG and CN: conflict with Genentech (Advisory board: Honorarium); all the remaining authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
The work was presented in part at the American Society of Hematology (ASH), 54th Annual Meeting, Georgia, Atlanta, December 2012 and the 12th International Conference on Malignant Lymphoma, Lugano, Switzerland, June 2013. The authors thank the University of Wisconsin Carbone Comprehensive Cancer Center (UWCCC) for use of its facilities to complete this research; the work was supported in part by NIH/NCI P30 CA014520 (UWCCC Support).
references
- 1.Evens AM, Gordon LI. Burkitt's and Burkitt-like lymphoma. Curr Treat Options Oncol. 2002;3:291–305. doi: 10.1007/s11864-002-0029-9. [DOI] [PubMed] [Google Scholar]
- 2.Costa LJ, Xavier AC, Wahlquist AE, et al. Trends in survival of patients with Burkitt lymphoma/leukemia in the USA: an analysis of 3691 cases. Blood. 2013;121(24):4861–4866. doi: 10.1182/blood-2012-12-475558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magrath I, Adde M, Shad A, et al. Adults and children with small non-cleaved-cell lymphoma have a similar excellent outcome when treated with the same chemotherapy regimen. J Clin Oncol. 1996;14:925–934. doi: 10.1200/JCO.1996.14.3.925. [DOI] [PubMed] [Google Scholar]
- 4.Mead GM, Sydes MR, Walewski J, et al. An international evaluation of CODOX-M and CODOX-M alternating with IVAC in adult Burkitt's lymphoma: results of United Kingdom Lymphoma Group LY06 study. Ann Oncol. 2002;13:1264–1274. doi: 10.1093/annonc/mdf253. [DOI] [PubMed] [Google Scholar]
- 5.Mead GM, Barrans SL, Qian W, et al. A prospective clinicopathologic study of dose-modified CODOX-M/IVAC in patients with sporadic Burkitt lymphoma defined using cytogenetic and immunophenotypic criteria (MRC/NCRI LY10 trial) Blood. 2008;112:2248–2260. doi: 10.1182/blood-2008-03-145128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lacasce A, Howard O, Lib S, et al. Modified magrath regimens for adults with Burkitt and Burkitt-like lymphomas: preserved efficacy with decreased toxicity. Leuk Lymphoma. 2004;45:761–767. doi: 10.1080/1042819031000141301. [DOI] [PubMed] [Google Scholar]
- 7.Wasterlid T, Brown PN, Hagberg O, et al. Impact of chemotherapy regimen and rituximab in adult Burkitt lymphoma: a retrospective population-based study from the Nordic Lymphoma Group. Ann Oncol. 2013;24:1879–1886. doi: 10.1093/annonc/mdt058. [DOI] [PubMed] [Google Scholar]
- 8.Barnes JA, Lacasce AS, Feng Y, et al. Evaluation of the addition of rituximab to CODOX-M/IVAC for Burkitt's lymphoma: a retrospective analysis. Ann Oncol. 2011;22:1859–1864. doi: 10.1093/annonc/mdq677. [DOI] [PubMed] [Google Scholar]
- 9.Maruyama D, Watanabe T, Maeshima AM, et al. Modified cyclophosphamide, vincristine, doxorubicin, and methotrexate (CODOX-M)/ifosfamide, etoposide, and cytarabine (IVAC) therapy with or without rituximab in Japanese adult patients with Burkitt lymphoma (BL) and B cell lymphoma, unclassifiable, with features intermediate between diffuse large B cell lymphoma and BL. Int J Hematol. 2010;92:732–743. doi: 10.1007/s12185-010-0728-0. [DOI] [PubMed] [Google Scholar]
- 10.Mohamedbhai SG, Sibson K, Marafioti T, et al. Rituximab in combination with CODOX-M/IVAC: a retrospective analysis of 23 cases of non-HIV related B-cell non-Hodgkin lymphoma with proliferation index >95% Br J Haematol. 2011;152:175–181. doi: 10.1111/j.1365-2141.2010.08447.x. [DOI] [PubMed] [Google Scholar]
- 11.Murphy SB, Bowman WP, Abromowitch M, et al. Results of treatment of advanced-stage Burkitt's lymphoma and B cell (SIg+) acute lymphoblastic leukemia with high-dose fractionated cyclophosphamide and coordinated high-dose methotrexate and cytarabine. J Clin Oncol. 1986;4:1732–1739. doi: 10.1200/JCO.1986.4.12.1732. [DOI] [PubMed] [Google Scholar]
- 12.Bowman WP, Shuster JJ, Cook B, et al. Improved survival for children with B-cell acute lymphoblastic leukemia and stage IV small noncleaved-cell lymphoma: a pediatric oncology group study. J Clin Oncol. 1996;14:1252–1261. doi: 10.1200/JCO.1996.14.4.1252. [DOI] [PubMed] [Google Scholar]
- 13.Thomas DA, Faderl S, O'Brien S, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006;106:1569–1580. doi: 10.1002/cncr.21776. [DOI] [PubMed] [Google Scholar]
- 14.Corazzelli G, Frigeri F, Russo F, et al. RD-CODOX-M/IVAC with rituximab and intrathecal liposomal cytarabine in adult Burkitt lymphoma and ‘unclassifiable’ highly aggressive B-cell lymphoma. Br J Haematol. 2012;156:234–244. doi: 10.1111/j.1365-2141.2011.08947.x. [DOI] [PubMed] [Google Scholar]
- 15.Ribrag VKS, Bouabdallah K. Addition of Rituximab Improves Outcome of HIV Negative Patients with Burkitt Lymphoma Treated with the Lmba Protocol: Results of the Randomized Intergroup (GRAALL-Lysa) LMBA02 Protocol. (IGR sponsored LMBA02, NCT00180882) Blood (ASH Annual Meeting Abstracts) 2012;120:685a. [Google Scholar]
- 16.Rubenstein JL, Combs D, Rosenberg J, et al. Rituximab therapy for CNS lymphomas: targeting the leptomeningeal compartment. Blood. 2003;101:466–468. doi: 10.1182/blood-2002-06-1636. [DOI] [PubMed] [Google Scholar]
- 17.Berinstein NL, Grillo-Lopez AJ, White CA, et al. Association of serum Rituximab (IDEC-C2B8) concentration and anti-tumor response in the treatment of recurrent low-grade or follicular non-Hodgkin's lymphoma. Ann Oncol. 1998;9:995–1001. doi: 10.1023/A:1008416911099. [DOI] [PubMed] [Google Scholar]
- 18.Jager U, Fridrik M, Zeitlinger M, et al. Rituximab serum concentrations during immuno-chemotherapy of follicular lymphoma correlate with patient gender, bone marrow infiltration and clinical response. Haematologica. 2012;97:1431–1438. doi: 10.3324/haematol.2011.059246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barth MJ, Goldman S, Smith L, et al. Rituximab pharmacokinetics in children and adolescents with de novo intermediate and advanced mature B-cell lymphoma/leukaemia: a Children's Oncology Group report. Br J Haematol. 2013;162:678–683. doi: 10.1111/bjh.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.